Cemiplimab as First Line Therapy in Advanced Penile Squamous Cell Carcinoma: A Real-World Experience
Abstract
:1. Introduction
2. Materials and Methods
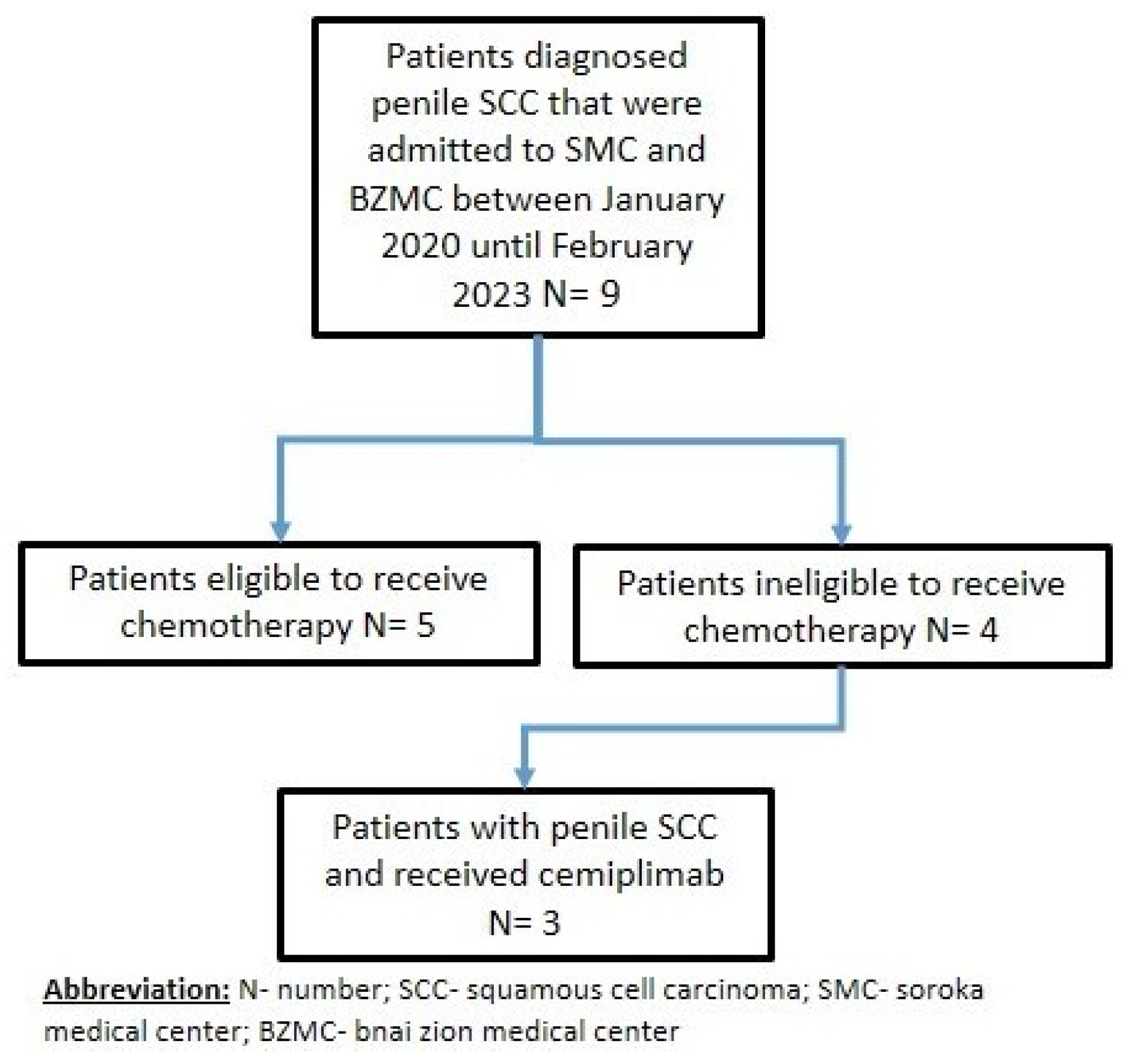
2.1. Selection of Patients
2.2. Clinical Data
2.2.1. Patient Data Collection
2.2.2. Treatment Response Evaluation
2.2.3. Pre-Treatment Evaluations
2.2.4. Monitoring
2.3. The Inclusion Criteria
2.4. Exclusion Criteria for the Study
3. Results
3.1. Elderly Patient with a Chronic Renal Failure
3.2. Eligible for Cisplatin but Refusing Surgery and Chemotherapy
3.3. Chronic Renal Failure with Extensive Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, C.B.; Feitoza, L.; Pinho, J.; Teixeira-Júnior, A.; Lages, J.; Calixto, J.; Coelho, R.; Nogueira, L.; Cunha, I.; Soares, F.; et al. Profile of patients with penile cancer in the region with the highest worldwide incidence. Sci. Rep. 2020, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Montes Cardona, C.E.; García-Perdomo, H.A. Incidence of penile cancer worldwide: Systematic review and meta-analysis. Rev. Panam. Salud Publica 2017, 41, e117. [Google Scholar] [CrossRef]
- Thomas, A.; Necchi, A.; Muneer, A.; Tobias-Machado, M.; Tran, A.T.H.; Van Rompuy, A.S.; Spiess, P.E.; Albersen, M. Penile cancer. Nat. Rev. Dis. Primers 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Douglawi, A.; Masterson, T.M. Opinion in Urology, and Undefined 2019: Penile Cancer Epidemiology and Risk Factors: A Contemporary Review. Available online: https://journals.lww.com/co-urology/Fulltext/2019/03000/Penile_cancer_epidemiology_and_risk_factors__a.14.aspx (accessed on 7 September 2022).
- Velazquez, E.F.; Cubilla, A.L. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: Frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am. J. Surg. Pathol. 2003, 27, 1448–1453. [Google Scholar] [CrossRef]
- Hakenberg, O.W.; Dräger, D.L.; Erbersdobler, A.; Naumann, C.M.; Jünemann, K.P.; Protzel, C. The Diagnosis and Treatment of Penile Cancer. Dtsch Arztebl Int. 2018, 115, 646–652. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, H. Introduction to Checkpoint Inhibitors and Cancer Immunotherapy. Immunol. Rev. 2017, 276, 5. [Google Scholar] [CrossRef] [PubMed]
- El Zarif, T.; Nassar, A.H.; Pond, G.R.; Zhuang, T.Z.; Master, V.; Nazha, B.; Niglio, S.; Simon, N.; Hahn, A.W.; Pettaway, C.A.; et al. Safety and efficacy of immune checkpoint inhibitors in advanced penile cancer: Report from the Global Society of Rare Genitourinary Tumors. J. Natl. Cancer Inst. 2023, djad155, Epub ahead of print. [Google Scholar] [CrossRef]
- de Vries, H.M.; Ottenhof, S.R.; Horenblas, S.; van der Heijden, M.S.; Jordanova, E.S. Defining the Tumor Microenvironment of Penile Cancer by Means of the Cancer Immunogram. Eur. Urol. Focus 2019, 5, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Vanthoor, J.; Vos, G.; Tsaur, I.; Albersen, M. Risk factors and molecular characterization of penile cancer: Impact on prognosis and potential targets for systemic therapy. Curr. Opin. Urol. 2020, 30, 202–207. [Google Scholar] [CrossRef]
- Shalata, W.; Yakobson, A.; Cohen, A.Y.; Goldstein, I.; Saleh, O.A.; Dudnik, Y.; Rouvinov, K. Unexpected Adverse Events of Immune Checkpoint Inhibitors. Life 2023, 13, 1657. [Google Scholar] [CrossRef]
- Yakobson, A.; Abu Jama, A.; Abu Saleh, O.; Michlin, R.; Shalata, W. PD-1 Inhibitors in Elderly and Immunocompromised Patients with Advanced or Metastatic Cutaneous Squamous Cell Carcinoma. Cancers 2023, 15, 4041. [Google Scholar] [CrossRef]
- Yakobson, A.; Rouvinov, K.; Cohen, A.Y.; Goldstein, I.; Abu Saleh, O.; Solomon, A.; Dudnik, Y.; Shalata, W. Carpal Tunnel Syndrome Associated with Immune Checkpoint Inhibitors. J. Pers. Med. 2023, 13, 1340. [Google Scholar] [CrossRef] [PubMed]
- Denis, C.; Sakalihasan, S.; Frères, P.; Withofs, N.; Sautois, B. Cemiplimab for cisplatin resistant metastatic penile cancer. Case Rep. Oncol. 2021, 14, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Chahoud, J.; Skelton, W.P.; Spiess, P.E.; Walko, C.; Dhillon, J.; Gage, K.L.; Johnstone, P.A.S.; Jain, R.K. Case report: Two cases of chemotherapy refractory metastatic penile squamous cell cancinoma with extreme durable response to pembrolizumab. Front. Oncol. 2020, 10, 615298. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Penile Cancer, Version 1.2023. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 20 February 2023).
- Burt, L.M.; Shrieve, D.C.; Tward, J.D. Stage presentation, care patterns, and treatment outcomes for squamous cell carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 94–100. [Google Scholar] [CrossRef]
- Marchionne, E.; Perez, C.; Hui, A.; Khachemoune, A. Penile squamous cell carcinoma: A review of the literature and case report treated with Mohs micrographic surgery. An. Bras. Dermatol. 2017, 92, 95–99. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Maldonado, J.L.; Pow-sang, J.; Giuliano, A.R. Incidence trends in primary malignant penile cancer [published correction appears in Urol Oncol. 2008 Jan-Feb;26:112. Guiliano, Anna R [corrected to Giuliano, Anna R]]. Urol. Oncol. 2007, 25, 361–367. [Google Scholar] [CrossRef]
- Brady, K.L.; Mercurio, M.G.; Brown, M.D. Malignant tumors of the penis. Dermatol. Surg. 2013, 39, 527–547. [Google Scholar] [CrossRef]
- Schlenker, B.; Schneede, P. The role of human papilloma virus in penile cancer prevention and new therapeutic agents. Eur. Urol. Focus 2019, 5, 42–45. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Approves Cemiplimab-Rwlc for Metastatic or Locally Advanced Cutaneous Squamous Cell Carcinoma. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-cemiplimab-rwlc-metastatic-or-locally-advanced-cutaneous-squamous-cell-carcinoma (accessed on 14 February 2023).
- Migden, M.R.; Rischin, D.; Schmults, C.D. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- National Institute of Health. Study of Cemiplimab in Patients with Type of Skin Cancer Stage II to IV Cutaneous Squamous Cell Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT04154943 (accessed on 22 February 2023).
- Even, C.; Torossian, N.; Ibrahim, T.; Martin, N.; Badis, L.M.; Ferrand Iacob, M.; Guigay, J.; Tourneau, C.L.; Daste, A.; Saâda-Bouzid, E.; et al. First-line versus second-line immunotherapy in recurrent/metastatic squamous cell carcinoma of the head and neck. Ann. Oncol. 2019, 30, v462. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Durable Tumor Regression and Overall Survival in Patients with Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J. Clin. Oncol. 2019, 37, 693–702. [Google Scholar] [CrossRef]
- Bsn, R.N.R. Immunotherapy Should Be First Line in Merkel Cell Carcinoma. Medscape. 18 August 2019. Available online: https://www.medscape.com/viewarticle/910547?form=fpf (accessed on 23 August 2019).
- Barlési, F. MS 05.02 First-line versus Second-Line Anti-PD-(L)1 Therapy for Patients with Positive PD-L1 Expression. J. Thorac. Oncol. 2017, 12, S1677. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Quhal, F.; Kawada, T.; Bekku, K.; Laukhtina, E.; Rajwa, P.; Deimling, M.V.; Chlosta, M.; Pradere, B.; Karakiewicz, P.I.; et al. Association between age and efficacy of first-line immunotherapy-based combination therapies for mRCC: A meta-analysis. Immunotherapy 2023, 15, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; Ye, L.; Sun, H.; He, Y. Comparison between the first-line and second-line immunotherapy drugs in the progression-free survival and overall survival in advanced non-small cell lung cancer: A systematic review and meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2021, 10, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Ben-Betzalel, G.; Steinberg-Silman, Y.; Stoff, R.; Asher, N.; Shapira-Frommer, R.; Schachter, J.; Markel, G. Immunotherapy comes of age in octagenarian and nonagenarian metastatic melanoma patients. Eur. J. Cancer 2019, 108, 61–68. [Google Scholar] [CrossRef]
- Kugel, C.H., 3rd; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef] [PubMed]
- Kacew, A.J.; Harris, E.J.; Lorch, J.H.; Haddad, R.I.; Chau, N.G.; Rabinowits, G.; LeBoeuf, N.R.; Schmults, C.D.; Thakuria, M.; MacConaill, L.E.; et al. Chromosome 3q arm gain linked to immunotherapy response in advanced cutaneous squamous cell carcinoma. Eur. J. Cancer 2019, 113, 1–9. [Google Scholar] [CrossRef]
- Udager, M.; Liu, T.-Z.; Skala, S.L.; Magers, M.J.; McDaniel, A.S.; Spratt, D.E.; Feng, F.Y.; Siddiqui, J.; Cao, X.; Fields, K.L.; et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: Potential opportunities for immunotherapeutic approaches. Ann. Oncol. 2016, 27, 1706–1712. [Google Scholar] [CrossRef]
- Shalata, W.; Peled, N.; Gabizon, I.; Abu Saleh, O.; Kian, W.; Yakobson, A. Associated Myocarditis: A Predictive Factor for Response? Case Rep. Oncol. 2020, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Komiya, K.; Nakamura, T.; Abe, T.; Ogusu, S.; Nakashima, C.; Takahashi, K.; Kimura, S.; Sueoka-Aragane, N. Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac. Cancer 2019, 10, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Zolnoorian, J.; Migliozzi, G.; Jama, A.A.; Dudnik, Y.; Cohen, A.Y.; Meirovitz, A.; Yakobson, A. Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience. Int. J. Mol. Sci. 2023, 24, 5938. [Google Scholar] [CrossRef] [PubMed]
- Sugano, T.; Seike, M.; Saito, Y.; Kashiwada, T.; Terasaki, Y.; Takano, N.; Hisakane, K.; Takahashi, S.; Tanaka, T.; Takeuchi, S.; et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac. Cancer 2020, 11, 1052–1060. [Google Scholar] [CrossRef]
- Wright, Q.; Cruz, J.L.G.; Wells, J.W.; Leggatt, G.R. PD-1 and beyond to Activate T Cells in Cutaneous Squamous Cell Cancers: The Case for 4-1BB and VISTA Antibodies in Combination Therapy. Cancers 2021, 13, 3310. [Google Scholar] [CrossRef]
- Trefny, M.P.; Kaiser, M.; Stanczak, M.A.; Herzig, P.; Savic, S.; Wiese, M.; Lardinois, D.; Laubli, H.; Uhlenbrock, F.; Zippelius, A. PD-1+ natural killer cells in human non-small cell lung cancer can be activated by PD-1/PD-L1 blockade. Cancer Immunol. Immunother. 2020, 69, 1505–1517. [Google Scholar] [CrossRef]
- Pesce, S.; Trabanelli, S.; Di Vito, C.; Greppi, M.; Obino, V.; Guolo, F.; Minetto, P.; Bozzo, M.; Calvi, M.; Zaghi, E.; et al. Cancer Immunotherapy by Blocking Immune Checkpoints on Innate Lymphocytes. Cancers 2020, 12, 3504. [Google Scholar] [CrossRef]
- Lee, H.; Quek, C.; Silva, I.; Tasker, A.; Batten, M.; Rizos, H.; Lim, S.Y.; Gide, T.N.; Shang, P.; Attrill, G.H.; et al. Integrated molecular and immunophenotypic analysis of NK cells in anti-PD-1 treated metastatic melanoma patients. Oncoimmunology 2019, 8, e1537581. [Google Scholar] [CrossRef]
- Shalata, W.; Yakobson, A.; Dudnik, Y.; Swaid, F.; Ahmad, M.S.; Abu Jama, A.; Cohen, A.Y.; Agbarya, A. Multi-Center Real-World Outcomes of Nivolumab Plus Ipilimumab and Chemotherapy in Patients with Metastatic Non-Small-Cell Lung Cancer. Biomedicines 2023, 11, 2438. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Weissmann, S.; Itzhaki Gabay, S.; Sheva, K.; Abu Saleh, O.; Jama, A.A.; Yakobson, A.; Rouvinov, K. A Retrospective, Single-Institution Experience of Bullous Pemphigoid as an Adverse Effect of Immune Checkpoint Inhibitors. Cancers 2022, 14, 5451. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Abu-Salman, A.; Steckbeck, R.; Mathew Jacob, B.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 5218. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Iraqi, M.; Bhattacharya, B.; Fuchs, V.; Roisman, L.C.; Cohen, A.Y.; Massalha, I.; Yakobson, A.; Prasad, M.; Elkabets, M.; et al. Rapid Response to the Combination of Lenvatinib and Pembrolizumab in Patients with Advanced Carcinomas (Lung Adenocarcinoma and Malignant Pleural Mesothelioma). Cancers 2021, 13, 3630. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, C.N.; Perez, S.A.; Papamichail, M. Cancer immunotherapy. Crit. Rev. Clin. Lab. Sci. 2009, 46, 167–189. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K. Basic Overview of Current Immunotherapy Approaches in Cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 298–308. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, Q.; Li, B. Evaluation of immunotherapy efficacy in gynecologic cancer. Front. Immunol. 2023, 14, 1061761. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Diaz, J.L.; Wanta, S.M.; Fishbein, T.M.; Kroemer, A. Developments in immunotherapy for gastrointestinal cancer. Minerva Chir. 2015, 70, 217–230. [Google Scholar]
- Gammon, J.M.; Dold, N.M.; Jewell, C.M. Improving the clinical impact of biomaterials in cancer immunotherapy. Oncotarget 2016, 7, 15421–15443. [Google Scholar] [CrossRef] [PubMed]
- Kassab, J.; Saba, L.; Kassab, R.; Kourie, H.R. Tsunami of immunotherapies in the management of esophageal cancer. Immunotherapy 2022, 14, 879–884. [Google Scholar] [CrossRef]
- Johnston, M.P.; Khakoo, S.I. Immunotherapy for hepatocellular carcinoma: Current and future. World J. Gastroenterol. 2019, 25, 2977–2989. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, S.; Pan, H.; Chen, W.; Wang, H. Cancer immunotherapy for metastasis: Past, present and future. Brief Funct. Genom. 2019, 18, 140–146. [Google Scholar] [CrossRef]
- Lynam, S.; Lugade, A.A.; Odunsi, K. Immunotherapy for Gynecologic Cancer: Current Applications and Future Directions. Clin. Obstet. Gynecol. 2020, 63, 48–63. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouvinov, K.; Mazor, G.; Kozlener, E.; Meirovitz, A.; Shrem, N.S.; Abu Saleh, O.; Shalata, S.; Yakobson, A.; Shalata, W. Cemiplimab as First Line Therapy in Advanced Penile Squamous Cell Carcinoma: A Real-World Experience. J. Pers. Med. 2023, 13, 1623. https://doi.org/10.3390/jpm13111623
Rouvinov K, Mazor G, Kozlener E, Meirovitz A, Shrem NS, Abu Saleh O, Shalata S, Yakobson A, Shalata W. Cemiplimab as First Line Therapy in Advanced Penile Squamous Cell Carcinoma: A Real-World Experience. Journal of Personalized Medicine. 2023; 13(11):1623. https://doi.org/10.3390/jpm13111623
Chicago/Turabian StyleRouvinov, Keren, Gal Mazor, Ella Kozlener, Amichay Meirovitz, Noa Shani Shrem, Omar Abu Saleh, Sondos Shalata, Alexander Yakobson, and Walid Shalata. 2023. "Cemiplimab as First Line Therapy in Advanced Penile Squamous Cell Carcinoma: A Real-World Experience" Journal of Personalized Medicine 13, no. 11: 1623. https://doi.org/10.3390/jpm13111623
APA StyleRouvinov, K., Mazor, G., Kozlener, E., Meirovitz, A., Shrem, N. S., Abu Saleh, O., Shalata, S., Yakobson, A., & Shalata, W. (2023). Cemiplimab as First Line Therapy in Advanced Penile Squamous Cell Carcinoma: A Real-World Experience. Journal of Personalized Medicine, 13(11), 1623. https://doi.org/10.3390/jpm13111623







