The Association of Endothelin-1 with Early and Long-Term Mortality in COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Director-General’s Opening Remarks at the Media Briefing. Available online: https://www.who.int/news-room/speeches/item/who-director-general-s-opening-remarks-at-the-media-briefing (accessed on 5 May 2023).
- Kumar, A.; Jatteppanvar, B.; Panda, P.K.; Dhangar, P.; Bahurupi, Y.A. Predictors of Mortality Among Post-COVID-19 Discharged Patients in Northern India: A Case-Control Study. Cureus 2023, 15, e36883. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Wang, X.Q.; Iwashyna, T.J.; Prescott, H.C. Readmission and Death After Initial Hospital Discharge Among Patients With COVID-19 in a Large Multihospital System. JAMA 2021, 325, 304. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-Dimensional Characterization of Post-Acute Sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Mainous, A.G.; Rooks, B.J.; Wu, V.; Orlando, F.A. COVID-19 Post-Acute Sequelae Among Adults: 12 Month Mortality Risk. Front. Med. 2021, 8, 778434. [Google Scholar] [CrossRef]
- Uusküla, A.; Jürgenson, T.; Pisarev, H.; Kolde, R.; Meister, T.; Tisler, A.; Suija, K.; Kalda, R.; Piirsoo, M.; Fischer, K. Long-Term Mortality Following SARS-CoV-2 Infection: A National Cohort Study from Estonia. Lancet Reg. Health-Eur. 2022, 18, 100394. [Google Scholar] [CrossRef]
- Freund, O.; Breslavsky, A.; Fried, S.; Givoli-Vilensky, R.; Cohen-Rubin, S.; Zacks, N.; Kleinhendler, E.; Unterman, A.; Frydman, S.; Wand, O.; et al. Interactions and Clinical Implications of Serological and Respiratory Variables 3 Months after Acute COVID-19. Clin. Exp. Med. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Maamar, M.; Artime, A.; Pariente, E.; Fierro, P.; Ruiz, Y.; Gutiérrez, S.; Tobalina, M.; Díaz-Salazar, S.; Ramos, C.; Olmos, J.M.; et al. Post-COVID-19 Syndrome, Low-Grade Inflammation and Inflammatory Markers: A Cross-Sectional Study. Curr. Med. Res. Opin. 2022, 38, 901–909. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Yantiss, R.K. The Pathogenesis of Coronavirus-19 Disease. J. Biomed. Sci. 2022, 29, 87. [Google Scholar] [CrossRef]
- Cambier, S.; Metzemaekers, M.; de Carvalho, A.C.; Nooyens, A.; Jacobs, C.; Vanderbeke, L.; Malengier-Devlies, B.; Gouwy, M.; Heylen, E.; Meersseman, P.; et al. Atypical Response to Bacterial Coinfection and Persistent Neutrophilic Bronchoalveolar Inflammation Distinguish Critical COVID-19 from Influenza. JCI Insight 2022, 7, e155055. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines Including Interleukin-6 in COVID-19 Induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Li, S.; Jiang, L.; Li, X.; Lin, F.; Wang, Y.; Li, B.; Jiang, T.; An, W.; Liu, S.; Liu, H.; et al. Clinical and Pathological Investigation of Patients with Severe COVID-19. JCI Insight 2020, 5, e138070. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakagawa, A.; Suzuki, K.; Yamashita, K.; Yamashita, S.; Iwanaga, N.; Tamada, E.; Noda, K.; Tomii, K. Type 1 Inflammatory Endotype Relates to Low Compliance, Lung Fibrosis, and Severe Complications in COVID-19. Cytokine 2021, 148, 155618. [Google Scholar] [CrossRef]
- ter Ellen, B.M.; Niewold, J.; Flikweert, A.; Muller Kobold, A.C.; Heeringa, P.; van Meurs, M.; Smit, J.M.; van der Voort, P.H.J.; Rodenhuis-Zybert, I.A.; Moser, J. Mediators of Obesity Do Not Influence SARS-CoV-2 Infection or Activation of Primary Human Lung Microvascular Endothelial Cells In Vitro. Front. Immunol. 2022, 13, 879033. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-Associated Coagulopathy: Evidence from a Single-Centre, Cross-Sectional Study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Weng, J. Endothelial Dysfunction in COVID-19: An Overview of Evidence, Biomarkers, Mechanisms and Potential Therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Motte, S.; McEntee, K.; Naeije, R. Endothelin Receptor Antagonists. Pharmacol. Ther. 2006, 110, 386–414. [Google Scholar] [CrossRef]
- Langleben, D.; Demarchie, M.; Laporta, D.; Spanier, A.H.; Schlesinger, R.D.; Stewart, D.J. Endothelin-1 in Acute Lung Injury and the Adult Respiratory Distress Syndrome. Am. Rev. Respir. Dis. 1993, 148, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Yacoub, M.H. The Role of Endothelin-1 in Pulmonary Arterial Hypertension. Glob. Cardiol. Sci. Pract. 2014, 2014, 29. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kleniewska, P.; Kolodziejczyk, M.; Skibska, B.; Goraca, A. The Role of Endothelin-1 and Endothelin Receptor Antagonists in Inflammatory Response and Sepsis. Arch. Immunol. Ther. Exp. 2015, 63, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.R.; Kuc, R.E.; Althage, M.; Greasley, P.J.; Ambery, P.; Maguire, J.J.; Wilkinson, I.B.; Hoole, S.P.; Cheriyan, J.; Davenport, A.P. Endothelin-1 Is Increased in the Plasma of Patients Hospitalised with Covid-19. J. Mol. Cell. Cardiol. 2022, 167, 92–96. [Google Scholar] [CrossRef]
- Willems, L.H.; Nagy, M.; ten Cate, H.; Spronk, H.M.H.; Groh, L.A.; Leentjens, J.; Janssen, N.A.F.; Netea, M.G.; Thijssen, D.H.J.; Hannink, G.; et al. Sustained Inflammation, Coagulation Activation and Elevated Endothelin-1 Levels without Macrovascular Dysfunction at 3 Months after COVID-19. Thromb. Res. 2022, 209, 106–114. [Google Scholar] [CrossRef]
- Omland, T.; Lie, R.T.; Aakvaag, A.; Aarsland, T.; Dickstein, K. Plasma Endothelin Determination as a Prognostic Indicator of 1-Year Mortality after Acute Myocardial Infarction. Circulation 1994, 89, 1573–1579. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Moukarbel, G.V.; Gupta, R.; Frank, S.M.; Anderson, A.M.; Liu, L.C.; Khouri, S.J. Endothelin 1 Is Associated with Heart Failure Hospitalization and Long-Term Mortality in Patients with Heart Failure with Preserved Ejection Fraction and Pulmonary Hypertension. Cardiology 2019, 143, 124–133. [Google Scholar] [CrossRef]
- Li, P.; Schmidt, I.M.; Sabbisetti, V.; Tio, M.C.; Opotowsky, A.R.; Waikar, S.S. Plasma Endothelin-1 and Risk of Death and Hospitalization in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 784–793. [Google Scholar] [CrossRef]
- Gregoriano, C.; Damm, D.; Kutz, A.; Koch, D.; Wolfisberg, S.; Haubitz, S.; Conen, A.; Bernasconi, L.; Hammerer-Lercher, A.; Fux, C.A.; et al. Association of Endothelial Activation Assessed through Endothelin-I Precursor Peptide Measurement with Mortality in COVID-19 Patients: An Observational Analysis. Respir. Res. 2021, 22, 148. [Google Scholar] [CrossRef]
- Vieceli Dalla Sega, F.; Fortini, F.; Spadaro, S.; Ronzoni, L.; Zucchetti, O.; Manfrini, M.; Mikus, E.; Fogagnolo, A.; Torsani, F.; Pavasini, R.; et al. Time Course of Endothelial Dysfunction Markers and Mortality in COVID-19 Patients: A Pilot Study. Clin. Transl. Med. 2021, 11, e283. [Google Scholar] [CrossRef]
- Diagnosis and Treatment Protocol for COVID-19 Patients (Trial Version 9). Health Care Sci. 2022, 1, 14–28. [CrossRef]
- Clinical Guidance of COVID-19 Diagnostics and Treatment in Adults of the Republic of Kazakhstan. Available online: https://diseases.medelement.com/disease/%D0%BA%D0%BE%D1%80%D0%BE%D0%BD%D0%B0%D0%B2%D0%B8%D1%80%D1%83%D1%81%D0%BD%D0%B0%D1%8F-%D0%B8%D0%BD%D1%84%D0%B5%D0%BA%D1%86%D0%B8%D1%8F-covid-19-%D1%83-%D0%B2%D0%B7%D1%80%D0%BE%D1%81%D0%BB%D1%8B%D1%85-2021/16540 (accessed on 5 May 2023).
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a Combined Comorbidity Index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Druml, W.; Steltzer, H.; Waldhäusl, W.; Lenz, K.; Hammerle, A.; Vierhapper, H.; Gasic, S.; Wagner, O.F. Endothelin-1 in Adult Respiratory Distress Syndrome. Am. Rev. Respir. Dis. 1993, 148, 1169–1173. [Google Scholar] [CrossRef]
- Shahbazi, S.; Vahdat Shariatpanahi, Z.; Shahbazi, E. Bosentan for High-Risk Outpatients with COVID-19 Infection: A Randomized, Double Blind, Placebo-Controlled Trial. EClinicalMedicine 2023, 62, 102117. [Google Scholar] [CrossRef]
- Miedema, J.; Schreurs, M.; van der Brugge, S.v.d.S.; Paats, M.; Baart, S.; Bakker, M.; Hoek, R.; Dik, W.A.; Endeman, H.; Van Der Velden, V.; et al. Antibodies Against Angiotensin II Receptor Type 1 and Endothelin A Receptor Are Associated With an Unfavorable COVID19 Disease Course. Front. Immunol. 2021, 12, 684142. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Mao, Z.; Xiao, M.; Wang, L.; Qi, S.; Zhou, F. Predictive Values of Neutrophil-to-Lymphocyte Ratio on Disease Severity and Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Crit. Care 2020, 24, 647. [Google Scholar] [CrossRef]
- Callender, L.A.; Curran, M.; Bates, S.M.; Mairesse, M.; Weigandt, J.; Betts, C.J. The Impact of Pre-Existing Comorbidities and Therapeutic Interventions on COVID-19. Front. Immunol. 2020, 11, 1991. [Google Scholar] [CrossRef]
- Freund, O.; Weiss, T.E.; Tau, L.; Meidan, R.; Liron, Y.; Tchebiner, J.Z.; Bornstein, G. Safety and Outcomes of an Early Discharge Strategy with Oxygen Home Therapy in Stable Severe COVID-19 Patients. Infect. Dis. 2023, 55, 292–298. [Google Scholar] [CrossRef]
- Lau, E.M.T.; Giannoulatou, E.; Celermajer, D.S.; Humbert, M. Epidemiology and Treatment of Pulmonary Arterial Hypertension. Nat. Rev. Cardiol. 2017, 14, 603–614. [Google Scholar] [CrossRef]
- Knowles, J.; Loizidou, M.; Taylor, I. Endothelin-1 and Angiogenesis in Cancer. Curr. Vasc. Pharmacol. 2005, 3, 309–314. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Morawietz, H.; Talanow, R.; Szibor, M.; Rueckschloss, U.; Schubert, A.; Bartling, B.; Darmer, D.; Holtz, J. Regulation of the Endothelin System by Shear Stress in Human Endothelial Cells. J. Physiol. 2000, 525, 761–770. [Google Scholar] [CrossRef]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular Consequences of Inflammation: A Position Statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef]
- Henkens, M.T.H.M.; Raafs, A.G.; Verdonschot, J.A.J.; Linschoten, M.; van Smeden, M.; Wang, P.; van der Hooft, B.H.M.; Tieleman, R.; Janssen, M.L.F.; ter Bekke, R.M.A.; et al. Age Is the Main Determinant of COVID-19 Related in-Hospital Mortality with Minimal Impact of Pre-Existing Comorbidities, a Retrospective Cohort Study. BMC Geriatr. 2022, 22, 184. [Google Scholar] [CrossRef]
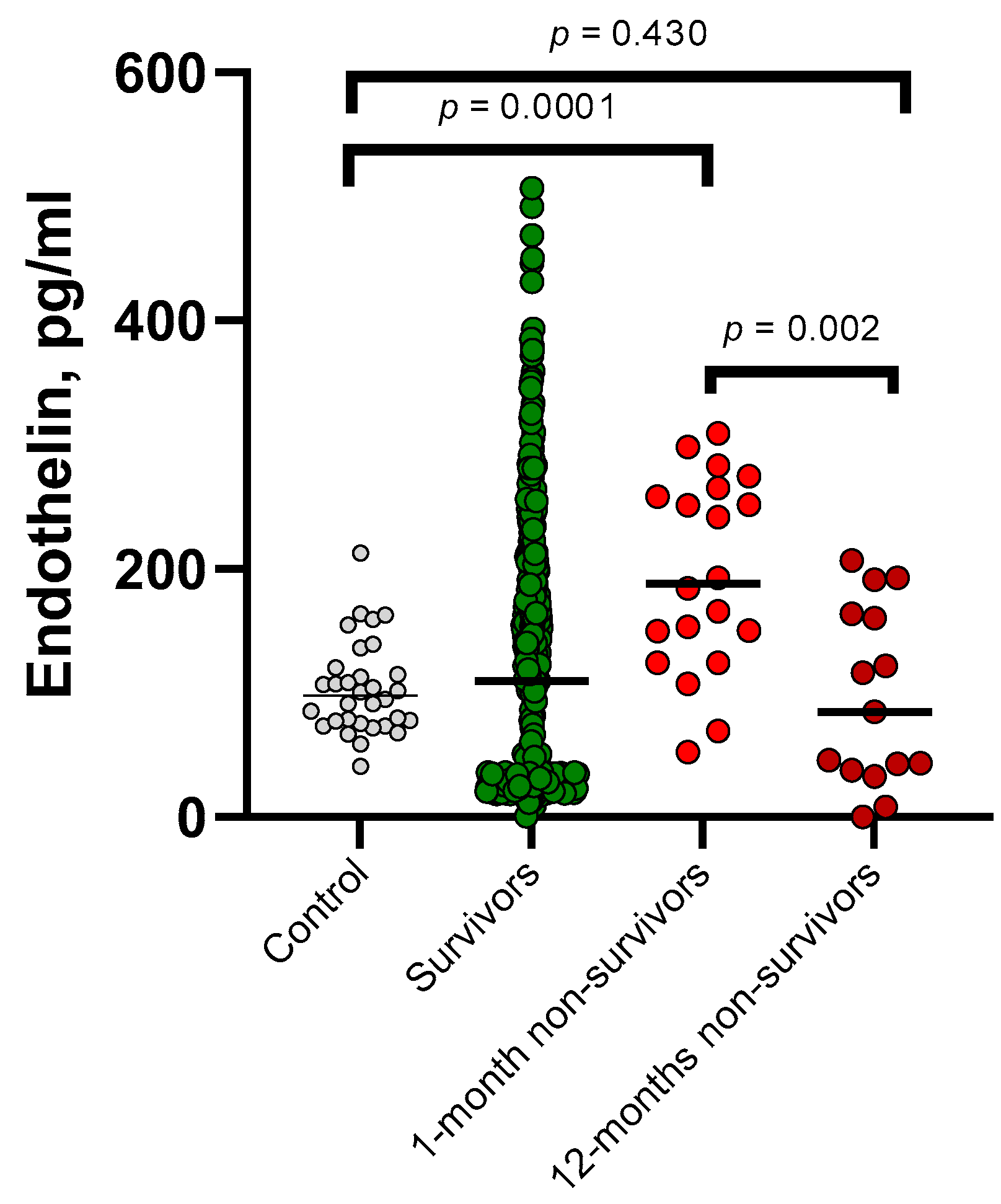

| Baseline Variable | Survivors, n = 435 | Non-Survivors 1-Month, n = 20 | Non-Survivors 12-Months, n = 15 | p-Value * | p-Value ** |
|---|---|---|---|---|---|
| Ме (Q25–Q75) | Ме (Q25–Q75) | Ме (Q25–Q75) | |||
| Demographic | |||||
| Age (y) | 61 (50–69) | 73 (65–81) | 72 (62–84) | 0.0001 | 0.002 |
| Gender, n (%) | |||||
| Male | 163 (37.5) | 11 (55) | 5 (33.3) | 0.112 | 0.745 |
| Female | 272 (62.5) | 9 (45) | 10 (66.7) | ||
| Comorbidities or coexisting disorders, n (%) | |||||
| Hypertension | 221 (50.8) | 15 (75) | 7 (46.7) | 0.033 | 0.753 |
| Chronic heart failure | 149 (34.3) | 14 (70) | 5 (33.3) | 0.001 | 0.941 |
| Diabetes mellitus | 74 (17.0) | 6 (30) | 4 (26.7) | 0.148 | 0.332 |
| Chronic obstructive pulmonary disease | 11 (2.5) | 1 (5) | 0 | 0.244 | 0.621 |
| Myocardial infarction in medical history | 10 (2.3) | 5 (25) | 6 (17.1) | 0.0001 | 0.282 |
| Chronic renal failure | 15 (3.4) | 2 (10) | 0 | 0.119 | 0.465 |
| Malignancy | 13 (3.0) | 0 | 2 (13.3) | 0.407 | 0.023 |
| BMI (kg/m2) | 28.3 (24.7–32.4) | 26.5 (23.9–32.4) | 29.0 (23.1–34.5) | 0.531 | 0.649 |
| Vital signs at day of sampling | |||||
| Heart rate (bpm) | 80 (76–86) | 82 (76–91) | 78 (76–88) | 0.171 | 0.837 |
| Respiratory rate (vpm) | 19 (18–20) | 22 (20–23) | 20 (19–22) | 0.0001 | 0.015 |
| Oxygen saturation, % | 96 (94–98) | 93 (91–98) | 95 (92–98) | 0.033 | 0.437 |
| Severity, n (%) | |||||
| Moderate | 364 (83.7) | 7 (35) | 11 (73.3) | 0.0001 | 0.291 |
| Severe | 71 (16.3) | 13 (65) | 4 (26.7) | ||
| Invasive mechanical ventilation, n (%) | 9 (2.1) | 12 (60) | 0 | 0.0001 | 0.574 |
| ICU admission (n, %) | 40 (9.2) | 13 (65.0) | 2 (13.3) | 0.0001 | 0.588 |
| Infiltrate on chest radiograph, % | 25 (12–40) | 45 (25–55) | 25 (15–45) | 0.005 | 0.609 |
| Hospital length of stay (days) | 10 (8–11) | 11 (7–14) | 10 (9–15) | 0.230 | 0.169 |
| Laboratory findings | |||||
| Hemoglobin (g/L) | 139 (129–153) | 134 (125–156) | 143 (118–153) | 0.452 | 0.729 |
| Leukocytes × 109/L | 4.9 (3.8–6.2) | 5.9 (4.2–8.2) | 6.1 (5.1–7.0) | 0.144 | 0.01 |
| Neutrophils × 109/L | 3.08 (2.19–4.25) | 4.36 (2.83–6.17) | 4.02 (3.54–5.51) | 0.025 | 0.005 |
| NLR | 2.4 (1.6–3.5) | 3.8 (2.5–8.7) | 3.0 (2.0–9.1) | 0.001 | 0.09 |
| Platelets × 109/L | 187 (155–218) | 189 (155–216) | 196 (176–210) | 0.656 | 0.481 |
| ESR, (mm/h) | 15 (10–23) | 15 (10–22) | 14 (4–20) | 0.836 | 0.243 |
| Creatinine (mmol/L) | 88 (80–96) | 89 (77–99) | 86 (82–97) | 0.780 | 0.967 |
| ALT (units/L) | 29 (23–33) | 24 (19–31) | 26 (18–32) | 0.100 | 0.169 |
| AST (units/L) | 26 (23–35) | 27 (25–32) | 30 (24–34) | 0.871 | 0.378 |
| Bilirubin (µmol/L) | 13.0 (11.8–15.0) | 14.0 (12.0–16.0) | 14.9 (11.6–16.0) | 0.462 | 0.341 |
| Glucose (mmol/L) | 6.5 (5.5–7.9) | 6.2 (5.1–12.2) | 8.3 (6.5–16.5) | 0.929 | 0.024 |
| CRP (mg/l) | 12 (6–52) | 39.9 (9.1–139.2) | 24 (6–103) | 0.062 | 0.596 |
| Ferritin (μg/l) | 218 (130–364) | 244 (206–681) | 242 (54–395) | 0.077 | 0.515 |
| D-dimer, (ng/mL) | 281 (165–424) | 434 (319–538) | 379 (231–582) | 0.002 | 0.05 |
| Endothelin-1, (pg/mL) | 100.4 (27.7–197.8) | 188.4 (130.6–263.6) | 84.7 (37.6–163.6) | 0.001 | 0.643 |
| Treatment | |||||
| Anticoagulants | 392 (89.9) | 18 (90) | 14 (93.3) | 0.997 | 0.664 |
| Glucocorticosteroids | 323 (53.2) | 17 (85) | 10 (66.7) | 0.006 | 0.305 |
| Antibiotic therapy | 308 (70.6) | 18 (90) | 14 (93.3) | 0.070 | 0.056 |
| Antiviral therapy | 93 (2) | 8 (40) | 3 (20) | 0.050 | 0.895 |
| Marker | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | CI | p-Value | HR | CI | p-Value | |
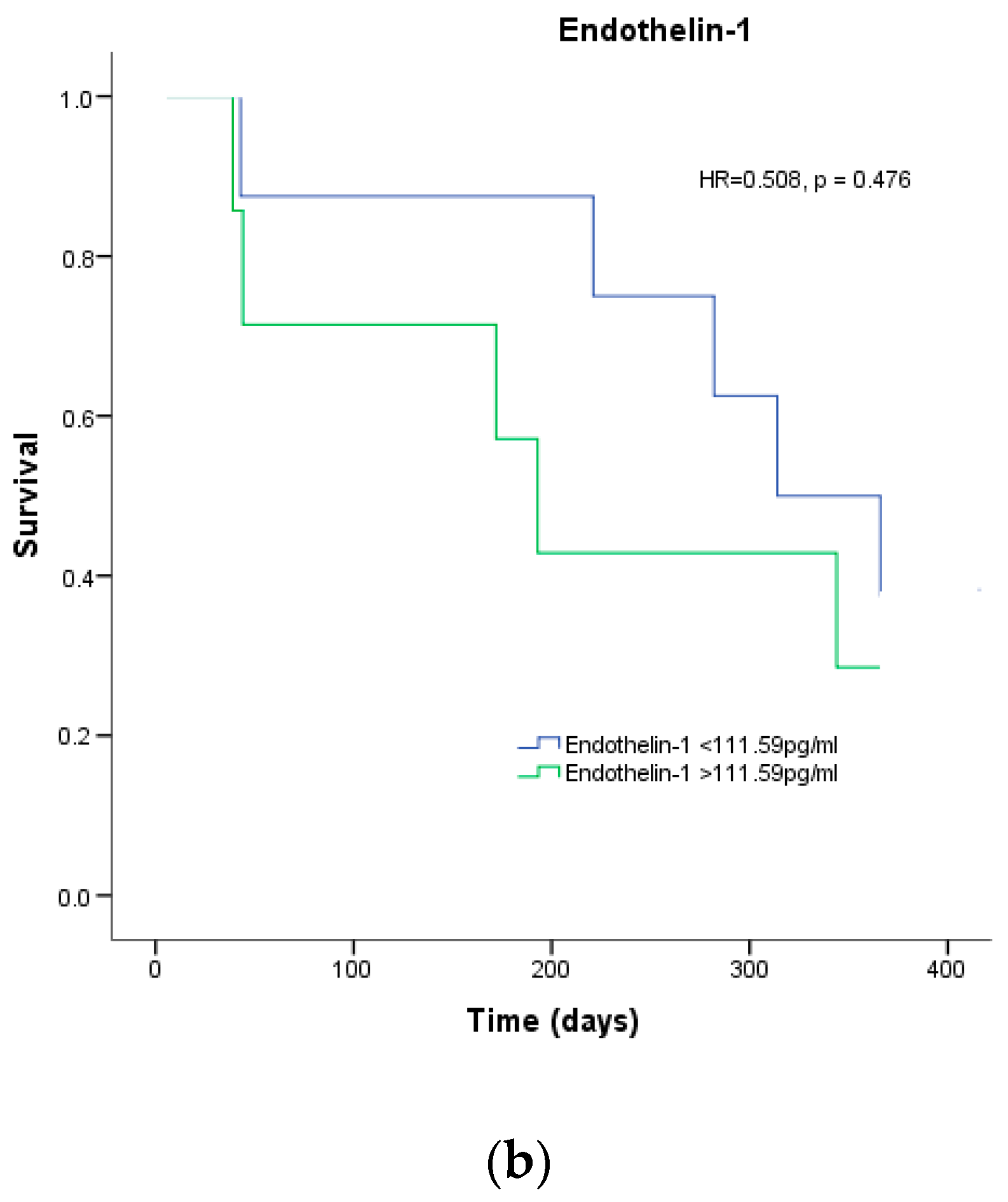
| Endothelin-1 | 1.002 | 1.000–1.004 | 0.036 | 1.004 | 1.001–1.007 | 0.009 |
| Severity | 8.538 | 3.406–21.403 | 0.0001 | 5.765 | 2.242–14.823 | 0.0001 |
| Gender (Male) | 2.032 | 0.842–4.903 | 0.115 | - | - | - |
| NLR | 1.118 | 1.065–1.173 | 0.0001 | 1.093 | 1.031–1.158 | 0.003 |
| Age | 1.073 | 1.035–1.112 | 0.0001 | 1.076 | 1.029–1.126 | 0.001 |
| Arterial hypertension | 2.843 | 1.033–7.824 | 0.043 | 0.745 | 0.245–2.262 | 0.603 |
| Myocardial infarction | 10.714 | 3.891–29.505 | 0.0001 | 5.742 | 1.867–17.659 | 0.002 |
| Chronic heart failure | 4.351 | 1.672–11.323 | 0.003 | 2.016 | 0.425–9.559 | 0.377 |
| Chronic obstructive pulmonary disease | 3.207 | 0.429–23.960 | 0.256 | - | - | - |
| Chronic kidney disease | 2.958 | 0.686–12.748 | 0.146 | - | - | - |
| Marker | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | CI | p-Value | HR | CI | p-Value | |
| Endothelin-1 | 0.996 | 0.990–1.003 | 0.304 | - | - | - |
| Severity | 3.290 | 0.787–13.757 | 0.103 | - | - | - |
| Gender (Male) | 1.126 | 0.318–3.989 | 0.854 | - | - | - |
| NLR | 1.106 | 1.019–1.200 | 0.016 | 1.093 | 1.003–1.192 | 0.043 |
| Age | 1.069 | 1.017–1.124 | 0.009 | 1.070 | 1.015–1.128 | 0.012 |
| Arterial hypertension | 0.646 | 0.182–2.288 | 0.498 | - | - | - |
| Myocardial infarction | 4.431 | 0.561–34.973 | 0.158 | - | - | - |
| Chronic heart failure | 0.819 | 0.212–3.165 | 0.772 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turgunova, L.; Mekhantseva, I.; Laryushina, Y.; Alina, A.; Bacheva, I.; Zhumadilova, Z.; Turmukhambetova, A. The Association of Endothelin-1 with Early and Long-Term Mortality in COVID-19. J. Pers. Med. 2023, 13, 1558. https://doi.org/10.3390/jpm13111558
Turgunova L, Mekhantseva I, Laryushina Y, Alina A, Bacheva I, Zhumadilova Z, Turmukhambetova A. The Association of Endothelin-1 with Early and Long-Term Mortality in COVID-19. Journal of Personalized Medicine. 2023; 13(11):1558. https://doi.org/10.3390/jpm13111558
Chicago/Turabian StyleTurgunova, Lyudmila, Irina Mekhantseva, Yelena Laryushina, Assel Alina, Irina Bacheva, Zhibek Zhumadilova, and Anar Turmukhambetova. 2023. "The Association of Endothelin-1 with Early and Long-Term Mortality in COVID-19" Journal of Personalized Medicine 13, no. 11: 1558. https://doi.org/10.3390/jpm13111558





