Skull Biomechanics and Simplified Cephalometric Lines for the Estimation of Muscular Lines of Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
- be of European (Caucasian) descent;
- malocclusion that could be corrected by orthodontic treatment alone, as determined by a specialized orthodontist;
- complete primary or permanent dentition (excluding third molars);
- a maximum difference of 3 mm in the distance between each crest and maxillary point from the mid-sagittal plane in the posterior-anterior projection according to Hwang et al. [24];
- no crossbite as reported in the patient’s records and confirmed by CBCT scan.
2.2. Exclusion Criteria Were as Follows
- missing molars or bicuspids;
- a history of orthodontic treatment;
- altered bone metabolism;
- skeletal asymmetry greater than 2 mm on the left and right cephalograms;
- syndromic disorders (acquired or congenital);
- patients requiring surgery were not evaluated.
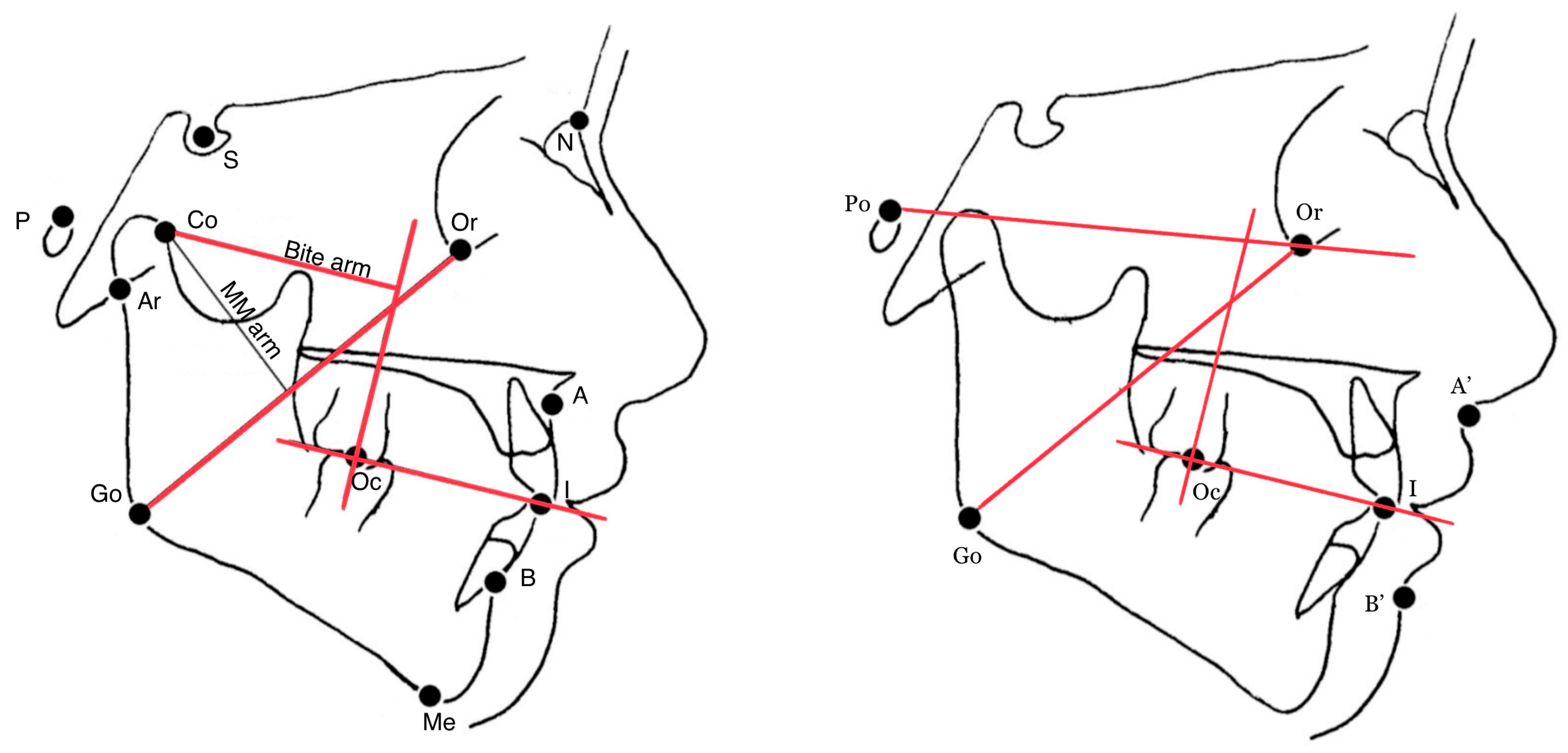
2.3. Measurements
- skeletal-cutaneous class (soft tissue equivalent of the Wits appraisal, i.e., the linear distance (mm) between the projections of the points A′ and B′ on the bisecting occlusal plane (OP), i.e., the plane bisecting the overbite of the molar and incisor teeth [19];
- the angle between the Go-Or line (estimated MM action line) and the perpendicular line to the bisecting occlusal plane through the molar occlusal point (Oc);
- the angle between the Go-Or line and the Frankfort plane (Po-Or).
2.4. Error Evaluation Method
2.5. Estimation of Sample Size
3. Results
3.1. Orthodontic Sample
3.2. Bolton Tracings
4. Discussion
5. Conclusions
Future Directions of Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patano, A.; Inchingolo, A.M.; Cardarelli, F.; Inchingolo, A.D.; Viapiano, F.; Giotta, M.; Bartolomeo, N.; Di Venere, D.; Malcangi, G.; Minetti, E.; et al. Effects of Elastodontic Appliance on the Pharyngeal Airway Space in Class II Malocclusion. J. Clin. Med. 2023, 12, 4280. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.W.; Huang, C.S.; Lo, L.J.; Chen, Y.R. Alteration of masticatory electromyographic activity and stability of orthognathic surgery in patients with skeletal class III malocclusion. J. Oral Maxillofac. Surg. 2013, 71, 1249–1260. [Google Scholar] [CrossRef]
- Wang, M.Q.; He, J.J.; Wang, K.; Svensson, P. Influence of changing occlusal support on jaw-closing muscle electromyographic activity in healthy men and women. Acta Odontol. Scand. 2009, 67, 187–192. [Google Scholar] [CrossRef]
- Mavreas, D.; Melsen, B. Changes in the mechanical advantage of the masseter and temporal muscles following surgical correction of mandibular prognathism. Int. J. Adult Orthod. Orthognath. Surg. 1997, 12, 215–225. [Google Scholar]
- Throckmorton, G.S.; Finn, R.A.; Bell, W.H. Biomechanics of differences in lower facial height. Am. J. Orthod. 1980, 77, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Throckmorton, G.S.; Dean, J.S. The relationship between jaw-muscle mechanical advantage and activity levels during isometric bites in humans. Arch. Oral Biol. 1994, 39, 429–437. [Google Scholar] [CrossRef]
- Dellavia, C.; Francetti, L.; Rosati, R.; Corbella, S.; Ferrario, V.F.; Sforza, C. Electromyographic assessment of jaw muscles in patients with All-on-Four fixed implant-supported prostheses. J. Oral Rehabil. 2012, 39, 896–904. [Google Scholar] [CrossRef]
- McNeill, C. (Ed.) Science and Practice of Occlusion; Quintessence Pub. Co.: Chicago, IL, USA, 1997; 538p. [Google Scholar]
- Throckmorton, G.S.; Throckmorton, L.S. Quantitative calculations of temporomandibular joint reaction forces—I. The importance of the magnitude of the jaw muscle forces. J. Biomech. 1985, 18, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Osborn, J.W. Orientation of the masseter muscle and the curve of Spee in relation to crushing forces on the molar teeth of primates. Am. J. Phys. Anthropol. 1993, 92, 99–106. [Google Scholar] [CrossRef]
- Gionhaku, N.; Lowe, A.A. Relationship between jaw muscle volume and craniofacial form. J. Dent. Res. 1989, 68, 805–809. [Google Scholar] [CrossRef]
- Hannam, A.G.; Wood, W.W. Relationships between the size and spatial morphology of human masseter and medial pterygoid muscles, the craniofacial skeleton, and jaw biomechanics. Am. J. Phys. Anthropol. 1989, 80, 429–445. [Google Scholar] [PubMed]
- Kasai, K.; Richards, L.C.; Kanazawa, E.; Ozaki, T.; Iwasawa, T. Relationship between attachment of the superficial masseter muscle and craniofacial morphology in dentate and edentulous humans. J. Dent. Res. 1994, 73, 1142–1149. [Google Scholar] [PubMed]
- Koolstra, J.H.; van Eijden, T.M.G.J.; van Spronsen, P.H.; Weijs, W.A.; Valk, J. Computer-assisted estimation of lines of action of human masticatory muscles reconstructed in vivo by means of magnetic resonance imaging of parallel sections. Arch. Oral Biol. 1990, 35, 549–556. [Google Scholar] [PubMed]
- Van Spronsen, P.H.; Weijs, W.A.; van Ginkel, E.; Prahl-Andersen, B. Jaw muscle orientation and moment arms of long-face and normal adults. J. Dent. Res. 1996, 75, 1372–1380. [Google Scholar]
- Broadbent, B.H.; Broadbent, B.H.; Golden, W.H. Bolton Standards of Dentofacial Developmental Growth; Mosby: Saint Louis, MI, USA, 1975; 166p. [Google Scholar]
- Van Eijden, T.M.G.J.; Korfage, J.A.M.; Brugman, P. Architecture of the human jaw-closing and jaw-opening muscles. Anat. Rec. 1997, 248, 464–474. [Google Scholar]
- Prado, F.B.; Rossi, A.C.; Freire, A.R.; Ferreira Caria, P.H. The application of finite element analysis in the skull biomechanics and dentistry. Indian J. Dent. Res. 2014, 25, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.C.; Dutel, H.; Watson, P.J.; Gröning, F.; Crumpton, N.; Fagan, M.J.; Evans, S.E. Assessment of the mechanical role of cranial sutures in the mammalian skull: Computational biomechanical modelling of the rat skull. J. Morphol. 2023, 284, e21555. [Google Scholar] [CrossRef]
- Watson, P.J.; Sharp, A.C.; Choudhary, T.; Fagan, M.J.; Dutel, H.; Evans, S.E.; Gröning, F. Computational biomechanical modelling of the rabbit cranium during mastication. Sci. Rep. 2021, 11, 13196. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Sforza, C.; Serrao, G.; Colombo, A.; Ciusa, V.; Bignotto, M. Reliability of soft tissue references for anteroposterior measurement of dental bases. Int. J. Adult Orthod. Orthognath. Surg. 1998, 13, 210–216. [Google Scholar]
- Ferrario, V.F.; Sforza, C.; Germano, D.; Dalloca, L.L.; Miani, A. Head posture and cephalometric analyses: An integrated photographic/radiographic technique. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 257–264. [Google Scholar]
- Lee, M.; Kanavakis, G.; Miner, R.M. Newly defined landmarks for a three-dimensionally based cephalometric analysis: A retrospective cone-beam computed tomography scan review. Angle Orthod. 2015, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lyros, I.; Ferdianakis, E.; Halazonetis, D.; Lykogeorgos, T.; Alexiou, A.; Alexiou, K.E.; Georgaki, M.; Vardas, E.; Yfanti, Z.; Tsolakis, A.I. Three-Dimensional Analysis of Posterior Mandibular Displacement in Rats. Vet. Sci. 2022, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Baldini, B.; Cavagnetto, D.; Baselli, G.; Sforza, C.; Tartaglia, G.M. Cephalometric measurements performed on CBCT and reconstructed lateral cephalograms: A cross-sectional study providing a quantitative approach of differences and bias. BMC Oral Health 2022, 22, 98. [Google Scholar]
- Krawczky, B.; Pacheco, A.G.; Mainenti, M.R. A systematic review of the angular values obtained by computerized photogrammetry in sagittal plane: A proposal for reference values. J. Manip. Physiol. Ther. 2014, 37, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Michler, L.; Möller, E. Occlusal control of mandibular elevator muscles. Eur. J. Oral Sci. 1992, 100, 284–291. [Google Scholar]
- Di Palma, E.; Tepedino, M.; Chimenti, C.; Tartaglia, G.; Sforza, C. Longitudinal effects of rapid maxillary expansion on masticatory muscles activity. J. Clin. Exp. Dent. 2017, 9, e635–e640. [Google Scholar] [CrossRef]
- Arat, F.E.; Arat, Z.M.; Acar, M.; Beyazova, M.; Tompson, B. Muscular and Condylar Response to rapid maxillary expansion. Part 1: Electromyographic study of anterior temporal and superficial masseter muscles. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 815–822. [Google Scholar]
- Piancino, M.G.; Farina, D.; Talpone, F.; Merlo, A.; Bracco, P. Muscular activation during reverse and non-reverse chewing cycles in unilateral posterior crossbite. Eur. J. Oral Sci. 2009, 117, 122–128. [Google Scholar]
- Alarcón, J.A.; Martín, C.; Palma, J.C.; Menéndez-Núñez, M. Activity of jaw muscles in unilateral cross-bite without mandibular shift. Arch. Oral Biol. 2009, 54, 108–114. [Google Scholar]
- Maspero, C.; Cavagnetto, D.; Abate, A.; Cressoni, P.; Farronato, M. Effects on the Facial Growth of Rapid Palatal Expansion in Growing Patients Affected by Juvenile Idiopathic Arthritis with Monolateral Involvement of the Temporomandibular Joints: A Case-Control Study on Posteroanterior and Lateral Cephalograms. J. Clin. Med. 2020, 9, 1159. [Google Scholar] [CrossRef]
- Raaj, V.; Raina, S.; Raina, R.; Kumari, M. Electromyographic Analysis of Masticatory and Accessory Muscles in Subjects with Implant-Supported Fixed Prostheses: A Three-Arm Comparative Clinical Study. Cureus 2023, 15, e33969. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.A. Oral anatomy. Fifth Edition. By Harry Sicher and E. Lloyd DuBrul. 502 pp. and 319 ill. C. V. Mosby Co., St. Louis. 1970. $17.50. Am. J. Phys. Anthropol. 1972, 36, 144–145. [Google Scholar] [CrossRef]
- Ellis, H. Gray’s anatomy. 37th ed. P. L. Williams, R. Warwick, M. Dyson, L.H. Bannister. 305 × 235mm. Pp. 1598. Illustrated. 1989. Edinburgh: Churchill Livingstone. £70.00. Br. J. Surg. 2005, 76, 1359. [Google Scholar] [CrossRef]
- Tortora, G.; Farronato, M.; Gaffuri, F.; Carloni, P.; Occhipinti, C.; Tucci, M.; Cenzato, N.; Maspero, C. Survey of oral hygiene habits and knowledge among school children: A cross-sectional study from Italy. Eur. J. Paediatr. Dent. 2023, 24, 1. [Google Scholar]
- Portelli, M.; Matarese, G.; Militi, A.; Logiudice, G.; Nucera, R.; Lucchese, A. Temporomandibular joint involvement in a cohort of patients with Juvenile Idiopatic Arthritis and evaluation of the effect induced by functional orthodontic appliance: Clinical and radiographic investigation. Eur. J. Paediatr. Dent. 2014, 15, 63–66. [Google Scholar]
- Park, J.H.; Hwang, H.W.; Moon, J.H.; Yu, Y.; Kim, H.; Her, S.B.; Srinivasan, G.; Aljanabi, M.N.A.; Donatelli, R.E.; Lee, S.J. Automated identification of cephalometric landmarks: Part 1—Comparisons between the latest deep-learning methods YOLOV3 and SSD. Angle Orthod. 2019, 89, 903–909. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Tsolakis, A.I.; Elshebiny, T.; Matthaios, S.; Palomo, J.M. Comparing a Fully Automated Cephalometric Tracing Method. to a Manual Tracing Method. for Orthodontic Diagnosis. J. Clin. Med. 2022, 11, 6854. [Google Scholar] [CrossRef] [PubMed]
- Ceratti, C.; Maspero, C.; Consonni, D.; Caprioglio, A.; Connelly, S.T.; Inchingolo, F.; Tartaglia, G.M. Cone-Beam Computed Tomographic Assessment of the Mandibular Condylar Volume in Different Skeletal Patterns: A Retrospective Study in Adult Patients. Bioengineering 2022, 9, 102. [Google Scholar] [CrossRef]
| Males | Females | All Subjects | 6–10 Years | 11–15 Years | 16–50 Years | |
|---|---|---|---|---|---|---|
| Orthodontic patients | 217 | 293 | 510 | 257 | 134 | 119 |
| Skeletal-cutaneous class (mm) | 4.7 (4.1) | 3.4 (3.7) | 4.2 (4.0) | 3.8 (3.4) | 4.8 (4.2) | 3.4 (5.8) |
| Go-Or to normal to OP (°) | 39.59 (0.27) | 39.12 (0.27) | 39.33 (0.19) | 39.92 (0.23) | 39.41 (0.37) | 36.87 (0.59) |
| Go-Or to Po-Or (°) | 41.99 (0.28) | 41.98 (0.22) | 41.99 (0.18) | 40.95 (0.19) | 42.34 (0.26) | 45.39 (0.50) |
| Age | Skeletal-Cutaneous Class | Go-Or to Po-Or | |
|---|---|---|---|
| Skeletal-cutaneous class | 0.025 | - | - |
| Go-Or to normal to OP | 0.255 | 0.444 | 0.322 |
| Go-Or to Po-Or | 0.449 | 0.057 | - |
| All Tracings | 6–10 Years | 11–15 Years | 16–18 Years | |
|---|---|---|---|---|
| Skeletal-cutaneous class (mm) | 3.5 (1.2) | 2.8 (1.5) | 4.2 (0.5) | 3.8 (0.7) |
| Go-Or to normal to OP (°) | 38.77 (0.47) | 39.04 (1.11) | 39.29 (0.20) | 37.39 (0.45) |
| Go-Or to Po-Or (°) | 40.72 (0.55) | 38.89 (0.49) | 41.16 (0.51) | 41.09 (0.56) |
| Age | Skeleto-Cutaneous Class | Go-Or to Po-Or | |
|---|---|---|---|
| Skeleto-cutaneous class | 0.410 | - | - |
| Go-Or to normal to OP | 0.397 | 0.530 | 0.481 |
| Go-Or to Po-Or | 0.965 | 0.367 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolci, C.; Cenzato, N.; Maspero, C.; Giannini, L.; Khijmatgar, S.; Dipalma, G.; Tartaglia, G.M.; Inchingolo, F. Skull Biomechanics and Simplified Cephalometric Lines for the Estimation of Muscular Lines of Action. J. Pers. Med. 2023, 13, 1569. https://doi.org/10.3390/jpm13111569
Dolci C, Cenzato N, Maspero C, Giannini L, Khijmatgar S, Dipalma G, Tartaglia GM, Inchingolo F. Skull Biomechanics and Simplified Cephalometric Lines for the Estimation of Muscular Lines of Action. Journal of Personalized Medicine. 2023; 13(11):1569. https://doi.org/10.3390/jpm13111569
Chicago/Turabian StyleDolci, Claudia, Niccolò Cenzato, Cinzia Maspero, Lucia Giannini, Shahnawaz Khijmatgar, Gianna Dipalma, Gianluca Martino Tartaglia, and Francesco Inchingolo. 2023. "Skull Biomechanics and Simplified Cephalometric Lines for the Estimation of Muscular Lines of Action" Journal of Personalized Medicine 13, no. 11: 1569. https://doi.org/10.3390/jpm13111569
APA StyleDolci, C., Cenzato, N., Maspero, C., Giannini, L., Khijmatgar, S., Dipalma, G., Tartaglia, G. M., & Inchingolo, F. (2023). Skull Biomechanics and Simplified Cephalometric Lines for the Estimation of Muscular Lines of Action. Journal of Personalized Medicine, 13(11), 1569. https://doi.org/10.3390/jpm13111569










