Role of Microenvironmental Components in Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Vascular Component of the HNSCC TME
3. The Role of Hypoxia in the TME of HNSCC
4. CAFs in HNSCC
4.1. Contribution of CAFs to HNSCC Progression
4.2. CAFs and Cancer Cell Proliferation
4.3. CAFs and Metastasis
4.4. CAFs and Angiogenesis
4.5. CAFs and Immune Response
4.6. CAFs and Metabolic Activity
5. Role of the ECM in HNSCC
| Main Components of the HNSCC ECM | What Are the Effects on HNSCC Progression? | |
|---|---|---|
| Collagens |
| |
| Elastin |
| |
| Fibronectin |
| |
| Laminins |
| |
| Hyaluronan |
| |
| Tenascin-C |
| |
| Integrins |
| |
| MMPs | MMP2 |
|
| MMP9 |
| |
| MMP13 |
| |
| MMP14 |
| |
| MMP17 | ||
| MMP20 |
| |
| Perlecan |
| |
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordemar, S.; Kronenwett, U.; Auer, G.; Högmo, A.; Lindholm, J.; Edström, S.; Tryggvasson, K.; Linder, S.; Munck-Wikland, E. Laminin-5 as a predictor of invasiveness in cancer in situ lesions of the larynx. Anticancer Res. 2001, 21, 509–512. [Google Scholar] [PubMed]
- Berndt, A.; Hyckel, P.; Könneker, A.; Katenkamp, D.; Kosmehl, H. Oral squamous cell carcinoma invasion is associated with a laminin-5 matrix re-organization but independent of basement membrane and hemidesmosome formation. Clues from an in vitro invasion model. Invasion Metastasis 1997, 17, 251–258. [Google Scholar]
- Meireles Da Costa, N.; Mendes, F.A.; Pontes, B.; Nasciutti, L.E.; Ribeiro Pinto, L.F.; Palumbo Júnior, A. Potential therapeutic significance of laminin in head and neck squamous carcinomas. Cancers 2021, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S.B.; Liu, W.; Lu, Y.; Mills, G.; Motter, T.; et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar]
- Zhang, P.; Li, S.; Zhang, T.; Cui, F.; Shi, J.H.; Zhao, F.; Sheng, X. Characterization of Molecular Subtypes in Head and Neck Squamous Cell Carcinoma with Distinct Prognosis and Treatment Responsiveness. Front. Cell Dev. Biol. 2021, 9, 711348. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Gil, Z.; Amit, M.; Yen, T.C.; Liao, C.T.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Kreppel, M.; Cernea, C.R.; et al. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: An international multicenter retrospective study. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 1138–1148. [Google Scholar] [CrossRef]
- Wreesmann, V.B.; Katabi, N.; Palmer, F.L.; Montero, P.H.; Migliacci, J.C.; Gönen, M.; Carlson, D.; Ganly, I.; Shah, J.P.; Ghossein, R.; et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck 2016, 38, E1192–E1199. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Nosé, V.; Lazar, A.J. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Familial Tumor Syndromes. Head Neck Pathol. 2022, 16, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Oropharynx and Nasopharynx. Head Neck Pathol. 2022, 16, 19–30. [Google Scholar] [CrossRef]
- Lemaire, F.; Millon, R.; Young, J.; Cromer, A.; Wasylyk, C.; Schultz, I.; Muller, D.; Marchal, P.; Zhao, C.; Melle, D.; et al. Differential expression profiling of head and neck squamous cell carcinoma (HNSCC). Br. J. Cancer 2003, 89, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Duray, A.; Demoulin, S.; Hubert, P.; Delvenne, P. Immune suppression in head and neck cancers: A review. Clin. Dev. Immunol. 2010, 2010, 701657. [Google Scholar]
- Plzák, J.; Bouček, J.; Bandúrová, V.; Kolář, M.; Hradilová, M.; Szabo, P.; Lacina, L.; Chovanec, M.; Smetana, K. The head and neck squamous cell carcinoma microenvironment as a potential target for cancer therapy. Cancers 2019, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, M.; Cheng, M.; Wang, X.; Li, K.; Chen, J.; Chen, Z.; Chen, S.; Chen, J.; Xiong, G.; et al. Tumor microenvironment in head and neck squamous cell carcinoma: Functions and regulatory mechanisms. Cancer Lett. 2021, 507, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Elmusrati, A.; Wang, J.; Wang, C.Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral Sci. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- Castells, M.; Thibault, B.; Delord, J.P.; Couderc, B. Implication of tumor microenvironment in chemoresistance: Tumor-associated stromal cells protect tumor cells from cell death. Int. J. Mol. Sci. 2012, 13, 9545–9571. [Google Scholar] [CrossRef]
- de Miranda, M.C.; de Melo, M.I.A.; da Silva Cunha, P.; Júnior, J.G.; Faria, J.A.Q.A.; Rodrigues, M.A.; Gomes, D.A. Roles of mesenchymal stromal cells in the head and neck cancer microenvironment. Biomed. Pharmacother. 2021, 144, 112269. [Google Scholar] [CrossRef]
- Almangush, A.; Alabi, R.O.; Troiano, G.; Coletta, R.D.; Salo, T.; Pirinen, M.; Mäkitie, A.A.; Leivo, I. Clinical significance of tumor-stroma ratio in head and neck cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 480. [Google Scholar] [CrossRef] [PubMed]
- Meads, M.B.; Gatenby, R.A.; Dalton, W.S. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat. Rev. Cancer 2009, 9, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Jumaniyazova, E.; Lokhonina, A.; Dzhalilova, D.; Kosyreva, A.; Fatkhudinov, T. Immune Cells in Head-and-Neck Tumor Microenvironments. J. Pers. Med. 2022, 12, 1521. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, P.A.; Cunha, I.W.; Ioannidis, J.P.A. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: A meta-analysis. Clin. Cancer Res. 2005, 11, 1434–1440. [Google Scholar] [CrossRef]
- Rad, H.S.; Shiravand, Y.; Radfar, P.; Ladwa, R.; Perry, C.; Han, X.; Warkiani, M.E.; Adams, M.N.; Hughes, B.G.; O’Byrne, K.; et al. Understanding the tumor microenvironment in head and neck squamous cell carcinoma. Clin. Transl. Immunol. 2022, 11, e1397. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Watanabe, S.; Kato, M.; Kotani, I.; Ryoke, K.; Hayashi, K. Lymphatic vessel density and vascular endothelial growth factor expression in squamous cell carcinomas of lip and oral cavity: A clinicopathological analysis with immunohistochemistry using antibodies to D2-40, VEGF-C and VEGF-D. Yonago Acta Med. 2013, 56, 29–37. [Google Scholar]
- Christopoulos, A.; Ahn, S.M.; Klein, J.D.; Kim, S. Biology of vascular endothelial growth factor and its receptors in head and neck cancer: Beyond angiogenesis. Head Neck 2010, 33, 1220–1229. [Google Scholar] [CrossRef]
- Carla, C.; Daris, F.; Cecilia, B.; Francesca, B.; Francesca, C.; Paolo, F. Angiogenesis in head and neck cancer: A review of the literature. J. Oncol. 2011, 2012, 358472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pradeep, C.R.; Sunila, E.S.; Kuttan, G. Expression of vascular endothelial growth factor (VEGF) and VEGF receptors in tumor angiogenesis and malignancies. Integr. Cancer Ther. 2005, 4, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.S.; Raica, M. Vascular Endothelial Growth Factor Family and Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2023, 43, 4315–4326. [Google Scholar] [CrossRef]
- Kim, K.J.; Cho, C.S.; Kim, W.U. Role of placenta growth factor in cancer and inflammation. Exp. Mol. Med. 2011, 44, 10–19. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell. Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, S.; Tamagnone, L. Multifaceted Role of Neuropilins in Cancer. Curr. Med. Chem. 2011, 18, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.M. The role of neuropilins in cancer. Mol. Cancer Ther. 2006, 5, 1099–1107. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Parra, M.; Verdin, E.; Bassel-Duby, R.; Olson, E.N. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc. Natl. Acad. Sci. USA 2008, 105, 7738–7743. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Breslin, J.W.; Pappas, P.J.; Cerveira, J.J.; Hobson, R.W.; Duran, W.N. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H92–H100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, K.; Kshirsagar, S.; Li, W.; Gui, L.; Ramakrishnan, S.; Gupta, P.; Law, P.Y.; Hebbel, R.P. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp. Cell Res. 1999, 247, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Reinmuth, N.; Liu, W.; Jung, Y.D.; Ahmad, S.A.; Shaheen, R.M.; Fan, F.; Bucana, C.D.; McMahon, G.; Gallick, G.E.; Ellis, L.M. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001, 15, 1239–1241. [Google Scholar] [CrossRef]
- Shigetomi, S.; Imanishi, Y.; Shibata, K.; Sakai, N.; Sakamoto, K.; Fujii, R.; Habu, N.; Otsuka, K.; Sato, Y.; Watanabe, Y.; et al. VEGF-C/Flt-4 axis in tumor cells contributes to the progression of oral squamous cell carcinoma via upregulating VEGF-C itself and contactin-1 in an autocrine manner. Am. J. Cancer Res. 2018, 8, 2046–2063. [Google Scholar] [PubMed]
- Campos, M.S.; Neiva, K.G.; Meyers, K.A.; Krishnamurthy, S.; Nör, J.E. Endothelial derived factors inhibit anoikis of head and neck cancer stem cells. Oral Oncol. 2012, 48, 26–32. [Google Scholar] [CrossRef]
- Ziogas, A.C.; Gavalas, N.G.; Tsiatas, M.; Tsitsilonis, O.; Politi, E.; Terpos, E.; Rodolakis, A.; Vlahos, G.; Thomakos, N.; Haidopoulos, D.; et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int. J. Cancer 2011, 130, 857–864. [Google Scholar] [CrossRef]
- Micaily, I.; Johnson, J.; Argiris, A. An update on angiogenesis targeting in head and neck squamous cell carcinoma. Cancers Head Neck 2020, 5, 5–7. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Curry, J.M.; Sprandio, J.; Cognetti, D.; Luginbuhl, A.; Bar-Ad, V.; Pribitkin, E.; Tuluc, M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin. Oncol. 2014, 41, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Neiva, K.G.; Zhang, Z.; Miyazawa, M.; Warner, K.A.; Karl, E.; Nör, J.E. Cross talk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. Neoplasia 2009, 11, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Herrington, H.; Reh, D.; Weber, S.; Bornstein, S.; Wang, D.; Li, A.G.; Tang, C.-F.; Siddiqui, Y.; Nord, J.; et al. Loss of transforming growth factor-β type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006, 20, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Smith, G.L.; Carter, D.; Sasaki, C.T.; Haffty, B.G. Prognostic significance of Vascular Endothelial Growth Factor protein levels in oral and oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2000, 18, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Mineta, H.; Miura, K.; Ogino, T.; Takebayashi, S.; Misawa, K.; Ueda, Y.; Suzuki, I.; Dictor, M.; Borg, Å.; Wennerberg, J. Prognostic value of vascular endothelial growth factor (VEGF) in head and neck squamous cell carcinomas. Br. J. Cancer 2000, 83, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.M.; Chan, A.W.H.; Yu, K.H.; King, A.D.; Wong, K.T.; Chen, G.G.; Tsang, R.K.Y.; Chan, A.B.W. Strong immunohistochemical expression of vascular endothelial growth factor predicts overall survival in head and neck squamous cell carcinoma. Ann. Surg. Oncol. 2007, 14, 3558–3565. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, R.; Macluskey, M.; Williams, H.; Ogden, G.R. Vascularity and expression of vascular endothelial growth factor in oral squamous cell carcinoma, resection margins, and nodal metastases. Br. J. Oral Maxillofac. Surg. 2006, 44, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.J.; Li, J.R.; Li, Z.B. Circulating levels of vascular endothelial growth factor in patients with oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2002, 31, 495–498. [Google Scholar] [CrossRef]
- Aggarwal, S.; Devaraja, K.; Sharma, S.C.; Das, S.N. Expression of vascular endothelial growth factor (VEGF) in patients with oral squamous cell carcinoma and its clinical significance. Clin. Chim. Acta 2014, 436, 35–40. [Google Scholar] [CrossRef]
- Nejad, A.E.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Javanmard, S.H.; Taherian, M.; Ahmadlou, M. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 1–26. [Google Scholar]
- White, R.A.; Malkoski, S.P.; Wang, X.J. TGFΒ signaling in head and neck squamous cell carcinoma. Oncogene 2010, 29, 5437–5446. [Google Scholar] [CrossRef] [PubMed]
- D’Ignazio, L.; Batie, M.; Rocha, S. Hypoxia and inflammation in cancer, focus on HIF and NF-κB. Biomedicines 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L. Hypoxia-A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010, 29, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Machiels, J.P. Targeting the Tumor Environment in Squamous Cell Carcinoma of the Head and Neck. Curr. Treat. Options Oncol. 2016, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—Implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef]
- Li, J.Z.; Gao, W.; Chan, J.Y.-W.; Ho, W.-K.; Wong, T.-S. Hypoxia in Head and Neck Squamous Cell Carcinoma. ISRN Otolaryngol. 2012, 2012, 708974. [Google Scholar] [CrossRef]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism: Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef]
- Palazón, A.; Aragonés, J.; Morales-Kastresana, A.; Ortiz De Landázuri, M.; Melero, I. Molecular pathways: Hypoxia response in immune cells fighting or promoting cancer. Clin. Cancer Res. 2012, 18, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Potluri, N.; Lu, J.; Kim, Y.; Rastinejad, F. Structural integration in hypoxia-inducible factors. Nature 2015, 524, 303–308. [Google Scholar]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [PubMed]
- Ratcliffe, P.; Koivunen, P.; Myllyharju, J.; Ragoussis, J.; Bovée, J.; Batinic-Haberle, I.; Vinatier, C.; Trichet, V.; Robriquet, F.; Oliver, L.; et al. Update on hypoxia-inducible factors and hydroxylases in oxygen regulatory pathways: From physiology to therapeutics. Hypoxia 2017, 5, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G. HIFα targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Shibasaki, F. Hypoxia-inducible factor as an angiogenic master switch. Front. Pediatr. 2015, 3, 33. [Google Scholar]
- Semenza, G.L. Oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 336–361. [Google Scholar] [CrossRef]
- Schödel, J.; Oikonomopoulos, S.; Ragoussis, J.; Pugh, C.W.; Ratcliffe, P.J.; Mole, D.R. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood J. Am. Soc. Hematol. 2011, 117, e207–e217. [Google Scholar] [CrossRef]
- Liao, D.; Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef]
- Bui, B.P.; Nguyen, P.L.; Lee, K.; Cho, J. Hypoxia-Inducible Factor-1: A Novel Therapeutic Target for the Management of Cancer, Drug Resistance, and Cancer-Related Pain. Cancers 2022, 14, 6054. [Google Scholar]
- Lv, X.; Li, J.; Zhang, C.; Hu, T.; Li, S.; He, S.; Yan, H.; Tan, Y.; Lei, M.; Wen, M.; et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2017, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, M.; Weijer, R.; Krekorian, M.; Ijssel, B.v.D.; Kos, M.; Alles, L.K.; van Wijk, A.C.; Bikadi, Z.; Hazai, E.; van Gulik, T.M.; et al. Inhibition of hypoxia-inducible factor 1 with acriflavine sensitizes hypoxic tumor cells to photodynamic therapy with zinc phthalocyanine-encapsulating cationic liposomes. Nano Res. 2016, 9, 1639–1662. [Google Scholar]
- Tang, N.; Wang, L.; Esko, J.; Giordano, F.J.; Huang, Y.; Gerber, H.P.; Ferrara, N.; Johnson, R.S. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004, 6, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Hapke, R.Y.; Haake, S.M. Hypoxia-induced epithelial to mesenchymal transition in cancer. Cancer Lett. 2020, 487, 10–20. [Google Scholar]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Simopoulos, C.; Turley, H.; Talks, K.; Gatter, K.C.; Harris, A.L. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int. J. Radiat. Oncol. 2002, 53, 1192–1202. [Google Scholar] [CrossRef]
- Hill, R.M.; Rocha, S.; Parsons, J.L. Overcoming the Impact of Hypoxia in Driving Radiotherapy Resistance in Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 4130. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Esteban, M.A.; Tran, M.G.B.; Harten, S.K.; Hill, P.; Castellanos, M.C.; Chandra, A.; Raval, R.; O’Brien, T.S.; Maxwell, P.H. Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res. 2006, 66, 3567–3575. [Google Scholar] [CrossRef]
- Kim, K.; Lu, Z.; Hay, E.D. Direct evidence for a role of β-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol. Int. 2002, 26, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Dudas, J.; Ladanyi, A.; Ingruber, J.; Steinbichler, T.B.; Riechelmann, H. Epithelial to Mesenchymal Transition: A Mechanism that Fuels Cancer Radio/Chemoresistance. Cells 2020, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.D.; Kang, N.; Choi, S.Y.; Kim, B.N.; Park, C.K.; Kim, J.W.; Kim, Y.K.; Kim, S.J. The role of hypoxia on the acquisition of epithelial-mesenchymal transition and cancer stemness: A possible link to epigenetic regulation. Korean J. Intern. Med. 2017, 32, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed]
- Lemos, D.R.; Duffield, J.S. Tissue-resident mesenchymal stromal cells: Implications for tissue-specific antifibrotic therapies. Sci. Transl. Med. 2018, 10, eaan5174. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gersten, A.; Moleirinho, S.; Gunn-Moore, F.J.; Reynolds, P.A.; Prystowsky, M.B. Fibroblasts in head and neck squamous cell carcinoma associated with perineural invasion have high-level nuclear yes-associated protein (YAP) expression. Acad. Pathol. 2015, 2, 2374289515616972. [Google Scholar] [CrossRef]
- Fotsitzoudis, C.; Koulouridi, A.; Messaritakis, I.; Konstantinidis, T.; Gouvas, N.; Tsiaoussis, J.; Souglakos, J. Cancer-Associated Fibroblasts: The Origin, Biological Characteristics and Role in Cancer—A Glance on Colorectal Cancer. Cancers 2022, 14, 4394. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Kojima, Y.; Acar, A.; Eaton, E.N.; Mellody, K.T.; Scheel, C.; Ben-Porath, I.; Onder, T.T.; Wang, Z.C.; Richardson, A.L.; Weinberg, R.A.; et al. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl. Acad. Sci. USA 2010, 107, 20009–20014. [Google Scholar] [CrossRef]
- Mueller, L.; Goumas, F.A.; Affeldt, M.; Sandtner, S.; Gehling, U.M.; Brilloff, S.; Walter, J.; Karnatz, N.; Lamszus, K.; Rogiers, X.; et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am. J. Pathol. 2007, 171, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, Y.; Wu, C.; Huang, Q. Heterogeneity of cancer-associated fibroblasts in head and neck squamous cell carcinoma: Opportunities and challenges. Cell Death Discov. 2023, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Cintrón, K.M.; Ayuso, J.M.; Humayun, M.; Gong, M.M.; Kerr, S.C.; Ponik, S.M.; Harari, P.M.; Virumbrales-Munoz, M.; Beebe, D.J. Primary Head and Neck Tumour-Derived Fibroblasts Promote Lymphangiogenesis in a Lymphatic Organotypic Co-culture Model. EBioMedicine 2021, 73, 103634. [Google Scholar] [CrossRef] [PubMed]
- Markwell, S.M.; Weed, S.A. Tumor and stromal-based contributions to head and neck squamous cell carcinoma invasion. Cancers 2015, 7, 382–406. [Google Scholar] [CrossRef] [PubMed]
- Custódio, M.; Biddle, A.; Tavassoli, M. Portrait of a CAF: The story of cancer-associated fibroblasts in head and neck cancer. Oral Oncol. 2020, 110, 104972. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Baba, H.; Yoshida, N.; Miyake, K.; Yasuda, T.; Uchihara, T.; Tan, P.; Ishimoto, T. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene 2019, 38, 4887–4901. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.S.; Hui, M.N.; Elsworth, B.L.; Wu, S.Z.; Roden, D.; Chan, C.L.; Skhinas, J.N.; Collot, R.; Yang, J.; Harvey, K.; et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Gál, P.; Varinská, L.; Fáber, L.; Novák, Š.; Szabo, P.; Mitrengová, P.; Mirossay, A.; Mučaji, P.; Smetana, K. How Signaling Molecules Regulate Tumor Microenvironment: Parallels to Wound Repair. Molecules 2017, 22, 1818. [Google Scholar] [CrossRef]
- Kolář, M.; Szabo, P.; Dvořánková, B.; Lacina, L.; Gabius, H.J.; Strnad, H.; Šáchová, J.; Vlček, Č.; Plzák, J.; Chovanec, M.; et al. Upregulation of IL-6, IL-8 and CXCL-1 production in dermal fibroblasts by normal/malignant epithelial cells in vitro: Immunohistochemical and transcriptomic analyses. Biol. Cell. 2012, 104, 738–751. [Google Scholar] [CrossRef]
- Wondergem, N.E.; Nauta, I.H.; Muijlwijk, T.; Leemans, C.R.; van de Ven, R. The Immune Microenvironment in Head and Neck Squamous Cell Carcinoma: On Subsets and Subsites. Curr. Oncol. Rep. 2020, 22, 81. [Google Scholar] [CrossRef]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Sarkar, M.; Nguyen, T.; Gundre, E.; Ogunlusi, O.; El-Sobky, M.; Giri, B.; Sarkar, T.R. Cancer-associated fibroblasts: The chief architect in the tumor microenvironment. Front. Cell Dev. Biol. 2023, 11, 1089068. [Google Scholar] [CrossRef] [PubMed]
- Seandel, M.; Noack-Kunnmann, K.; Zhu, D.; Aimes, R.T.; Quigley, J.P. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood 2001, 97, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. 2010, 15, 166–179. [Google Scholar] [CrossRef]
- Principe, S.; Mejia-Guerrero, S.; Ignatchenko, V.; Sinha, A.; Ignatchenko, A.; Shi, W.; Pereira, K.; Su, S.; Huang, S.H.; O’Sullivan, B.; et al. Proteomic Analysis of Cancer-Associated Fibroblasts Reveals a Paracrine Role for MFAP5 in Human Oral Tongue Squamous Cell Carcinoma. J. Proteome Res. 2018, 17, 2045–2059. [Google Scholar] [CrossRef]
- Kamdje, A.H.N.; Etet, P.F.S.; Kipanyula, M.J.; Vecchio, L.; Simo, R.T.; Njamnshi, A.K.; Lukong, K.E.; Mimche, P.N. Insulin-like growth factor-1 signaling in the tumor microenvironment: Carcinogenesis, cancer drug resistance, and therapeutic potential. Front. Endocrinol. 2022, 13, 927390. [Google Scholar]
- Li, X.; Xu, Q.; Wu, Y.; Li, J.; Tang, D.; Han, L.; Fan, Q. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis 2014, 35, 1362–1370. [Google Scholar] [CrossRef]
- Chen, W.J.; Ho, C.C.; Chang, Y.L.; Chen, H.Y.; Lin, C.A.; Ling, T.Y.; Yu, S.L.; Yuan, S.S.; Chen, Y.J.L.; Lin, C.Y.; et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 2014, 5, 3472. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Teijeiro, S.; García-Inclán, C.; Villaronga, M.Á.; Casado, P.; Hermida-Prado, F.; Granda-Díaz, R.; Rodrigo, J.P.; Calvo, F.; Del-Río-Ibisate, N.; Gandarillas, A.; et al. Factors secreted by cancer-associated fibroblasts that sustain cancer stem properties in head and neck squamous carcinoma cells as potential therapeutic targets. Cancers 2018, 10, 334. [Google Scholar] [CrossRef]
- Powell, D.W.; Mifflin, R.C.; Valentich, J.D.; Crowe, S.E.; Saada, J.I.; West, A.B. Myofibroblasts. I. Paracrine cells important in health and disease. Am. J. Physiol. Cell Physiol. 1999, 277, C1–C19. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, Z.; Kong, J.; Zhang, F.; Fang, S.; Li, X.; Li, W.; Yang, X.; Luo, Y.; Lin, B.; et al. Carcinoma-associated fibroblasts lead the invasion of salivary gland adenoid cystic carcinoma cells by creating an invasive track. PLoS ONE 2016, 11, e0150247. [Google Scholar] [CrossRef] [PubMed]
- Asif, P.J.; Longobardi, C.; Hahne, M.; Medema, J.P. The role of cancer-associated fibroblasts in cancer invasion and metastasis. Cancers 2021, 13, 4720. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Neri, S.; Ishii, G.; Hashimoto, H.; Kuwata, T.; Nagai, K.; Date, H.; Ochiai, A. Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int. J. Cancer 2015, 137, 784–796. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, C.H.; Tan, L.D.; Wang, Q.S.; Li, X.Q.; Feng, Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef]
- Zhuang, J.; Lu, Q.; Shen, B.; Huang, X.; Shen, L.; Zheng, X.; Huang, R.; Yan, J.; Guo, H. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep. 2015, 5, 11924. [Google Scholar] [CrossRef]
- Brabletz, S.; Brabletz, T. The ZEB/miR-200 feedback loop-a motor of cellular plasticity in development and cancer? EMBO Rep. 2010, 11, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Hong, J.; Du, W.; Lin, Y.W.; Ren, L.L.; Wang, Y.C.; Su, W.Y.; Wang, J.L.; Cui, Y.; Wang, Z.H.; et al. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J. Biol. Chem. 2012, 287, 5819–5832. [Google Scholar] [CrossRef] [PubMed]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, Q.; Li, J.; Song, J.; Gu, Y. MiR-124 down-regulation is critical for cancer associated fibroblasts-enhanced tumor growth of oral carcinoma. Exp. Cell Res. 2017, 351, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Dudás, J.; Riml, A.; Tuertscher, R.; Pritz, C.; Steinbichler, T.B.; Schartinger, V.H.; Sprung, S.; Glueckert, R.; Schrott-Fischer, A.; Chacko, L.J.; et al. Brain-Derived Neurotrophin and TrkB in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 272. [Google Scholar]
- Nie, J.Z.; Wang, M.-T.; Nie, D. Regulations of Tumor Microenvironment by Prostaglandins. Cancers 2023, 15, 3090. [Google Scholar]
- Jiffar, T.; Yilmaz, T.; Lee, J.; Miller, Y.; Feng, L.; El-Naggar, A.; Kupferman, M.E. Brain derived neutrophic factor (BDNF) coordinates lympho-vascular metastasis through a fibroblast-governed paracrine axis in the tumor microenvironment. Cancer Cell Microenviron. 2017, 4, e1566. [Google Scholar] [CrossRef]
- Kupferman, M.E.; Jiffar, T.; El-Naggar, A.; Yilmaz, T.; Zhou, G.; Xie, T.; Feng, L.; Wang, J.; Holsinger, F.C.; Yu, D.; et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene 2010, 29, 2047–2059. [Google Scholar] [CrossRef]
- Abrahao, A.C.; Castilho, R.M.; Squarize, C.H.; Molinolo, A.A.; Santos-Pinto, D.; Dos Gutkind, J.S. A role for COX2-derived PGE2 and PGE2-receptor subtypes in head and neck squamous carcinoma cell proliferation. Oral Oncol. 2010, 46, 880–887. [Google Scholar] [CrossRef]
- Cohen, E.G.; Almahmeed, T.; Du, B.; Golijanin, D.; Boyle, J.O.; Soslow, R.A.; Subbaramaiah, K.; Dannenberg, A.J. Microsomal prostaglandin E synthase-1 is overexpressed in head and neck squamous cell carcinoma. Clin. Cancer Res. 2003, 9, 3425–3430. [Google Scholar]
- Knops, A.M.; South, A.; Rodeck, U.; Martinez-Outschoorn, U.; Harshyne, L.A.; Johnson, J.; Luginbuhl, A.J.; Curry, J.M. Cancer-Associated Fibroblast Density, Prognostic Characteristics, and Recurrence in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2020, 10, 565306. [Google Scholar] [CrossRef] [PubMed]
- Pietras, K.; Pahler, J.; Bergers, G.; Hanahan, D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008, 5, e19. [Google Scholar] [CrossRef] [PubMed]
- Dudás, J.; Fullár, A.; Bitsche, M.; Schartinger, V.; Kovalszky, I.; Sprinzl, G.M.; Riechelmann, H. Tumor-produced, active Interleukin-1 β regulates gene expression in carcinoma-associated fibroblasts. Exp. Cell Res. 2011, 317, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, N.; Kawanishi, S.; Hiraku, Y.; Oikawa, S.; Xie, Y.; Zhang, Z.; Huang, G.; Murata, M. Relationships of alpha-SMA-positive fibroblasts and SDF-1-positive tumor cells with neoangiogenesis in nasopharyngeal carcinoma. BioMed Res. Int. 2014, 2014, 507353. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Reh, D.; Li, A.G.; Woods, J.; Corless, C.L.; Kulesz-Martin, M.; Wang, X.J. Overexpression of transforming growth factor β1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 2004, 64, 4405–4410. [Google Scholar] [CrossRef]
- Alcolea, S.; Antón, R.; Camacho, M.; Soler, M.; Alfranca, A.; Avilés-Jurado, F.X.; Redondo, J.-M.; Quer, M.; León, X.; Vila, L. Interaction between head and neck squamous cell carcinoma cells and fibroblasts in the biosynthesis of PGE 2. J. Lipid Res. 2012, 53, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bao, H.; Fan, Y.J.; Jiang, Z.L.; Qi, Y.X.; Han, Y. Platelet-derived microvesicles promote endothelial progenitor cell proliferation in intimal injury by delivering TGF-β1. FEBS J. 2020, 287, 5196–5217. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakakura, K.; Kudo, T.; Toyoda, M.; Kaira, K.; Oyama, T.; Chikamatsu, K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 2017, 8, 8633. [Google Scholar] [CrossRef]
- Takahashi, H.; Rokudai, S.; Kawabata-iwakawa, R.; Sakakura, K.; Oyama, T.; Nishiyama, M.; Chikamatsu, K. Akt3 is a novel regulator of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancers 2021, 13, 1233. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakakura, K.; Kawabata-Iwakawa, R.; Rokudai, S.; Toyoda, M.; Nishiyama, M.; Chikamatsu, K. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2015, 64, 1407–1417. [Google Scholar] [CrossRef]
- Kumar, A.T.; Knops, A.; Swendseid, B.; Martinez-Outschoom, U.; Harshyne, L.; Philp, N.; Rodeck, U.; Luginbuhl, A.; Cognetti, D.; Johnson, J. Prognostic Significance of Tumor-Associated Macrophage Content in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2019, 9, 447799. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Russo, L.L.; Cirillo, N.; Muzio, L.L. Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Z.; Xu, S.; Liao, C.; Chen, X.; Li, B.; Peng, J.; Li, D.; Yang, L. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020, 478, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Lin, Z.; Trimmer, C.; Flomenberg, N.; Wang, C.; Pavlides, S.; Pestell, R.G.; Howell, A.; Sotgia, F.; Lisanti, M.P. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: Implications for PET imaging of human tumors. Cell Cycle 2011, 10, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Achreja, A.; Yeung, T.L.; Mangala, L.S.; Jiang, D.; Han, C.; Baddour, J.; Marini, J.C.; Ni, J.; Nakahara, R.; et al. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metab. 2016, 24, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Auciello, F.R.; Bulusu, V.; Oon, C.; Tait-Mulder, J.; Berry, M.; Bhattacharyya, S.; Tumanov, S.; Allen-Petersen, B.L.; Link, J.; Kendsersky, N.D.; et al. A stromal lysolipid–autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov. 2019, 9, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Raudenská, M.; Svobodová, M.; Gumulec, J.; Falk, M.; Masařík, M. The importance of cancer-associated fibroblasts in the pathogenesis of head and neck cancers. Klin. Onkol. 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Zhao, M.; Ding, L.; Yang, X.; Jing, Y.; Song, Y.; Chen, S.; Hu, Q.; Ni, Y. ITGB2-mediated metabolic switch in CAFs promotes OSCC proliferation by oxidation of NADH in mitochondrial oxidative phosphorylation system. Theranostics 2020, 10, 12044. [Google Scholar] [CrossRef]
- Kumar, D.; New, J.; Vishwakarma, V.; Joshi, R.; Enders, J.; Lin, F.; Dasari, S.; Gutierrez, W.R.; Leef, G.; Ponnurangam, S.; et al. Cancer-associated fibroblasts drive glycolysis in a targetable signaling loop implicated in head and neck squamous cell carcinoma progression. Cancer Res 2018, 78, 3769–3782. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. Embo Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Rigoglio, N.N.; Rabelo, A.C.S.; Borghesi, J.; de Sá Schiavo Matias, G.; Fratini, P.; Prazeres, P.H.D.M.; Pimentel, C.M.M.M.; Birbrair, A.; Miglino, M.A. The tumor microenvironment: Focus on extracellular matrix. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Clause, K.C.; Barker, T.H. Extracellular matrix signaling in morphogenesis and repair. Curr. Opin. Biotechnol. 2013, 24, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Jiang, A.; Jiang, E.; Panigrahy, D.; Kieran, M.W.; Mammoto, A. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. Am. J. Pathol. 2013, 183, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Hingorani, S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 2013, 108, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Sala, M.; Ros, M.; Saltel, F. A Complex and Evolutive Character: Two Face Aspects of ECM in Tumor Progression. Front. Oncol. 2020, 10, 1620. [Google Scholar] [CrossRef]
- Le, Q.T.; Harris, J.; Magliocco, A.M.; Kong, C.S.; Diaz, R.; Shin, B.; Cao, H.; Trotti, A.; Erler, J.T.; Chung, C.H.; et al. Validation of lysyl oxidase as a prognostic marker for metastasis and survival in head and neck squamous cell carcinoma: Radiation Therapy Oncology Group trial 90-03. J. Clin. Oncol. 2009, 27, 4281–4286. [Google Scholar] [CrossRef]
- Kass, L.; Erler, J.T.; Dembo, M.; Weaver, V.M. Mammary epithelial cell: Influence of extracellular matrix composition and organization during development and tumorigenesis. Cell Biol. 2007, 39, 1987–1994. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Saint, A.; Van Obberghen-Schillinga, E. The role of the tumor matrix environment in progression of head and neck cancer. Curr. Opin. Oncol. 2021, 33, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef] [PubMed]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Bao, L.; Xu, T.; Yi, X.; Chen, J.; Wang, S.; Pan, Z.; Huang, P.; Ge, M. The tumor ecosystem in head and neck squamous cell carcinoma and advances in ecotherapy. Mol. Cancer 2023, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Radwanska, A.; Beghelli-de la Forest Divonne, S.; et al. Fibronectin-guided migration of carcinoma collectives. Nat. Commun. 2017, 8, 14105. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Clauser, K.R.; Öhlund, D.; Rickelt, S.; Huang, Y.; Gupta, M.; Mani, D.R.; Carr, S.A.; Tuveson, D.A.; Hynes, R.O. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc. Natl. Acad. Sci. USA 2019, 116, 19609–19618. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 2020, 20, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.L.; Tan, M.L.; Hollows, R.J.; Robinson, M.; Ibrahim, M.; Margielewska, S.; Parkinson, E.K.; Ramanathan, A.; Zain, R.B.; Mehanna, H.; et al. Collagen induces a more proliferative, migratory and chemoresistant phenotype in head and neck cancer via DDR1. Cancers 2019, 11, 1766. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Discoidin domain receptors: Microenvironment sensors that promote cellular migration and invasion. Cell Adhes. Migr. 2018, 12, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B. Discoidin domain receptor functions in physiological and pathological conditions. Int. Rev. Cell Mol. Biol. 2014, 310, 39–87. [Google Scholar] [PubMed]
- Hidalgo-Carcedo, C.; Hooper, S.; Chaudhry, S.I.; Williamson, P.; Harrington, K.; Leitinger, B.; Sahai, E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat. Cell Biol. 2010, 13, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Gartland, A.; Erler, J.T. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res 2016, 76, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Mayorca-Guiliani, A.; Erler, J.T. The potential for targeting extracellular LOX proteins in human malignancy. OncoTargets Ther. 2013, 6, 1729–1735. [Google Scholar]
- Sanada, T.; Islam, A.; Kaminota, T.; Kirino, Y.; Tanimoto, R.; Yoshimitsu, H.; Yano, H.; Mizuno, Y.; Okada, M.; Mitani, S.; et al. Elevated exosomal lysyl oxidase like 2 is a potential biomarker for head and neck squamous cell carcinoma. Laryngoscope 2019, 130, E327–E334. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.E.; Yano, H.; Nakashiro, K.I.; Hamakawa, H.; Tanaka, J. Premetastatic vasculogenesis in oral squamous cell carcinoma xenograft-draining lymph nodes. Oral Oncol. 2012, 48, 663–670. [Google Scholar] [CrossRef]
- Zhou, W.H.; Du, W.D.; Li, Y.F.; Al-Aroomi, M.A.; Yan, C.; Wang, Y.; Zhang, Z.Y.; Liu, F.Y.; Sun, C.F. The Overexpression of Fibronectin 1 Promotes Cancer Progression and Associated with M2 Macrophages Polarization in Head and Neck Squamous Cell Carcinoma Patients. Int. J. Gen. Med. 2022, 15, 5027–5042. [Google Scholar] [CrossRef] [PubMed]
- Marzeda, A.M.; Midwood, K.S. Internal Affairs: Tenascin-C as a Clinically Relevant, Endogenous Driver of Innate Immunity. J. Histochem. Cytochem. 2018, 66, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.R.; Krushel, L.A.; Crossin, K.L. Domains of tenascin involved in glioma migration. J. Cell Sci. 1998, 111, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Chiquet-Ehrismann, R.; Battegay, E.J. The fibrinogen globe of tenascin-C promotes basic fibroblast growth factor-induced endothelial cell elongation. Mol. Biol. Cell 1999, 10, 2933–2943. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tremble, P.; Chiquet-Ehrismann, R.; Werb, Z. The extracellular matrix ligands fibronectin and tenascin collaborate in regulating collagenase gene expression in fibroblasts. Mol. Biol. Cell 1994, 5, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Lochtest, A.; Sympson, C.J.; Huey, B.; Rougier, J.P.; Gray, J.W.; Pinkel, D.; Bissell, M.J.; Werb, Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999, 98, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Spenle, C.; Loustau, T.; Murdamoothoo, D.; Erne, W.; Beghelli-De la Forest Divonne, S.; Veber, R.; Petti, L.; Bourdely, P.; Mörgelin, M.; Brauchle, E.-M.; et al. Tenascin-C orchestrates an immune-suppressive tumor microenvironment in oral squamous cell carcinoma. Cancer Immunol. Res. 2020, 8, 1122–1138. [Google Scholar] [CrossRef]
- Marangon Junior, H.; Rocha, V.N.; Leite, C.F.; de Aguiar, M.C.F.; Souza, P.E.A.; Horta, M.C.R. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J. Oral Pathol. Med. 2013, 43, 199–204. [Google Scholar] [CrossRef]
- Leitinger, B. Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290. [Google Scholar] [CrossRef]
- Beaulieu, J.F. Integrin α6β4 in colorectal cancer: Expression, regulation, functional alterations and use as a biomarker. Cancers 2020, 12, 41. [Google Scholar] [CrossRef]
- Li, H.X.; Zheng, J.H.; Fan, H.X.; Li, H.P.; Gao, Z.X.; Chen, D. Expression of αvβ6 integrin and collagen fibre in oral squamous cell carcinoma: Association with clinical outcomes and prognostic implications. J. Oral Pathol. Med. 2013, 42, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Regezi, J.A.; Ramos, D.M.; Pytela, R.; Dekker, N.P.; Jordan, R.C.K. Tenascin and β6 integrin are overexpressed in floor of mouth in situ carcinomas and invasive squamous cell carcinomas. Oral Oncol. 2002, 38, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.C.; Tian, H.; Wang, Y.; Liu, X.B. Integrin-α5 promoted the progression of oral squamous cell carcinoma and modulated PI3K/AKT signaling pathway. Arch. Oral Biol. 2019, 101, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, Z. The roles of integrin αvβ6 in cancer. Cancer Lett. 2017, 403, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Herrera, Z.; Molina-Frechero, N.; Damián-Matsumura, P.; Bologna-Molina, R. Proteoglycans as potential biomarkers in odontogenic tumors. J. Oral Maxillofac. Pathol. 2018, 22, 98–103. [Google Scholar] [PubMed]
- Gubbiotti, M.A.; Neill, T.; Iozzo, R.V. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biol. 2017, 57–58, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Karamanos, N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019, 75–76, 220–259. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.A.; Vennin, C.; Papanicolaou, M.; Chambers, C.R.; Herrmann, D.; Morton, J.P.; Cox, T.R.; Timpson, P. CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends Cancer 2019, 5, 724–741. [Google Scholar] [CrossRef]
- Netti, P.A.; Berk, D.A.; Swartz, M.A.; Grodzinsky, A.J.; Jain, R.K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000, 60, 2497–2503. [Google Scholar]
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049–6058. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Raavé, R.; van Kuppevelt, T.H.; Daamen, W.F. Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J. Control. Release 2018, 274, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2018, 120, 2782–2790. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, R.; Zhang, X.; Di Russo, J.; Li, L.; Song, J.; Hannocks, M.J.; Sorokin, L. The regulation of immune cell trafficking by the extracellular matrix. Curr. Opin. Cell Biol. 2015, 36, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wu, A.; Kong, L.Y.; Wang, Y.; Fuller, G.; Fokt, I.; Melillo, G.; Priebe, W.; Heimberger, A.B. Hypoxia potentiates glioma-mediated immunosuppression. PLoS ONE 2011, 6, e16195. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy article. Cell Death Disease 2018, 9, 115. [Google Scholar] [CrossRef]
- Ostroukhova, M.; Qi, Z.; Oriss, T.B.; Dixon-McCarthy, B.; Ray, P.; Ray, A. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-β. J. Clin. Investig. 2006, 116, 996–1004. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAILmediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Choi, H.; Li, F.; Wong, S.; Altorki, N.K.; Mittal, V.; Gao, D. Abstract 4721: Epithelial to mesenchymal transition is not required for breast to lung metastasis but contributes to chemoresistance. Cancer Res. 2015, 75 (Suppl. S15), 4721. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Branch, K.M.; Hoshino, D.; Weaver, A.M. Adhesion rings surround invadopodia and promote maturation. Biol. Open 2012, 1, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.S.; Robertson, A.E.; Stoletov, K.; Leith, S.J.; Chin, C.A.; Chien, A.E.; Hague, M.N.; Ablack, A.; Carmine-Simmen, K.; McPherson, V.A.; et al. Invadopodia Are Required for Cancer Cell Extravasation and Are a Therapeutic Target for Metastasis. Cell Rep. 2014, 8, 1558–1570. [Google Scholar] [CrossRef]
- Paz, H.; Pathak, N.; Yang, J. Invading one step at a time: The role of invadopodia in tumor metastasis. Oncogene 2014, 33, 4193–4202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Hema, K.N. Extracellular matrix in invasion and metastasis of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2019, 23, 10–16. [Google Scholar] [CrossRef]
- Culhaci, N.; Metin, K.; Copcu, E.; Dikicioglu, E. Elevated expression of MMP-13 and TIMP-1 in head and neck squamous cell carcinomas may reflect increased tumor invasiveness. BMC Cancer 2004, 4, 42. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int. J. Mol. Sci. 2022, 23, 146. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, P.; Yang, L. The role of nanotherapy in head and neck squamous cell carcinoma by targeting tumor microenvironment. Front. Immunol. 2023, 14, 1189323. [Google Scholar] [CrossRef]
- Bates, A.M.; Hernandez, M.P.G.; Lanzel, E.A.; Qian, F.; Brogden, K.A. Matrix metalloproteinase (MMP) and immunosuppressive biomarker profiles of seven head and neck squamous cell carcinoma (HNSCC) cell lines. Transl. Cancer Res. 2018, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Ginos, M.A.; Page, G.P.; Michalowicz, B.S.; Patel, K.J.; Volker, S.E.; Pambuccian, S.E.; Ondrey, F.G.; Adams, G.L.; Gaffney, P.M. Identification of a Gene Expression Signature Associated with Recurrent Disease in Squamous Cell Carcinoma of the Head and Neck. Cancer Res 2004, 64, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, L.; Liu, Y.; Yang, S.; Rao, Y.; Chen, X.; Nie, M.; Liu, X. Identification of the MMP family as therapeutic targets and prognostic biomarkers in the microenvironment of head and neck squamous cell carcinoma. J. Transl. Med. 2023, 21, 208. [Google Scholar] [CrossRef]
- Peng, C.H.; Liao, C.T.; Peng, S.C.; Chen, Y.J.; Cheng, A.J.; Juang, J.L.; Tsai, C.Y.; Chen, T.C.; Chuang, Y.J.; Tang, C.Y.; et al. A novel molecular signature identified by systems genetics approach predicts prognosis in oral squamous cell carcinoma. PLoS ONE 2011, 6, e23452. [Google Scholar] [CrossRef]
- Ye, H.; Yu, T.; Temam, S.; Ziober, B.L.; Wang, J.; Schwartz, J.L.; Mao, L.; Wong, D.T.; Zhou, X. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genom. 2008, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Matrisian, L.M. Matrix metalloproteases in head and neck cancer. Head Neck 2006, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Koontongkaew, S.; Amornphimoltham, P.; Monthanpisut, P.; Saensuk, T.; Leelakriangsak, M. Fibroblasts and extracellular matrix differently modulate MMP activation by primary and metastatic head and neck cancer cells. Med Oncol. 2011, 29, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Ishida, M.; Yamaka, R.; Ueno, Y.; Sakagami, T.; Fujisawa, T.; Iwai, H.; Tsuta, K. MMP14 expression levels accurately predict the presence of extranodal extensions in oral squamous cell carcinoma: A retrospective cohort study. BMC Cancer 2023, 23, 142. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Huang, Y.; Huang, D.; Li, Y.; Wu, J.; Duan, M. Expression of COX-2, CD44v6 and CD147 and Relationship with Invasion and Lymph Node Metastasis in Hypopharyngeal Squamous Cell Carcinoma. PLoS ONE 2013, 8, e71048. [Google Scholar] [CrossRef]
- Su, C.W.; Su, B.F.; Chiang, W.L.; Yang, S.F.; Chen, M.K.; Lin, C.W. Plasma levels of the tissue inhibitor matrix metalloproteinase-3 as a potential biomarker in oral cancer progression. Int. J. Med. Sci. 2017, 14, 37. [Google Scholar] [CrossRef]
- Kudo, Y.; Kitajima, S.; Ogawa, I.; Hiraoka, M.; Sargolzaei, S.; Keikhaee, M.R.; Sato, S.; Miyauchi, M.; Takata, T. Invasion and metastasis of oral cancer cells require methylation of E-cadherin and/or degradation of membranous β-catenin. Clin. Cancer Res. 2004, 10, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Costanza, B.; Umelo, I.A.; Bellier, J.; Castronovo, V.; Turtoi, A. Stromal modulators of TGF-β in cancer. J. Clin. Med. 2017, 6, 7. [Google Scholar] [CrossRef]
- Chen, J.M.; Chen, P.Y.; Lin, C.C.; Hsieh, M.C.; Lin, J.T. Antimetastatic effects of sesamin on human head and neck squamous cell Carcinoma through regulation of matrix metalloproteinase-2. Molecules 2020, 25, 2248. [Google Scholar] [CrossRef] [PubMed]
- Utispan, K.; Niyomtham, N.; Yingyongnarongkul, B.E.; Koontongkaew, S. Ethanolic Extract of Ocimum sanctum Leaves Reduced Invasion and Matrix Metalloproteinase Activity of Head and Neck Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2020, 21, 363. [Google Scholar] [CrossRef] [PubMed]
- Juin, A.; Billotteta, C.; Moreau, V.; Destaing, O.; Albiges-Rizo, C.; Rosenbaum, J.; Génot, E.; Saltel, F. Physiological type I collagen organization induces the formation of a novel class of linear invadosomes. Mol. Biol. Cell 2012, 23, 297–309. [Google Scholar] [CrossRef]
- Zhong, C.; Tao, B.; Tang, F.; Yang, X.; Peng, T.; You, J.; Xia, K.; Xia, X.; Chen, L.; Peng, L. Remodeling cancer stemness by collagen/fibronectin via the AKT and CDC42 signaling pathway crosstalk in glioma. Theranostics 2021, 11, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Hayashido, Y.; Kitano, H.; Sakaue, T.; Fujii, T.; Suematsu, M.; Sakurai, S.; Okamoto, T. Overexpression of integrin αv facilitates proliferation and invasion of oral squamous cell carcinoma cells via mek/erk signaling pathway that is activated by interaction of integrin αvβ8 with type I collagen. Int. J. Oncol. 2014, 45, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Provenzano, P.P. Aligned forces: Origins and mechanisms of cancer dissemination guided by extracellular matrix architecture. Curr. Opin. Cell Biol. 2021, 72, 63–71. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Prabhudesai, S.A.; Carvalho, K.; Dhupar, A.; Spadigam, A. Elastin remodeling: Does it play a role in priming the malignant phenotype of oral mucosa? Indian J. Pathol. Microbiol. 2023, 66, 332. [Google Scholar]
- Von Au, A.; Vasel, M.; Kraft, S.; Sens, C.; Hackl, N.; Marx, A.; Stroebel, P.; Hennenlotter, J.; Todenhöfer, T.; Stenzl, A.; et al. Circulating fibronectin controls tumor growth. Neoplasia 2013, 15, 925–938. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Dazin, P.; Kapila, Y. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: Fibronectin and integrin αv mediate survival signals through focal adhesion kinase. J. Biol. Chem. 2004, 279, 48342–48349. [Google Scholar] [CrossRef] [PubMed]
- Degen, M.; Natarajan, E.; Barron, P.; Widlund, H.R.; Rheinwald, J.G. MAPK/ERK-dependent translation factor hyperactivation and dysregulated laminin γ2 expression in oral dysplasia and squamous cell carcinoma. Am. J. Pathol. 2012, 180, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Earle, C.; Shiina, M. Activation of matrix Hyaluronan-Mediated CD44 signaling, epigenetic regulation and chemoresistance in head and neck cancer stem cells. Int. J. Mol. Sci. 2017, 18, 1849. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.T.; Sugimoto, H.; Cooke, V.G.; MacDonald, B.A.; Mehta, A.I.; LeBleu, V.S.; Dewar, R.; Rocha, R.M.; Brentani, R.R.; Resnick, M.B.; et al. VEGF-A and Tenascin-C produced by S100A4 + stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. USA 2011, 108, 16002–16007. [Google Scholar] [CrossRef]
- Miller, S.E.; Veale, R.B. Environmental modulation of αv, α2 and β1 integrin subunit expression in human oesophageal squamous cell carcinomas. Cell Biol. Int. 2001, 25, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.H.; Zhou, Y.G.; Pan, J.Q.; Zhu, W.; Xu, P. Overexpression of integrin αv in the human nasopharyngeal carcinoma associated with metastasis and progression. Cancer Biomark. 2013, 13, 323–328. [Google Scholar] [CrossRef]
- Ou, J.; Luan, W.; Deng, J.; Sa, R.; Liang, H. αV integrin induces multicellular radioresistance in human nasopharyngeal carcinoma via activating SAPK/JNK pathway. PLoS ONE 2012, 7, e38737. [Google Scholar] [CrossRef]
- Terry, S.Y.A.; Abiraj, K.; Frielink, C.; Van Dijk, L.K.; Bussink, J.; Oyen, W.J.; Boerman, O.C. Imaging integrin αvβ3 on blood vessels with 111In-RGD2 in head and neck tumor xenografts. J. Nucl. Med. 2014, 55, 281–286. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Wang, C.W. S100A4 promotes squamous cell laryngeal cancer Hep-2 cell invasion via NF-kB/MMP-9 signal. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1361–1367. [Google Scholar]
- Vilen, S.T.; Salo, T.; Sorsa, T.; Nyberg, P. Fluctuating roles of matrix metalloproteinase-9 in oral squamous cell carcinoma. Sci. World J. 2013, 2013, 920595. [Google Scholar] [CrossRef]
- Kudo, Y.; Iizuka, S.; Yoshida, M.; Tsunematsu, T.; Kondo, T.; Subarnbhesaj, A.; Deraz, E.M.; Siriwardena, S.B.S.M.; Tahara, H.; Ishimaru, N.; et al. Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. J. Biol. Chem. 2012, 287, 38716–38728. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cao, N.; Chen, Y.; Lan, H.Y.; Cha, J.H.; Yang, W.H.; Yang, M.H. MT4-MMP promotes invadopodia formation and cell motility in FaDu head and neck cancer cells. Biochem. Biophys. Res. Commun. 2020, 522, 1009–1014. [Google Scholar] [CrossRef]
- Aseervatham, J.; Ogbureke, K.U.E. Effects of DSPP and MMP20 silencing on adhesion, metastasis, angiogenesis, and epithelial-mesenchymal transition proteins in oral squamous cell carcinoma cells. Int. J. Mol. Sci. 2020, 21, 4734. [Google Scholar] [CrossRef]
- Kawahara, R.; Granato, D.C.; Carnielli, C.M.; Cervigne, N.K.; Oliveria, C.E.; Martinez, C.A.R.; Yokoo, S.; Fonseca, F.P.; Lopes, M.; Santos-Silva, A.R.; et al. Agrin and perlecan mediate tumorigenic processes in oral squamous cell carcinoma. PLoS ONE 2014, 9, e115004. [Google Scholar] [CrossRef] [PubMed]
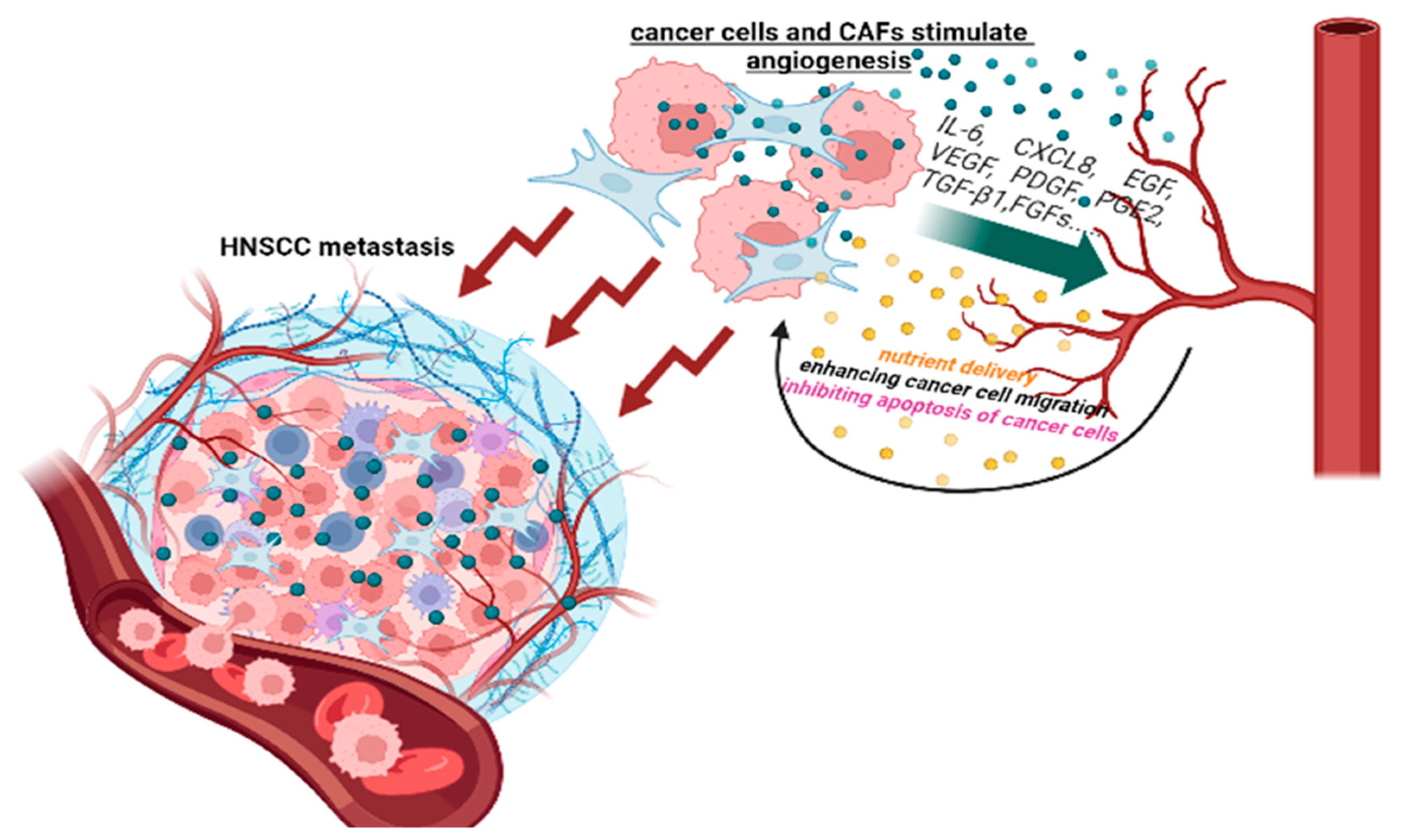
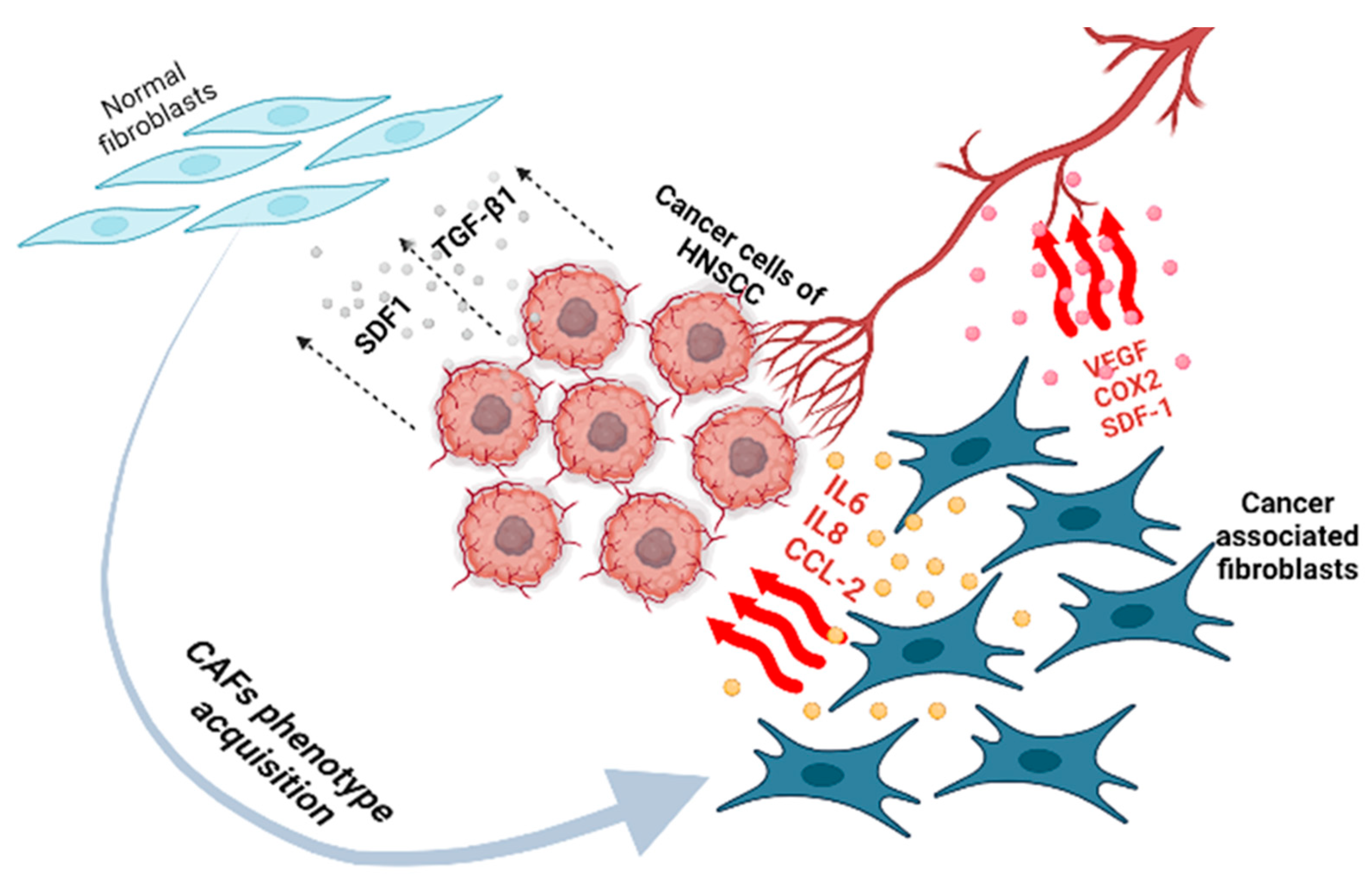
| Process | CAFs Function |
|---|---|
| Proliferation |
|
| Metastasis |
|
| Immune response |
|
| Angiogenesis |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumaniyazova, E.; Lokhonina, A.; Dzhalilova, D.; Kosyreva, A.; Fatkhudinov, T. Role of Microenvironmental Components in Head and Neck Squamous Cell Carcinoma. J. Pers. Med. 2023, 13, 1616. https://doi.org/10.3390/jpm13111616
Jumaniyazova E, Lokhonina A, Dzhalilova D, Kosyreva A, Fatkhudinov T. Role of Microenvironmental Components in Head and Neck Squamous Cell Carcinoma. Journal of Personalized Medicine. 2023; 13(11):1616. https://doi.org/10.3390/jpm13111616
Chicago/Turabian StyleJumaniyazova, Enar, Anastasiya Lokhonina, Dzhuliia Dzhalilova, Anna Kosyreva, and Timur Fatkhudinov. 2023. "Role of Microenvironmental Components in Head and Neck Squamous Cell Carcinoma" Journal of Personalized Medicine 13, no. 11: 1616. https://doi.org/10.3390/jpm13111616
APA StyleJumaniyazova, E., Lokhonina, A., Dzhalilova, D., Kosyreva, A., & Fatkhudinov, T. (2023). Role of Microenvironmental Components in Head and Neck Squamous Cell Carcinoma. Journal of Personalized Medicine, 13(11), 1616. https://doi.org/10.3390/jpm13111616






