A Case of Prostatic Signet-Ring Cell-like Carcinoma with Pagetoid Spread and Intraductal Carcinoma and Long-Term Survival: PD-L1 and Mismatch Repair System Proteins (MMR) Immunohistochemical Evaluation with Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Our Case
2.2. Systematic Literature Review
- Population: men with a diagnosis of primary prostatic SRCC;
- Intervention: any;
- Comparison: none;
- Outcomes: patients’ clinical outcomes (survival and recurrence rates, status at last follow-up).
- Eligibility/inclusion criteria: studies reporting human cases of primary prostatic SRCCs tested for PD-L1, mismatch repair system proteins, and/or microsatellite instability by IHC or molecular assays.
- Exclusion criteria: unclear diagnosis; signet ring cell carcinomas originating from other sites; non-analyzable results (too aggregated data); review articles; cases not tested for the investigated markers.
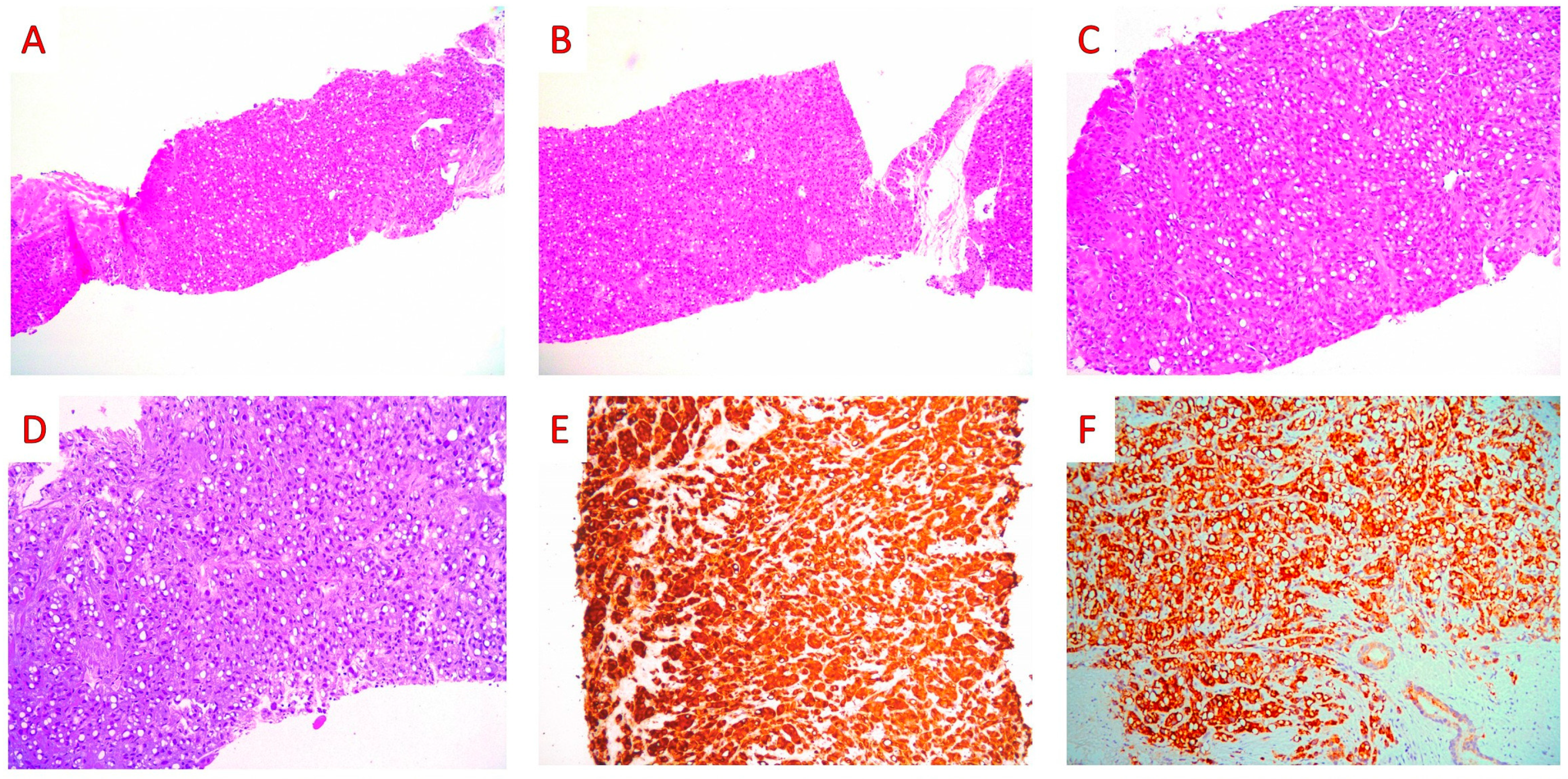
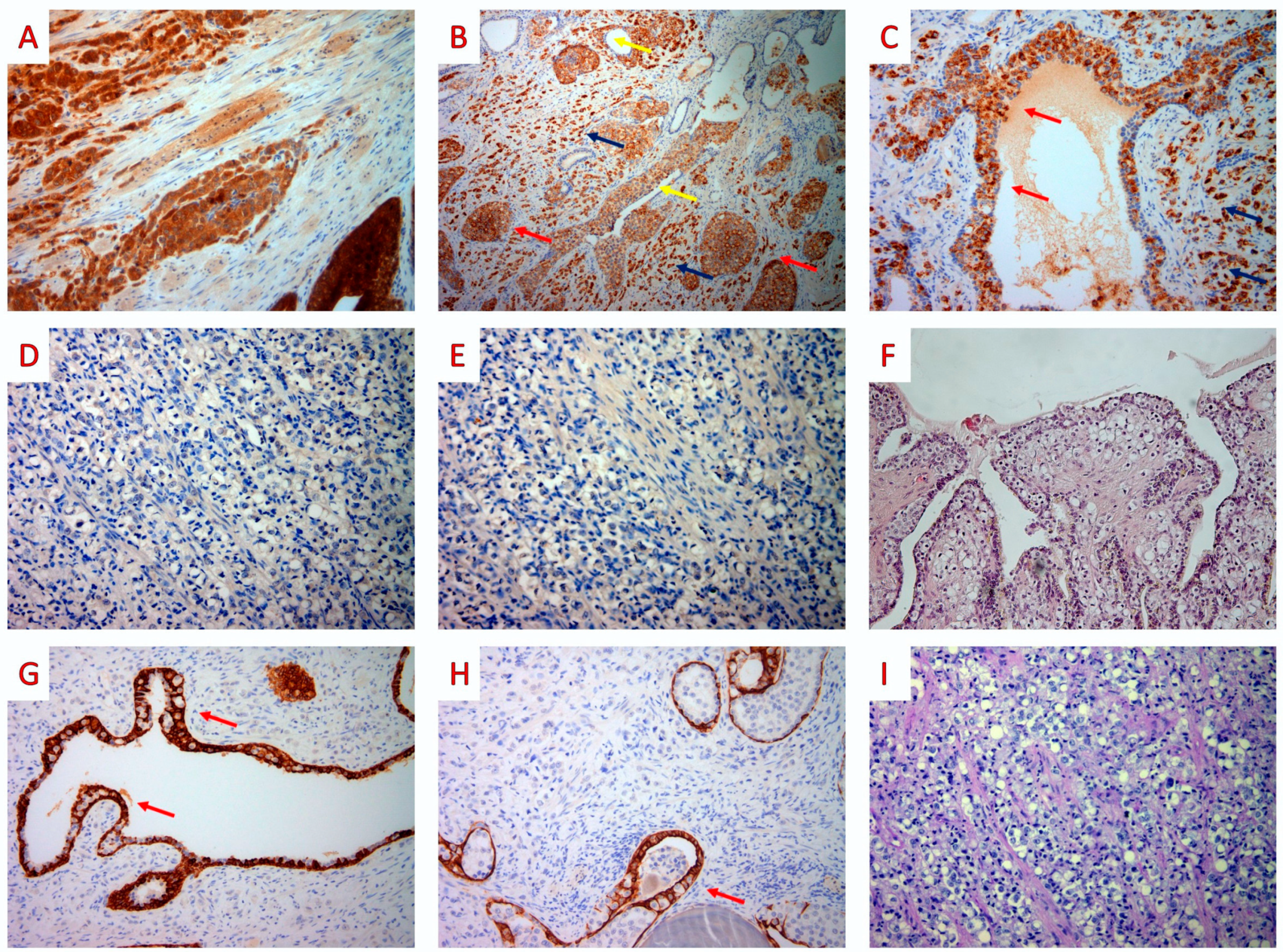
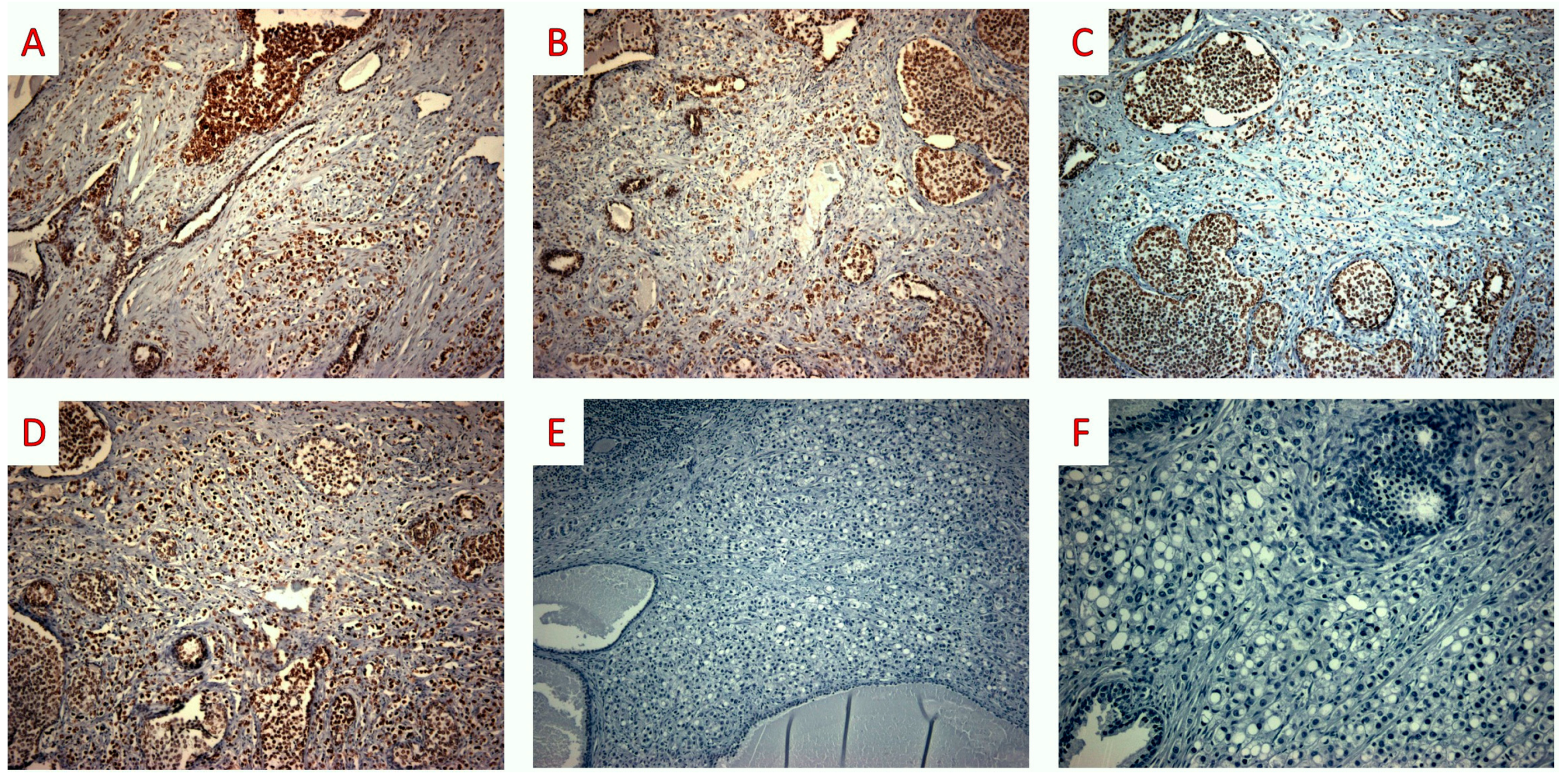
3. Case Report
4. Discussion
- (1)
- At least focal IHC expression of PD-L1 was found in the tumor cells of 29% acinar PCs (0–100% as to various studies), 7% ductal PAs, and 46% neuroendocrine carcinomas/tumors. Only 1 case of prostatic SRCC (reported by an abstract of Hashimoto et al.) was tested for PD-L1; however, the authors identified 2/110 (2%) PD-L1+ PAs in a series including 105 acinar, 4 ductal, and 1 SRCC PA, but they did not clearly report which histotypes resulted positive [67].
- (2)
- About one-third of the tested PAs (280/883, 32%) revealed MSI (210/263, 80%) or MMR IHC loss; at least 1, ≥2, ≥3, or 4 MMR proteins were lost by IHC in 12%, 4%, 1% and 0.4% of PA cases, respectively. MSH6 was the most frequently lost (45/60, 75%), followed by PMS2 (29/60, 48%), MSH2 (11/60, 18%), and MLH1 (3/60, 5%).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and prevention of prostate cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef]
- Teng, Q.L. Immunohistochemical analysis of PD-L1 and tumor-infiltrating immune cells expression in the tumor microenvironment of primary signet ring cell carcinoma of the prostate. Asian J. Androl. 2022, 24, 525. [Google Scholar] [CrossRef]
- Giltman, L.I. Signet ring adenocarcinoma of the prostate. J. Urol. 1981, 126, 134–135. [Google Scholar] [CrossRef]
- Torbenson, M.; Dhir, R.; Nangia, A.; Becich, M.J.; Kapadia, S.B. Prostatic carcinoma with signet ring cells: A clinicopathologic and immunohistochemical analysis of 12 cases, with review of the literature. Mod. Pathol. 1998, 11, 552–559. [Google Scholar]
- Sidhu, S.K.; Sharin, M.F.M.; Ghani, K.A.M.; Zainuddin, S.A.M.; Azizan, N.; Hayati, F. Primary prostatic signet ring cell carcinoma in elderly with obstructive uropathy: A case report. Afr. J. Urol. 2022, 28, 16. [Google Scholar] [CrossRef]
- Gök, A.; Tuygun, C.; Akmansu, M.; Uslu, A.A.; Kartal, I.G.; Sandikçi, F.; Karabacak, O.R.; Sağnak, A.L.; Topaloğlu, H.; Ersoy, H. Primary Signet Ring Cell Carcinoma of the Prostate: A Rare Case Report. J. Clin. Med. 2018, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Blas, L.; Vitagliano, G.; Rios-Pita, H.; Roberti, J.; Guglielmi, J.M.; Ameri, C. Signet ring cell carcinoma of the prostate. Report of 5 cases and literature review. Arch. Esp. Urol. 2019, 72, 1051–1055. [Google Scholar]
- Celik, O.; Budak, S.; Ekin, G.; Akarken, I.; Ilbey, Y.O. A case with primary signet ring cell adenocarcinoma of the prostate and review of the literature. Arch. Ital. Urol. Androl. 2014, 86, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.N.; Nakamura, L.Y.; Pacelli, A.; Humphreys, M.R.; Castle, E.P. Primary signet ring cell carcinoma of the prostate. Mayo. Clin. Proc. 2010, 85, 1130–1136. [Google Scholar] [CrossRef]
- Xing, D.; Berrebi, A.A.; Liu, C.; Richmond, A.; Vang, R.; Ronnett, B.M. An epithelioid smooth muscle neoplasm mimicking a signet ring cell carcinoma in the ovary. Int. J. Gynecol. Pathol. 2019, 38, 464–469. [Google Scholar] [CrossRef]
- Wang, H.; Herath, C. Signet ring cell mesothelioma; A diagnostic challenge. Pathol. Res. Pract. 2019, 215, 152462. [Google Scholar] [CrossRef] [PubMed]
- Richard, H.T. Pathology of pediatric oligodendroglioma. In Book Oligodendroglioma; Paleologos, N.A., Newton, H.B., Eds.; Academic Press: London, UK, 2019; Volume 11, pp. 129–135. [Google Scholar]
- Hysek, M.; Jatta, K.; Stenman, A.; Darai-Ramqvist, E.; Zedenius, J.; Höög, A.; Juhlin, C.C. Signet ring cell variant of follicular thyroid carcinoma: Report of two cases with focus on morphological, expressional and genetic characteristics. Diagn. Pathol. 2019, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Yalta, T.; Elagoz, S.; Uyar, M.; Topuz, O.; Ozer, H.; Tuncer, E. Signet ring cell adenoma of the thyroid: A very rare entity. Med. Princ. Pract. 2010, 19, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Hui, P.; Buza, N. Bilateral Signet-ring Stromal Tumor of the Ovary: A Case Report with Next-generation Sequencing Analysis and FOXL2 Mutation Testing. Int. J. Gynecol. Pathol. 2020, 39, 193–198. [Google Scholar] [CrossRef]
- Mishra, P.; Adhya, A.K.; Kar, M.; Parihar, M.; Samal, S.; Sable, M. Signet ring cell lymphoma of follicular type with BCL2 gene rearrangement: A rare case with a short review of literature. J. Cancer Res. Ther. 2022, 18, 807. [Google Scholar] [CrossRef]
- Kuroda, N.; Yamasaki, I.; Nakayama, H.; Tamura, K.; Yamamoto, Y.; Miyazaki, E.; Naruse, K.; Kiyoku, H.; Hiroi, M.; Enzan, H. Prostatic signet-ring cell carcinoma: Case report and literature review. Pathol. Int. 1999, 49, 457–461. [Google Scholar] [CrossRef]
- Varma, M.; Delahunt, B.; Egevad, L.; Samaratunga, H.; Kristiansen, G. Intraductal carcinoma of the prostate: A critical re-appraisal. Virchows Arch. 2019, 474, 525–534. [Google Scholar] [CrossRef]
- Tsuzuki, T. Intraductal carcinoma of the prostate: A comprehensive and updated review. Int. J. Urol. 2015, 22, 140–145. [Google Scholar] [CrossRef]
- Grignon, D.J. Unusual subtypes of prostate cancer. Mod. Pathol. 2004, 17, 316–327. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines. Treatment by Cancer Type. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 1 May 2023).
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.N.; Rescigno, P.; Liu, D.; Yuan, W.; Carreira, S.; Lambros, M.B.; Seed, G.; Mateo, J.; Riisnaes, R.; Mullane, S.; et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J. Clin. Investig. 2018, 128, 4441–4453. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Dong, H.; Kwon, E.; Karnes, R.J. Positive Pelvic Lymph Nodes in Prostate Cancer Harbor Immune Suppressor Cells to Impair Tumor-reactive T Cells. Eur. Urol. Focus 2018, 4, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Loriot, Y.; Shaffer, D.R.; Braiteh, F.; Powderly, J.; Harshman, L.C.; Conkling, P.; Delord, J.P.; Gordon, M.; Kim, J.W.; et al. Safety and Clinical Activity of Atezolizumab in Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase I Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 3360–3369. [Google Scholar] [CrossRef]
- Graff, J.N.; Beer, T.M.; Alumkal, J.J.; Slottke, R.E.; Redmond, W.L.; Thomas, G.V.; Thompson, R.F.; Wood, M.A.; Koguchi, Y.; Chen, Y.; et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J. Immunother. Cancer 2020, 8, e000642. [Google Scholar] [CrossRef]
- Shaw, K.; Calagua, C.; Russo, J.; Einstein, D.; Balk, S.; Ye, H. Tumor PD-L1 Expression is Detected in a Significant Subset of High-Risk Localized and Metastatic Prostate Cancer but is Rare in Ductal Subtype. Abstracts from USCAP 2019: Genitourinary Pathology (including renal tumors) (776–992). Mod. Pathol. 2019, 32, 143–144. [Google Scholar]
- Pal, S.K.; Moreira, D.; Won, H.; White, S.W.; Duttagupta, P.; Lucia, M.; Jones, J.; Hsu, J.; Kortylewski, M. Reduced T-cell Numbers and Elevated Levels of Immunomodulatory Cytokines in Metastatic Prostate Cancer Patients De Novo Resistant to Abiraterone and/or Enzalutamide Therapy. Int. J. Mol. Sci. 2019, 20, 1831. [Google Scholar] [CrossRef]
- Haffner, M.C.; Guner, G.; Taheri, D.; Netto, G.J.; Palsgrove, D.N.; Zheng, Q.; Guedes, L.B.; Kim, K.; Tsai, H.; Esopi, D.M.; et al. Comprehensive Evaluation of Programmed Death-Ligand 1 Expression in Primary and Metastatic Prostate Cancer. Am. J. Pathol. 2018, 188, 1478–1485. [Google Scholar] [CrossRef]
- Calagua, C.; Russo, J.; Sun, Y.; Schaefer, R.; Lis, R.; Zhang, Z.; Mahoney, K.; Bubley, G.J.; Loda, M.; Taplin, M.E.; et al. Expression of PD-L1 in Hormone-naïve and Treated Prostate Cancer Patients Receiving Neoadjuvant Abiraterone Acetate plus Prednisone and Leuprolide. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6812–6822. [Google Scholar] [CrossRef]
- Graff, J.N.; Alumkal, J.J.; Drake, C.G.; Thomas, G.V.; Redmond, W.L.; Farhad, M.; Cetnar, J.P.; Ey, F.S.; Bergan, R.C.; Slottke, R.; et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016, 7, 52810–52817. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, Q.; Zeng, X.; Yu, W.; Xu, G. Pembrolizumab with or without enzalutamide in selected populations of men with previously untreated metastatic castration-resistant prostate cancer harbouring programmed cell death ligand-1 staining: A retrospective study. BMC Cancer 2021, 21, 399. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Urinary and Male Genital Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Guerin, D.; Hasan, N.; Keen, C. Signet ring cell differentiation in adenocarcinoma of the prostate: A study of five cases. Histopathology 1993, 22, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Sugao, H.; Gotoh, T.; Yokomizo, S.; Itoh, Y. Primary signet ring cell carcinoma of the prostate: Report and review of 42 cases. Int. J. Urol. 2004, 11, 178–181. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Z.; Bao, M.; Li, J.; Meng, X.; Liu, B.; Tang, M. Diagnosis and Management of Primary Prostatic Signet Ring Cell Carcinoma: Single-Center Experience. Am. J. Mens. Health. 2022, 16, 1–8. [Google Scholar] [CrossRef]
- Inamura, K. Prostatic cancers: Understanding their molecular pathology and the 2016 WHO classification. Oncotarget 2018, 9, 14723. [Google Scholar] [CrossRef]
- Guerrieri, C.; Jobbagy, Z.; Hudacko, R. Expression of CDX2 in metastatic prostate cancer. Pathologica 2019, 111, 105. [Google Scholar] [CrossRef]
- Herawi, M.; De Marzo, A.M.; Kristiansen, G.; Epstein, J.I. Expression of CDX2 in benign tissue and adenocarcinoma of the prostate. Hum. Pathol. 2007, 38, 72–78. [Google Scholar] [CrossRef]
- Chang, A.; Amin, A.; Gabrielson, E.; Illei, P.; Roden, R.B.; Sharma, R.; Epstein, J.I. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am. J. Surg. Pathol. 2012, 36, 1472–1476. [Google Scholar] [CrossRef]
- Azizan, N.; Hayati, F.; Tizen, N.M.S.; Farouk, W.I.; Masir, N. Role of co-expression of estrogen receptor beta and Ki67 in prostate adenocarcinoma. Investig. Clin. Urol. 2018, 59, 232–237. [Google Scholar] [CrossRef]
- Ro, J.Y.; El-Naggar, A.; Ayala, A.G.; Mody, D.R.; Ordóñez, N.G. Signet-ring-cell carcinoma of the prostate. Electron-microscopic and immunohistochemical studies of eight cases. Am. J. Surg. Pathol. 1988, 12, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Roma, A.A.; Magi-Galluzzi, C.; Wood, H.; Fergany, A.; McKenney, J.K. Metastatic prostate adenocarcinoma to the penis presenting as pagetoid carcinoma: A phenomenon not previously reported. Am. J. Surg. Pathol. 2015, 39, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Petcu, E.B.; Gonzalez-Serva, A.; Wright, R.G.; Slevin, M.; Brinzaniuc, K. Prostate carcinoma metastatic to the skin as an extrammamary Paget’s disease. Diagn. Pathol. 2012, 7, 106. [Google Scholar] [CrossRef]
- Segal, R.; Penneys, N.S.; Nahass, G. Metastatic prostatic carcinoma histologically mimicking malignant melanoma. J. Cutan. Pathol. 1994, 21, 280–282. [Google Scholar] [CrossRef]
- Akgul, M.; Bomeisl, P.B.; MacLennan, G.T. Extravesical Pagetoid Spread of Urothelial Carcinoma in Situ: A Report of Five Cases. Anal. Quant. Cytopathol. Histpathol. 2015, 37, 364–368. [Google Scholar] [PubMed]
- Hondo, N.; Miyagawa, Y.; Kitazawa, M.; Muranaka, F.; Tokumaru, S.; Koyama, M.; Takahata, S.; Soejima, Y. Laparoscopic abdominosacral resection for rectal and anal canal carcinoma with pagetoid spread. Asian J. Endosc. Surg. 2021, 14, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.R.; Cho, H.Y.; Baek, J.H.; Jeong, J.; Ha, S.Y.; Seok, J.Y.; Park, S.W.; Sym, S.J.; Lee, K.C.; Chung, D.H. Rare Case of Anal Canal Signet Ring Cell Carcinoma Associated with Perianal and Vulvar Pagetoid Spread. J. Pathol. Transl. Med. 2016, 50, 231–237. [Google Scholar] [CrossRef]
- Kanomata, N.; Kozuka, Y.; Moriya, T. Prostatic intraepithelial pagetoid histiocyte: A potential diagnostic pitfall. Pathol. Ιnt. 2011, 61, 551. [Google Scholar] [CrossRef]
- Xiao, G.Q.; Unger, P.D. Focal Signet Ring Cell High-Grade Prostatic Intraepithelial Neoplasia on Needle Biopsy. Int. J. Surg. Pathol. 2017, 25, 344–347. [Google Scholar] [CrossRef]
- Bronkema, C.; Arora, S.; Sood, A.; Dalela, D.; Keeley, J.; Borchert, A.; Baumgartner, L.; Rogers, C.G.; Peabody, J.O.; Menon, M.; et al. Rare histological variants of prostate adenocarcinoma: A national cancer database analysis. J. Urol. 2020, 204, 260–266. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.W.; Hemstreet, G.P. Younger age is an independent predictor for poor survival in patients with signet ring prostate carcinoma. Prostate Cancer 2011, 2011, 216169. [Google Scholar] [CrossRef]
- Akagashi, K.; Tanda, H.; Kato, S.; Ohnishi, S.; Nakajima, H.; Nanbu, A.; Nitta, T.; Koroku, M. Signet-ring cell carcinoma of the prostate effectively treated with maximal androgen blockade. Int. J. Urol. 2003, 10, 456–458. [Google Scholar] [CrossRef]
- Lilleby, W.; Axcrona, K.; Cecilie Alfsen, G.; Urnes, T.; Hole, K.H. Diagnosis and treatment of primary signet-ring cell carcinoma of the prostate. Acta. Oncol. 2007, 46, 1195–1197. [Google Scholar] [CrossRef]
- Yoshimura, K.; Fukui, I.; Ishikawa, Y.; Maeda, H.; Yamauchi, T.; Kawai, T. Locally-Confined Signet-Ring Cell Carcinoma of the Prostate: A Case Report of a Long-Term Survivor. Int. J. Urol. 1996, 3, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Nayak, B.; Seth, A. Good response of an aggressive rare variant of signet ring cell carcinoma of prostate with hormonal therapy. BMJ Case Rep. 2017, 2017, bcr2016217567. [Google Scholar] [CrossRef] [PubMed]
- Roldan, A.M.; Nunez, N.F.; Grande, E.; Garcia, A.A.; Anton-Aparicio, L.M. A primary signet ring cell carcinoma of the prostate with bone metastasis with impressive response to FOLFOX and cetuximab. Clin. Genitourin. Cancer 2012, 10, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, A.; Hiura, M. Primary signet ring cell adenocarcinoma of the prostate treated by radical prostatectomy after preoperative androgen deprivation. Int. J. Urol. 1997, 4, 522–523. [Google Scholar] [CrossRef]
- Bilusic, M.; Madan, R.A.; Gulley, J.L. Immunotherapy of Prostate Cancer: Facts and Hopes Prostate Cancer Immunotherapy. Clin. Cancer Res. 2017, 23, 6764–6770. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Shenderov, E.; Eisenberger, M.A.; Kachhap, S.; Pardoll, D.M.; Denmeade, S.R.; Antonarakis, E.S. Extreme responses to immune checkpoint blockade following bipolar androgen therapy and enzalutamide in patients with metastatic castration resistant prostate cancer. Prostate 2020, 80, 407–411. [Google Scholar] [CrossRef]
- Palicelli, A.; Bonacini, M.; Croci, S.; Magi-Galluzzi, C.; Cañete-Portillo, S.; Chaux, A.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; et al. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review. Part 1: Focus on Immunohistochemical Results with Discussion of Pre-Analytical and Interpretation Variables. Cells 2021, 10, 3166. [Google Scholar] [CrossRef]
- Palicelli, A.; Bonacini, M.; Croci, S.; Magi-Galluzzi, C.; Cañete-Portillo, S.; Chaux, A.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; et al. What do We have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review. Part 2: Clinic–Pathologic Correlations. Cells 2021, 10, 3165. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What do We have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review (Part 6): Correlation of PD-L1 Expression with the Status of Mismatch Repair System, BRCA, PTEN, and Other Genes. Biomedicines 2022, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Poorman, K.; Catalano, F.; Seeber, A.; Goldberg, R.M.; Salem, M.E.; Shields, A.F.; Berger, M.D.; Battaglin, F.; Tokunaga, R.; et al. Molecular profiling of signet-ring-cell carcinoma (SRCC) from the stomach and colon reveals potential new therapeutic targets. Oncogene 2022, 41, 3455–3460. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Imai, A.; Hatakeyama, S.; Yoneyama, T.; Koie, T.; Ohyama, C. PD-L1 over expression may predict disease aggressiveness in prostate cancer. Meeting Abstract: 291P. Ann. Oncol. 2016, 27, ix91–ix92. [Google Scholar] [CrossRef]


| Authors | Age (Years) | PSA (°) (ng/mL) | Symptoms | GG | Stage | Treatment | FU (mo) |
|---|---|---|---|---|---|---|---|
| Teng et al., 2022: case 1 [2] | 75 | 49.73 | NR | 3 | T2cNxM0 | biopsy + HT (FLU) | NED, 117 |
| Teng et al., 2022: case 2 [2] | 70 | 10.09 | hematuria (2 weeks) | 4 | pT1NxMx (*) | biopsy + HT (BIC + ZOL) + RP | NED, 106 |
| Teng et al., 2022: case 3 [2] | 69 | >100 | hydronephrosis | 5 | M1 (bone) | biopsy + HT (FLU + ZOL + zoledronic acid) + TURP + BO + Cht (DOC)/ZOL | AWD, 142 |
| Teng et al., 2022: case 4 [2] | 67 | 65.87 | difficult urination (1 week) | 5 | NR | biopsy | AWD, 20 |
| Our case | 63 | 16.39 | acute urinary retention, hematuria | 5 | pT3b cN0 cM0 | biopsy + RT + HT (Gn-RH, VER, LEU) + RT | NED, 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koufopoulos, N.; Ieronimaki, A.-I.; Zacharatou, A.; Gouloumis, A.R.; Leventakou, D.; Boutas, I.; Dimas, D.T.; Kontogeorgi, A.; Sitara, K.; Khaldi, L.; et al. A Case of Prostatic Signet-Ring Cell-like Carcinoma with Pagetoid Spread and Intraductal Carcinoma and Long-Term Survival: PD-L1 and Mismatch Repair System Proteins (MMR) Immunohistochemical Evaluation with Systematic Literature Review. J. Pers. Med. 2023, 13, 1016. https://doi.org/10.3390/jpm13061016
Koufopoulos N, Ieronimaki A-I, Zacharatou A, Gouloumis AR, Leventakou D, Boutas I, Dimas DT, Kontogeorgi A, Sitara K, Khaldi L, et al. A Case of Prostatic Signet-Ring Cell-like Carcinoma with Pagetoid Spread and Intraductal Carcinoma and Long-Term Survival: PD-L1 and Mismatch Repair System Proteins (MMR) Immunohistochemical Evaluation with Systematic Literature Review. Journal of Personalized Medicine. 2023; 13(6):1016. https://doi.org/10.3390/jpm13061016
Chicago/Turabian StyleKoufopoulos, Nektarios, Argyro-Ioanna Ieronimaki, Andriani Zacharatou, Alina Roxana Gouloumis, Danai Leventakou, Ioannis Boutas, Dionysios T. Dimas, Adamantia Kontogeorgi, Kyparissia Sitara, Lubna Khaldi, and et al. 2023. "A Case of Prostatic Signet-Ring Cell-like Carcinoma with Pagetoid Spread and Intraductal Carcinoma and Long-Term Survival: PD-L1 and Mismatch Repair System Proteins (MMR) Immunohistochemical Evaluation with Systematic Literature Review" Journal of Personalized Medicine 13, no. 6: 1016. https://doi.org/10.3390/jpm13061016
APA StyleKoufopoulos, N., Ieronimaki, A.-I., Zacharatou, A., Gouloumis, A. R., Leventakou, D., Boutas, I., Dimas, D. T., Kontogeorgi, A., Sitara, K., Khaldi, L., Zanelli, M., & Palicelli, A. (2023). A Case of Prostatic Signet-Ring Cell-like Carcinoma with Pagetoid Spread and Intraductal Carcinoma and Long-Term Survival: PD-L1 and Mismatch Repair System Proteins (MMR) Immunohistochemical Evaluation with Systematic Literature Review. Journal of Personalized Medicine, 13(6), 1016. https://doi.org/10.3390/jpm13061016








