Predicting 90-Day Prognosis in Ischemic Stroke Patients Post Thrombolysis Using Machine Learning
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
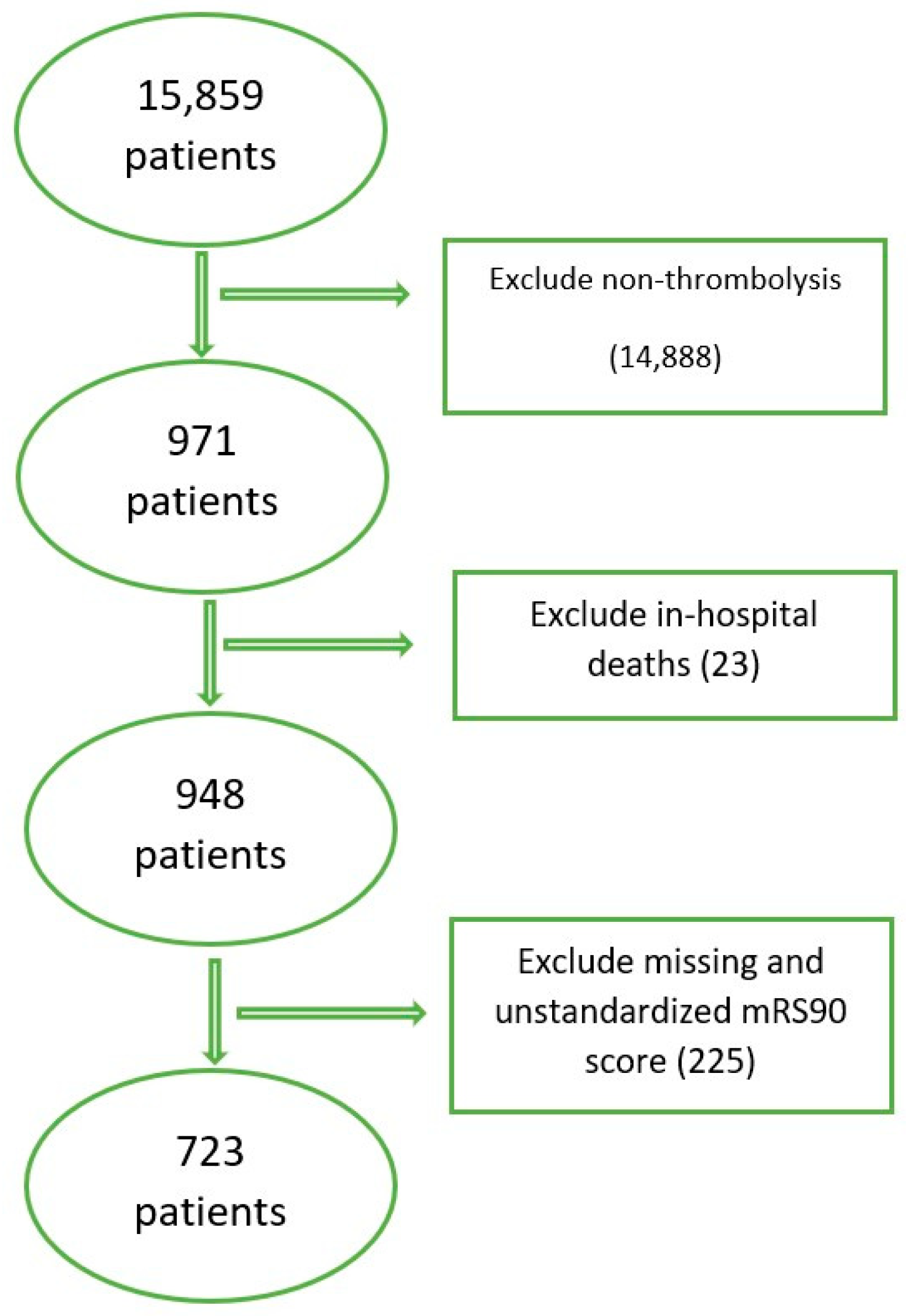
2.2. Inclusion/Exclusion Criteria
2.3. Baseline Variables
2.4. Outcome Variable
2.5. Handling Missing Data
2.6. Model Training and Evaluation
3. Results
3.1. Model Evaluation
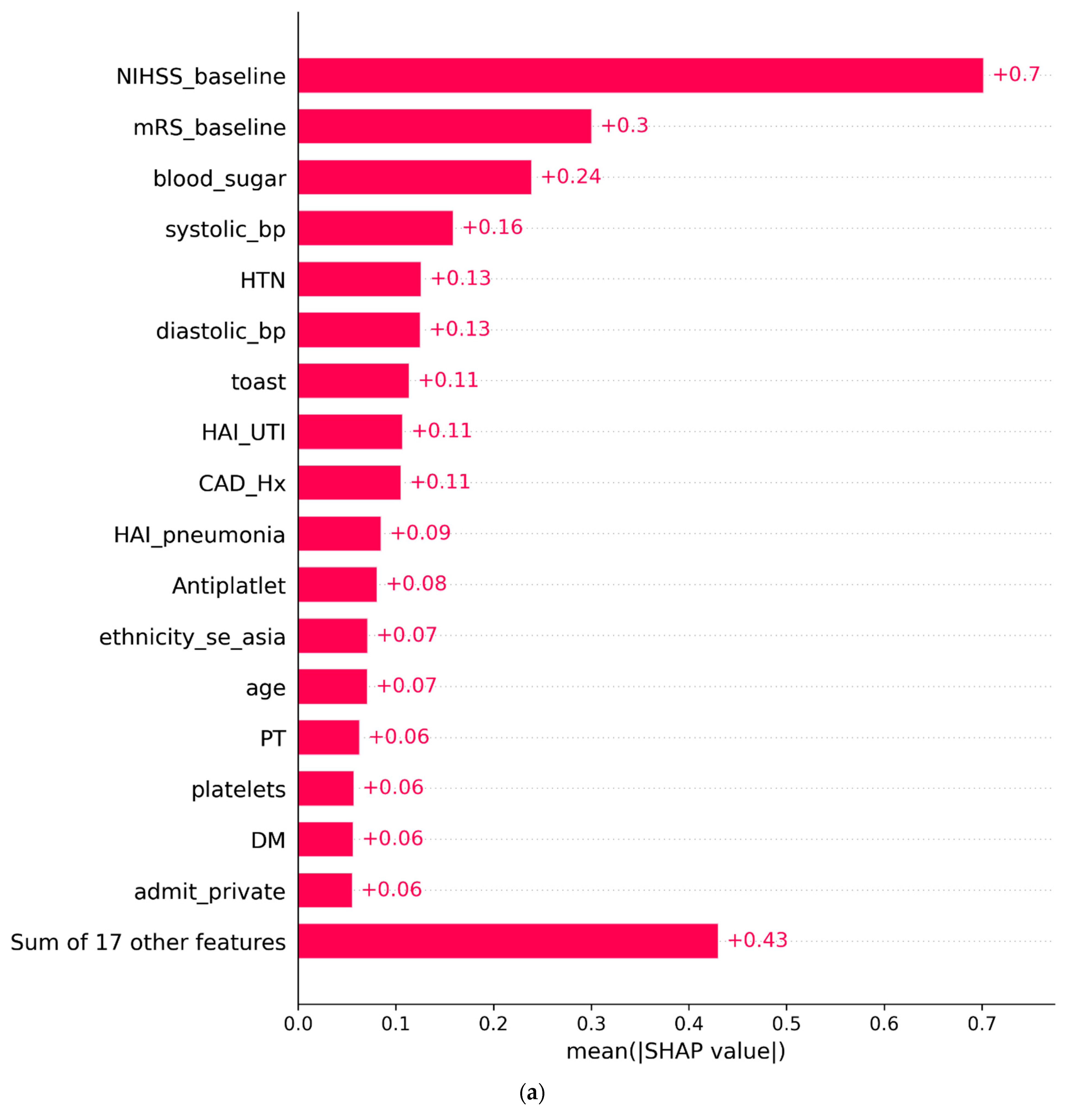
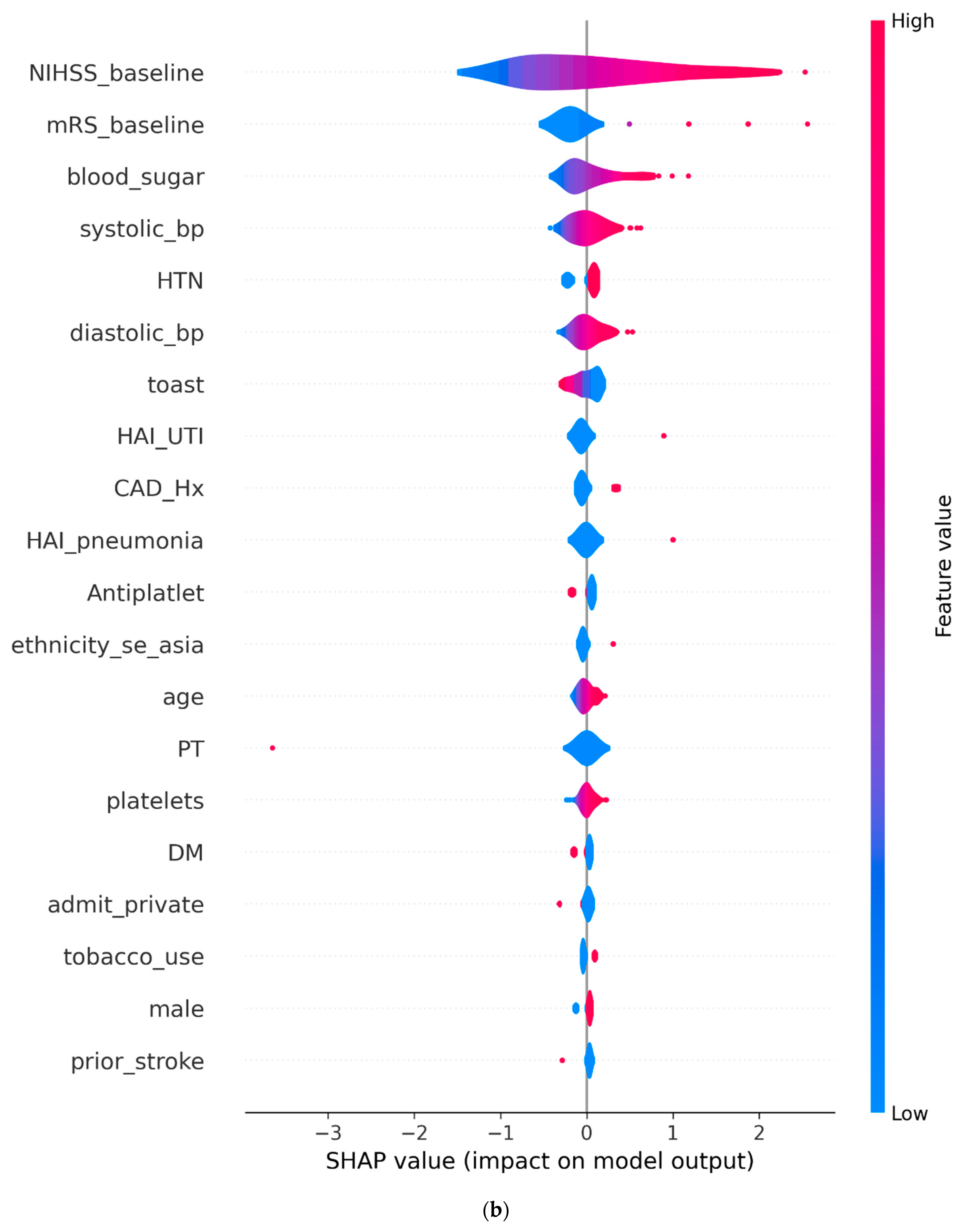
3.2. SHAP Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global stroke fact sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Phipps, M.S.; Cronin, C.A. Management of acute ischemic stroke. BMJ 2020, 368, l6983. [Google Scholar] [CrossRef]
- Fekete, K.E.; Héja, M.; Márton, S.; Tóth, J.; Harman, A.; Horváth, L.; Fekete, I. Predictors and long-term outcome of intracranial hemorrhage after thrombolytic therapy for acute ischemic stroke—A prospective single-center study. Front. Neurol. 2023, 14, 1080046. [Google Scholar] [CrossRef] [PubMed]
- Cerasuolo, J.O.; Mandzia, J.; Cipriano, L.E.; Kapral, M.K.; Fang, J.; Hachinski, V.; Sposato, L.A. Intravenous thrombolysis after first-ever ischemic stroke and reduced incident dementia rate. Stroke 2022, 53, 1170–1177. [Google Scholar] [CrossRef]
- Chwojnicki, K.; Kozera, G.; Sobolewski, P.; Fryze, W.; Nyka, W. Intravenous thrombolysis and three-year ischemic stroke mortality. Acta Neurol. Scand. 2017, 135, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Imam, Y.Z.; Kamran, S.; Saqqur, M.; Ibrahim, F.; Chandra, P.; Perkins, J.D.; Malik, R.A.; Akhtar, N.; Al-Jerdi, S.; Deleu, D. Stroke in the adult Qatari population (Q-stroke) a hospital-based retrospective cohort study. PLoS ONE 2020, 15, e0238865. [Google Scholar] [CrossRef]
- Van Wijngaarden, J.D.; Dirks, M.; Huijsman, R.; Niessen, L.W.; Fabbricotti, I.N.; Dippel, D.W.; The Promoting Acute Thrombolysis for Ischaemic Stroke (PRACTISE) Investigators. Hospital rates of thrombolysis for acute ischemic stroke: The influence of organizational culture. Stroke 2009, 40, 3390–3392. [Google Scholar] [CrossRef]
- Imam, Y.Z.; Kamran, S.; Akhtar, N.; Deleu, D.; Singh, R.; Malik, R.A.; Abdelmoneim, M.; Bermejo, P.; Bourke, P.; Morgan, D. Incidence, clinical features and outcomes of atrial fibrillation and stroke in Qatar. Int. J. Stroke 2020, 15, 85–89. [Google Scholar] [CrossRef]
- Deb-Chatterji, M.; Schlemm, E.; Flottmann, F.; Meyer, L.; Alegiani, A.; Brekenfeld, C.; Fiehler, J.; Gerloff, C.; Thomalla, G. Sex differences in outcome after thrombectomy for acute ischemic stroke are explained by confounding factors. Clin. Neuroradiol. 2021, 31, 1101–1109. [Google Scholar] [CrossRef]
- Haranhalli, N.; Javed, K.; Boyke, A.; Dardick, J.; Naidu, I.; Ryvlin, J.; Kadaba, D.; Fluss, R.; Derby, C.; Altschul, D. A Predictive Model for Functional Outcome in Patients with Acute Ischemic Stroke Undergoing Endovascular Thrombectomy. J. Stroke Cerebrovasc. Dis. 2021, 30, 106054. [Google Scholar] [CrossRef]
- Reid, J.M.; Dai, D.; Delmonte, S.; Counsell, C.; Phillips, S.J.; MacLeod, M.J. Simple prediction scores predict good and devastating outcomes after stroke more accurately than physicians. Age Ageing 2017, 46, 421–426. [Google Scholar] [CrossRef]
- Thompson, D.D.; Murray, G.D.; Sudlow, C.L.M.; Dennis, M.; Whiteley, W.N. Comparison of Statistical and Clinical Predictions of Functional Outcome after Ischemic Stroke. PLoS ONE 2014, 9, e110189. [Google Scholar] [CrossRef]
- Pirson, F.A.V.; Boodt, N.; Brouwer, J.; Bruggeman, A.A.; den Hartog, S.J.; Goldhoorn, R.-J.B.; Langezaal, L.C.; Staals, J.; van Zwam, W.H.; van der Leij, C. Endovascular treatment for posterior circulation stroke in routine clinical practice: Results of the multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands registry. Stroke 2022, 53, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Teng, J.; Liebeskind, D.; Miao, W.; Du, R. Predictors of Infarct Growth Measured by Apparent Diffusion Coefficient Quantification in Patients with Acute Ischemic Stroke. World Neurosurg. 2019, 123, e797–e802. [Google Scholar] [CrossRef]
- Kelion, A.D.; Banning, A.P.; Shahi, M.; Bell, J.A. The effect of reduction of door-to-needle times on the administration of thrombolytic therapy for acute myocardial infarction. Postgrad. Med. J. 1998, 74, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Murray, V.; Berge, E.; Del Zoppo, G.J. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev. 2014, 2014, CD000213. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, W.; Wu, B.; Sun, X. Intravenous thrombolysis for acute ischemic stroke with extended time window. Chin. Med. J. 2021, 134, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Campbell, B.C.V.; Parsons, M.W.; Churilov, L.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Wijeratne, T.; Curtze, S.; Dewey, H.M.; et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N. Engl. J. Med. 2019, 380, 1795–1803. [Google Scholar] [CrossRef]
- Brouwer, J.; Smaal, J.A.; Emmer, B.J.; de Ridder, I.R.; van den Wijngaard, I.R.; de Leeuw, F.-E.; Hofmeijer, J.; van Zwam, W.H.; Martens, J.M.; Roos, Y.B. Endovascular thrombectomy in young patients with stroke: A MR CLEAN registry study. Stroke 2022, 53, 34–42. [Google Scholar] [CrossRef]
- Chen, S.-D.; You, J.; Yang, X.-M.; Gu, H.-Q.; Huang, X.-Y.; Liu, H.; Feng, J.-F.; Jiang, Y.; Wang, Y.-J. Machine learning is an effective method to predict the 90-day prognosis of patients with transient ischemic attack and minor stroke. BMC Med. Res. Methodol. 2022, 22, 195. [Google Scholar] [CrossRef]
- Purrucker, J.; Hametner, C.; Engelbrecht, A.; Bruckner, T.; Popp, E.; Poli, S. Comparison of Stroke Recognition and Stroke Severity Scores for Stroke Detection in a Single Cohort. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1021. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of Acute Cerebral Infarction: A Clinical Examination Scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Adams, H.; Bendixen, B.; Kappelle, L.; Biller, J.; Love, B.; Gordon, D.; Marsh, E. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Center of Disease Control (CDC). Defining Adult Overweight & Obesity; Center of Disease Control (CDC): Atlanta, GA, USA, 2022.
- Saqqur, M.; Salam, A.; Ayyad, A.; Akhtar, N.; Ali, M.; Khan, A.; Imam, Y.; Joseph, S.; Al Jerdi, S.; Shuaib, A. The Prevalence, Mortality Rate and Functional Outcome of Intracerebral Hemorrhage According to Age Sex and Ethnic Group in the State of Qatar. Clin. Neurol. Neurosurg. 2020, 199, 106255. [Google Scholar] [CrossRef]
- Seizing the Opportunity: Ending AIDS in the Middle East and North Africa Amman; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2019.
- Imam, Y.Z.; Akhtar, N.; Kamran, S.; Garcia-Bermejo, P.; Al Jerdi, S.; Zakaria, A.; Own, A.; Patro, S. Rescue Stent Placement for Acute Ischemic Stroke with Large Vessel Occlusion Refractory to Mechanical Thrombectomy: A Multiethnic Middle Eastern/African/Asian Cohort. J. Vasc. Interv. Radiol. 2023, 34, 1740–1748. [Google Scholar] [CrossRef]
- Gulli, G.; Rutten-Jacobs, L.; Kalra, L.; Rudd, A.; Wolfe, C.; Markus, H. Differences in the Distribution of Stroke Subtypes in a UK Black Stroke Population—Final Results from the South London Ethnicity and Stroke Study. BMC Med. 2016, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.; Marotta, C. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials: A Literature Review and Synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple imputation by chained equations: What is it and how does it work? Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef]
- Lolak, S.; Attia, J.; McKay, G.J.; Thakkinstian, A. Comparing Explainable Machine Learning Approaches with Traditional Statistical Methods for Evaluating Stroke Risk Models: Retrospective Cohort Study. JMIR Cardio 2023, 7, e47736. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Harshfield, E.L.; Bell, S.; Burkhart, M.; Tuladhar, A.M.; Hilal, S.; Tozer, D.J.; Chappell, F.M.; Makin, S.D.J.; Lo, J.W.; et al. Predicting incident dementia in cerebral small vessel disease: Comparison of machine learning and traditional statistical models. Cereb. Circ.-Cogn. Behav. 2023, 5, 100179. [Google Scholar] [CrossRef] [PubMed]
- Dharmarathne, G.; Hanea, A.; Robinson, A.P. Improving the Computation of Brier Scores for Evaluating Expert-Elicited Judgements. Front. Appl. Math. Stat. 2021, 7, 669546. [Google Scholar] [CrossRef]
- Dodge, Y. The Concise Encyclopedia of Statistics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef]
- Vujović, Ž. Classification model evaluation metrics. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 599–606. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 4768–4777. [Google Scholar]
- Johnson, K.; Wei, W.; Weeraratne, D.; Frisse, M.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J. Precision medicine, AI, and the future of personalized health care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Karamchandani, R.R.; Prasad, T.; Strong, D.; Rhoten, J.B.; Asimos, A.W. A tool to improve stroke outcome prediction: The charlotte large artery occlusion endovascular therapy outcome score. J. Stroke Cerebrovasc. Dis. 2022, 31, 106393. [Google Scholar] [CrossRef]
- Abedi, V.; Avula, V.; Razavi, S.; Bavishi, S.; Chaudhary, D.; Shahjouei, S.; Wang, M.; Griessenauer, C.; Li, J.; Zand, R. Predicting short and long-term mortality after acute ischemic stroke using EHR. J. Neurol. Sci. 2021, 427, 117560. [Google Scholar] [CrossRef]
- Li, H.; Ye, S.-s.; Wu, Y.-L.; Huang, S.-M.; Li, Y.-X.; Lu, K.; Huang, J.-B.; Chen, L.; Li, H.-Z.; Wu, W.-J. Predicting mortality in acute ischaemic stroke treated with mechanical thrombectomy: Analysis of a multicentre prospective registry. BMJ Open 2021, 11, e043415. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Oyama, N.; Kitano, T.; Iwamoto, T.; Yamashita, S.; Takai, H.; Matsubara, S.; Uno, M.; Yagita, Y. Prestroke Conditions of Acute Ischemic Stroke Patients are Associated with Functional Outcome after Mechanical Thrombectomy. J. Stroke Cerebrovasc. Dis. 2020, 29, 104540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, G.; Zhang, J.; Wang, J.; You, W.; Zhu, J. Blood glucose level affects prognosis of patients who received intravenous thrombolysis after acute ischemic stroke? A meta-analysis. Front. Endocrinol. 2023, 14, 1120779. [Google Scholar] [CrossRef]
- Ho, W.-M.; Lin, J.-R.; Wang, H.-H.; Liou, C.-W.; Chang, K.-C.; Lee, J.-D.; Peng, T.-Y.; Yang, J.-T.; Chang, Y.-J.; Chang, C.-H. Prediction of in-hospital stroke mortality in critical care unit. Springerplus 2016, 5, 1051. [Google Scholar] [CrossRef]
- Skafida, A.; Mitrakou, A.; Georgiopoulos, G.; Alevizaki, M.; Spengos, K.; Takis, K.; Ntaios, G.; Thomadakis, C.; Vemmos, K. In-hospital dynamics of glucose, blood pressure and temperature predict outcome in patients with acute ischaemic stroke. Eur. Stroke J. 2018, 3, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Tziomalos, K.; Giampatzis, V.; Bouziana, S.D.; Spanou, M.; Papadopoulou, M.; Kostaki, S.; Dourliou, V.; Papagianni, M.; Savopoulos, C.; Hatzitolios, A.I. Elevated Diastolic but Not Systolic Blood Pressure Increases Mortality Risk in Hypertensive but Not Normotensive Patients with Acute Ischemic Stroke. Am. J. Hypertens. 2015, 28, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhu, Y.; Chen, Z.; Li, W.; Li, L.; Li, Y.; Xia, Y.; Zhang, T.; Feng, Q.; Wu, J.; et al. Relationship between mean blood pressure during hospitalization and clinical outcome after acute ischemic stroke. BMC Neurol. 2023, 23, 156. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Minhas, J.S.; Moullaali, T.J.; Di Tanna, G.L.; Lindley, R.I.; Chen, X.; Arima, H.; Chen, G.; Delcourt, C.; Bath, P.M.; et al. Associations of Early Systolic Blood Pressure Control and Outcome After Thrombolysis-Eligible Acute Ischemic Stroke: Results from the ENCHANTED Study. Stroke 2022, 53, 779–787. [Google Scholar] [CrossRef]
- Namale, G.; Kamacooko, O.; Makhoba, A.; Mugabi, T.; Ndagire, M.; Ssanyu, P.; Ddamulira, J.; Yperzeele, L.; Cras, P.; Ddumba, E.; et al. Predictors of 30-day and 90-day mortality among hemorrhagic and ischemic stroke patients in urban Uganda: A prospective hospital-based cohort study. BMC Cardiovasc. Disord. 2020, 20, 442. [Google Scholar] [CrossRef]
- Ozkara, B.B.; Karabacak, M.; Hamam, O.; Wang, R.; Kotha, A.; Khalili, N.; Hoseinyazdi, M.; Chen, M.M.; Wintermark, M.; Yedavalli, V.S. Prediction of Functional Outcome in Stroke Patients with Proximal Middle Cerebral Artery Occlusions Using Machine Learning Models. J. Clin. Med. 2023, 12, 839. [Google Scholar] [CrossRef]
- Wei, W.; Li, S.; San, F.; Zhang, S.; Shen, Q.; Guo, J.; Zhang, L. Retrospective analysis of prognosis and risk factors of patients with stroke by TOAST. Medicine 2018, 97, e0412. [Google Scholar] [CrossRef]
- Miceli, G.; Basso, M.G.; Rizzo, G.; Pintus, C.; Cocciola, E.; Pennacchio, A.R.; Tuttolomondo, A. Artificial Intelligence in Acute Ischemic Stroke Subtypes According to Toast Classification: A Comprehensive Narrative Review. Biomedicines 2023, 11, 1138. [Google Scholar] [CrossRef] [PubMed]
- Grieten, J.; Chevalier, P.; Lesenne, A.; Ernon, L.; Vandermeulen, E.; Panis, E.; Mesotten, D. Hospital-acquired infections after acute ischaemic stroke and its association with healthcare-related costs and functional outcome. Acta Neurol. Belg. 2022, 122, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]

| Variable | Feature | Favorable (mRS ≤ 2) | Unfavorable (mRS > 2) | Total |
|---|---|---|---|---|
| Age (year) | <mean (54.1) | 218 | 164 | 382 |
| ≥mean (54.1) | 163 | 178 | 341 | |
| Mean ± SD (54.1 ± 13.1)-IQR 17 | ||||
| Sex | 1: Male | 320 | 284 | 604 |
| 2: Female | 61 | 58 | 119 | |
| Ethnicity | 1: Qatari | 41 | 49 | 90 |
| 2: MENA | 77 | 74 | 151 | |
| 3: South Asian | 202 | 178 | 380 | |
| 4: Southeast Asian | 38 | 30 | 68 | |
| 5: Other | 23 | 11 | 34 | |
| Modified Rankin Score (mRS) pre-stroke onset | <mean (0.2) | 364 | 300 | 664 |
| ≥mean (0.2) | 17 | 42 | 59 | |
| Mean ± SD (0.2 ± 0.72)-IQR 9 | ||||
| NIHSS at admission | <mean (10.3) | 281 | 147 | 428 |
| ≥mean (10.3) | 100 | 195 | 295 | |
| Mean ± SD (10.3 ± 5.9)-IQR 0 | ||||
| Mode of arrival | 1: Ambulance | 332 | 306 | 638 |
| 2: Private vehicle | 45 | 26 | 71 | |
| 3: In-hospital | 4 | 10 | 14 | |
| Body Mass Index (BMI) | 1: Underweight | 11 | 10 | 21 |
| 2: Normal weight | 112 | 107 | 219 | |
| 3: Overweight | 153 | 151 | 304 | |
| 4: Obese | 82 | 39 | 121 | |
| 5: Extremely Obese | 23 | 35 | 58 | |
| Time from onset to hospital arrival (hour) | 1: ≤hours | 347 | 309 | 656 |
| 2: >3 h | 34 | 33 | 67 | |
| Diabetes Mellitus (DM) | 0: No | 299 | 289 | 588 |
| 1: Yes | 82 | 53 | 135 | |
| Hypertension (HTN) | 0: No | 142 | 101 | 243 |
| 1: Yes | 239 | 241 | 480 | |
| Dyslipidemia | 0: No | 201 | 189 | 390 |
| 1: Yes | 180 | 153 | 333 | |
| Prior stroke | 0: No | 357 | 315 | 672 |
| 1: Yes | 24 | 27 | 51 | |
| Atrial Fibrillation (AF) | 0: No | 366 | 313 | 679 |
| 1: Yes | 15 | 29 | 44 | |
| Coronary artery disease (CAD) | 0: No | 336 | 282 | 618 |
| 1: Yes | 45 | 60 | 105 | |
| Tobacco use | 0: No | 277 | 267 | 544 |
| 1: Yes | 104 | 75 | 179 | |
| RBS at admission (mmol/L) | <mean (9.3) | 262 | 206 | 468 |
| ≥mean (9.3) | 117 | 135 | 252 | |
| Mean ± SD (9.3 ± 4.6)-IQR 5 | ||||
| SBP at admission (mmHg) | <mean (152.4) | 212 | 173 | 385 |
| ≥mean (152.4) | 168 | 169 | 337 | |
| Mean ± SD (152.4 ± 27.7)-IQR 37 | ||||
| DBP at admission (mmHg) | <mean (90.11) | 225 | 176 | 401 |
| ≥mean (90.11) | 155 | 165 | 320 | |
| Mean ± SD (90.11 ± 16.9)-IQR 22 | ||||
| HR at admission (bpm) | <mean (84.3) | 208 | 172 | 380 |
| ≥mean (84.3) | 168 | 169 | 337 | |
| Mean ± SD (84.3 ± 16.2)-IQR 21 | ||||
| Anti-platelets | 0: No | 310 | 269 | 579 |
| 1: Yes | 71 | 73 | 144 | |
| Anticoagulants | 0: No | 362 | 323 | 685 |
| 1: Yes | 19 | 19 | 38 | |
| Platelet count at admission | <mean (259.5) | 201 | 178 | 388 |
| ≥mean (259.5) | 171 | 149 | 320 | |
| Mean ± SD (259.5 ± 78.15)-IQR 88 | ||||
| Prothrombin Time (PT) | <mean (10.4) | 192 | 150 | 342 |
| ≥mean (10.4) | 166 | 177 | 343 | |
| Mean ± SD (10.4 ± 4.5)-IQR 2.4 | ||||
| Partial Thromboplastin Time (PTT) | <mean (26.5) | 218 | 203 | 421 |
| ≥mean (26.5) | 138 | 121 | 259 | |
| Mean ± SD (26.5 ± 10.9)-IQR 3.7 | ||||
| Door-to-needle time (DNT) in minutes | <mean (61.7) | 222 | 225 | 447 |
| ≥mean (61.7) | 159 | 117 | 276 | |
| Mean ± SD (61.7 ± 40.1)-IQR 40 | ||||
| Hospital-Acquired Pneumonia | 0: No | 375 | 292 | 667 |
| 1: Yes | 6 | 50 | 56 | |
| UTI | 0: No | 378 | 310 | 688 |
| 1: Yes | 3 | 32 | 35 | |
| Ischemic stroke subtype (TOAST) | Large Vessel Disease (LVD) | 101 | 137 | 238 |
| Cardioembolism (embolic) | 102 | 76 | 178 | |
| Small Vessel Disease (SVD) | 104 | 73 | 177 | |
| Stroke of other Determined Etiology (SDO) | 53 | 24 | 77 | |
| Stroke of Undetermined Etiology (SUO) | 21 | 32 | 53 | |
| 90-day mRS | 381 | 342 | 723 | |
| Model | Accuracy | Precision | Specificity | Recall | F1-Score | AUC | MCC | Log Loss | Brier Score |
|---|---|---|---|---|---|---|---|---|---|
| XGB Classifier | 0.676 | 0.569 | 0.648 | 0.719 | 0.636 | 0.756 | 0.359 | 0.761 | 0.235 |
| Random Forest Classifier (RF) | 0.683 | 0.580 | 0.670 | 0.702 | 0.635 | 0.758 | 0.364 | 0.584 | 0.200 |
| Support Vector Machine (SVM) | 0.697 | 0.600 | 0.705 | 0.684 | 0.639 | 0.762 | 0.382 | 0.594 | 0.201 |
| Logistic regression (LR) | 0.655 | 0.549 | 0.636 | 0.684 | 0.609 | 0.719 | 0.313 | 0.636 | 0.220 |
| Decision Tree Classifier (CART) | 0.621 | 0.514 | 0.614 | 0.632 | 0.567 | 0.623 | 0.240 | 13.672 | 0.379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abujaber, A.A.; Albalkhi, I.; Imam, Y.; Nashwan, A.J.; Yaseen, S.; Akhtar, N.; Alkhawaldeh, I.M. Predicting 90-Day Prognosis in Ischemic Stroke Patients Post Thrombolysis Using Machine Learning. J. Pers. Med. 2023, 13, 1555. https://doi.org/10.3390/jpm13111555
Abujaber AA, Albalkhi I, Imam Y, Nashwan AJ, Yaseen S, Akhtar N, Alkhawaldeh IM. Predicting 90-Day Prognosis in Ischemic Stroke Patients Post Thrombolysis Using Machine Learning. Journal of Personalized Medicine. 2023; 13(11):1555. https://doi.org/10.3390/jpm13111555
Chicago/Turabian StyleAbujaber, Ahmad A., Ibrahem Albalkhi, Yahia Imam, Abdulqadir J. Nashwan, Said Yaseen, Naveed Akhtar, and Ibraheem M. Alkhawaldeh. 2023. "Predicting 90-Day Prognosis in Ischemic Stroke Patients Post Thrombolysis Using Machine Learning" Journal of Personalized Medicine 13, no. 11: 1555. https://doi.org/10.3390/jpm13111555
APA StyleAbujaber, A. A., Albalkhi, I., Imam, Y., Nashwan, A. J., Yaseen, S., Akhtar, N., & Alkhawaldeh, I. M. (2023). Predicting 90-Day Prognosis in Ischemic Stroke Patients Post Thrombolysis Using Machine Learning. Journal of Personalized Medicine, 13(11), 1555. https://doi.org/10.3390/jpm13111555







