Diastole/Body Mass Index Ratio Can Predict Post-Thoracoscopic Surgery Metastasis in Stage I Lung Adenocarcinoma
Abstract
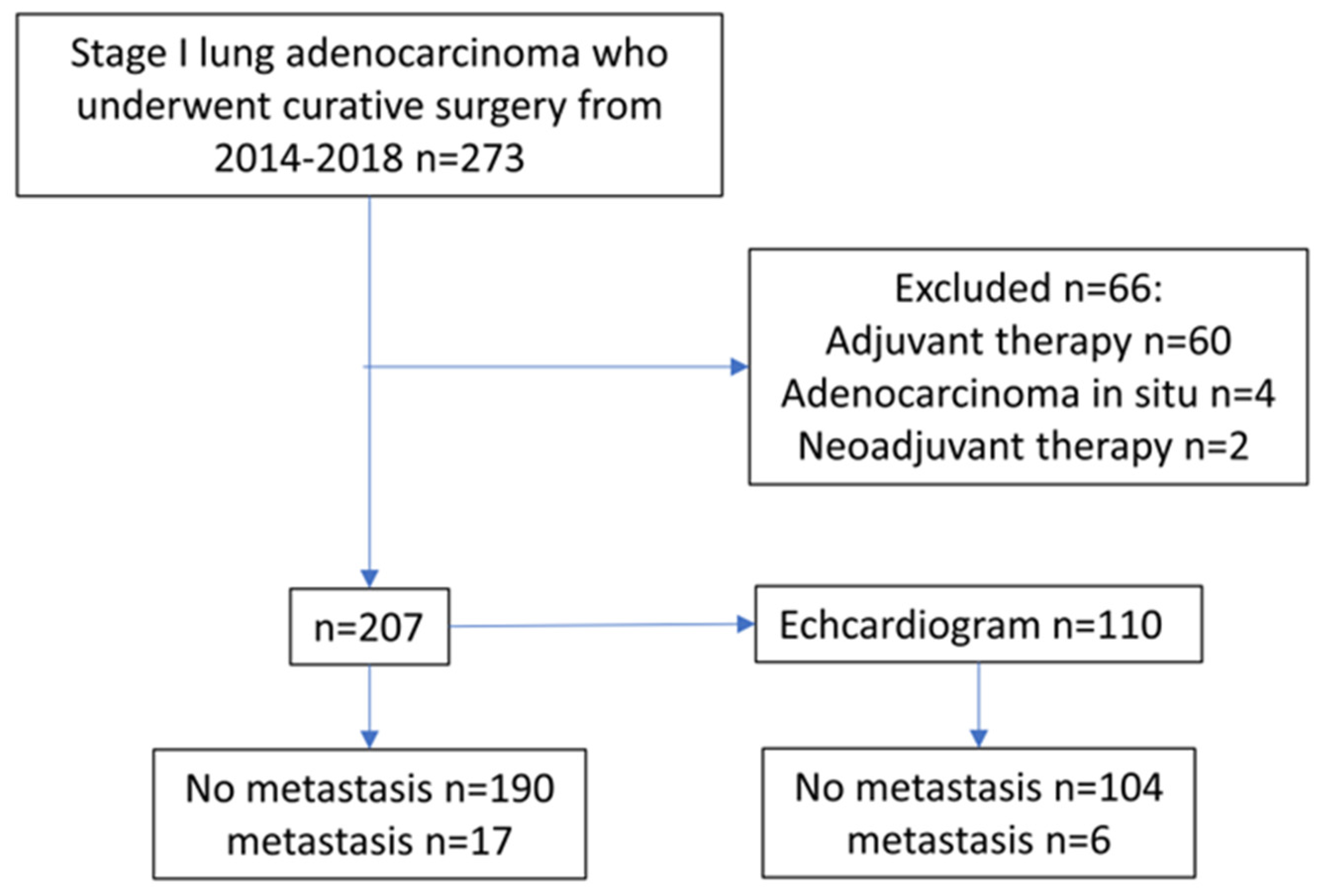
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Patient Follow-Up Evaluation
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tseng, C.H.; Tsuang, B.J.; Chiang, C.J.; Ku, K.C.; Tseng, J.S.; Yang, T.Y.; Hsu, K.H.; Chen, K.C.; Yu, S.L.; Lee, W.C.; et al. The Relationship Between Air Pollution and Lung Cancer in Nonsmokers in Taiwan. J. Thorac. Oncol. 2019, 14, 784–792. [Google Scholar] [CrossRef]
- Ekeke, C.N.; Mitchell, C.; Schuchert, M.; Dhupar, R.; Luketich, J.D.; Okusanya, O.T. Early Distant Recurrence in Patients with Resected Stage I Lung Cancer: A Case Series of “Blast Metastasis”. Clin. Lung Cancer 2021, 22, e132–e135. [Google Scholar] [CrossRef]
- Boyd, A.C.; Schiller, N.B.; Thomas, L. Principles of transthoracic echocardiographic evaluation. Nat. Rev. Cardiol. 2015, 12, 426–440. [Google Scholar] [CrossRef]
- Shim, C.Y. Preoperative cardiac evaluation with transthoracic echocardiography before non-cardiac surgery. Korean J. Anesthesiol. 2017, 70, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Simmons, J.K.; Hildreth, B.E., 3rd; Supsavhad, W.; Elshafae, S.M.; Hassan, B.B.; Dirksen, W.P.; Toribio, R.E.; Rosol, T.J. Animal Models of Bone Metastasis. Vet. Pathol. 2015, 52, 827–841. [Google Scholar] [CrossRef]
- Campbell, J.P.; Merkel, A.R.; Masood-Campbell, S.K.; Elefteriou, F.; Sterling, J.A. Models of bone metastasis. J. Vis. Exp. 2012, 67, e4260. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, S. Body mass index and lung cancer risk in never smokers: A meta-analysis. BMC Cancer 2018, 18, 635. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Sanikini, H.; Yuan, J.M.; Butler, L.M.; Koh, W.P.; Gao, Y.T.; Steffen, A.; Johansson, M.; Vineis, P.; Goodman, G.E.; Barnett, M.J.; et al. Body mass index and lung cancer risk: A pooled analysis based on nested case-control studies from four cohort studies. BMC Cancer 2018, 18, 220. [Google Scholar] [CrossRef] [Green Version]
- Shepshelovich, D.; Xu, W.; Lu, L.; Fares, A.; Yang, P.; Christiani, D.; Zhang, J.; Shiraishi, K.; Ryan, B.M.; Chen, C.; et al. Body Mass Index (BMI), BMI Change, and Overall Survival in Patients With SCLC and NSCLC: A Pooled Analysis of the International Lung Cancer Consortium. J. Thorac. Oncol. 2019, 14, 1594–1607. [Google Scholar] [CrossRef]
- Hung, J.J.; Jeng, W.J.; Hsu, W.H.; Chou, T.Y.; Huang, B.S.; Wu, Y.C. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1115–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chu, Y.; Li, J.; Zeng, F.; Wu, M.; Wang, T.; Sun, L.; Chen, Q.; Wang, P.; Zhang, X.; et al. Development of a prediction model with serum tumor markers to assess tumor metastasis in lung cancer. Cancer Med. 2020, 9, 5436–5445. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Goldberg, S.B.; Canavan, M.; Herrin, J.; Hoag, J.R.; Salazar, M.C.; Papageorge, M.; Ermer, T.; Boffa, D.J. Association of Survival With Adjuvant Chemotherapy Among Patients With Early-Stage Non-Small Cell Lung Cancer With vs Without High-Risk Clinicopathologic Features. JAMA Oncol. 2020, 6, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Zhou, C.; Zhou, Q. Extent of Visceral Pleural Invasion Affects Prognosis of Resected Non-small Cell Lung Cancer: A meta-analysis. Sci. Rep. 2017, 7, 1527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Gold, K.A.; Lin, H.Y.; Swisher, S.G.; Xing, Y.; Lee, J.J.; Kim, E.S.; William, W.N., Jr. Relationship between tumor size and survival in non-small-cell lung cancer (NSCLC): An analysis of the surveillance, epidemiology, and end results (SEER) registry. J. Thorac. Oncol. 2015, 10, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity with Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F. Left Ventricular Diastolic Function: Understanding Pathophysiology, Diagnosis, and Prognosis With Echocardiography. JACC Cardiovasc. Imaging 2020, 13, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef] [PubMed]
- Ohlwein, S.; Klumper, C.; Vossoughi, M.; Sugiri, D.; Stolz, S.; Vierkotter, A.; Schikowski, T.; Kara, K.; Germing, A.; Quass, U.; et al. Air pollution and diastolic function in elderly women-Results from the SALIA study cohort. Int. J. Hyg. Environ. Health 2016, 219, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Waddingham, M.T.; Sonobe, T.; Tsuchimochi, H.; Edgley, A.J.; Sukumaran, V.; Chen, Y.C.; Hansra, S.S.; Schwenke, D.O.; Umetani, K.; Aoyama, K.; et al. Diastolic dysfunction is initiated by cardiomyocyte impairment ahead of endothelial dysfunction due to increased oxidative stress and inflammation in an experimental prediabetes model. J. Mol. Cell. Cardiol. 2019, 137, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.L.; Ocampo, P.S.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.A.; Chino, J.P.; Ready, N.; D’Amico, T.A.; Berry, M.F.; Sporn, T.; Boyd, J.; Kelsey, C.R. Lymphovascular invasion in non-small-cell lung cancer: Implications for staging and adjuvant therapy. J. Thorac. Oncol. 2012, 7, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.B.; Kim, H.; Mino-Kenudson, M.; Cho, S.; Kwon, H.J.; Lee, K.R.; Kwon, S.; Lee, J.; Kim, K.; Jheon, S.; et al. Tumor spread through air spaces (STAS): Prognostic significance of grading in non-small cell lung cancer. Mod. Pathol. 2021, 34, 549–561. [Google Scholar] [CrossRef]


| No Metastasis (N = 190) | POM (N = 17) | p-Value | ||
|---|---|---|---|---|
| SEX (%) | Male (44.4) | 86 (93.5) | 6 (6.5) | 0.43 |
| Female (55.6) | 104 (90.4) | 11 (9.6) | ||
| AGE (YEAR-OLD) | 60.36 ± 11.19 | 62.41 ± 8.04 | 0.46 | |
| BMI (KG/M2) | 24.04 ± 3.99 | 23.54 ± 2.99 | 0.61 | |
| SURGICAL TYPE (%) | Lobectomy (60.7) | 115 (91.3) | 11 (8.7) | 0.74 |
| Sublobar (39.3) | 75 (92.6) | 6 (7.4) | ||
| TUMOR SIZE (CM) | 1.52 ± 0.64 | 2.04 ± 0.56 | 0.001 | |
| ADENOCARCINOMA SUBTYPE (%) | Lepidic (21.7) | 45 (100) | 0 (0) | <0.001 |
| Acinar (54.6) | 106 (94.6) | 6 (5.4) | 0.050 | |
| Papillary (15.9) | 26 (78.8) | 7 (21.2) | 0.022 | |
| Micropapillary (1.4) | 2 (66.7) | 1 (33.3) | 0.45 | |
| Solid (6.4) | 9 (75) | 3 (25) | 0.027 | |
| T STATUS (%) | T1a (31.9) | 66 (100) | 0 (0) | 0.003 |
| T1b (21.7) | 42 (93.3) | 3 (6.7) | 0.67 | |
| T1c (4.3) | 7 (77.8) | 2 (22.2) | 0.19 | |
| T2a (42.1) | 75 (86.2) | 12 (13.8) | 0.013 | |
| TUMOR GRADE (%) | Well (10.1) | 21 (100) | 0 (0) | 0.15 |
| Moderate to poor (89.9) | 169 (90.9) | 17 (9.1) | ||
| STAS (%) | Not identified (35.7) | 72 (97.3) | 2 (2.7) | 0.070 |
| Present (13.0) | 25 (92.6) | 2 (7.4) | ||
| Not mentioned (51.3) | 93 | 13 | ||
| LYMPHOVASCULAR INVASION (%) | Not identified (89.7) | 172 (93.0) | 13 (7.0) | 0.17 |
| Present (8.7) | 15 (83.3) | 3 (16.7) | ||
| Not mentioned (1.6) | 3 | 1 | ||
| PERCENTAGE OF ADENOCARCINOMA SUBTYPE | Lepidic | 27.89 ± 29.01 | 10.00 ± 12.87 | <0.001 |
| Acinar | 49.05 ± 29.34 | 34.41 ± 28.88 | 0.050 | |
| Papillary | 14.87 ± 22.16 | 27.94 ± 25.25 | 0.022 | |
| Micropapillary | 1.95 ± 8.49 | 4.71 ± 14.63 | 0.45 | |
| Solid | 5.05 ± 14.72 | 22.35 ± 29.05 | 0.027 | |
| BLOOD PRESSURE (MMHG) | Systolic | 134.82 ± 18.70 | 133.77 ± 15.12 | 0.85 |
| Diastolic | 78.39 ± 11.49 | 77.85 ± 13.17 | 0.87 | |
| CARDIOVASCULAR DISEASES (%) | Yes (33.8) | 65 (92.9) | 5 (7.1) | 0.69 |
| No (66.2) | 125 (91.2) | 12 (8.8) |
| Univariate Regression Analysis | ||||
|---|---|---|---|---|
| HR | 95% CI | p-Value | ||
| TUMOR SIZE | 3.636 | 1.595 | 8.290 | 0.002 |
| T1A | 0.026 | 0.00 | 1.784 | 0.091 |
| T2A | 3.485 | 1.227 | 9.894 | 0.019 |
| SUBTYPE | ||||
| LEPIDIC | 0.033 | 0.00 | 3.884 | 0.16 |
| ACINAR | 0.436 | 0.161 | 1.180 | 0.10 |
| PAPILLARY | 4.089 | 1.554 | 10.756 | 0.004 |
| SOLID | 4.070 | 1.168 | 14.181 | 0.028 |
| PERCENTAGE | ||||
| LEPIDIC | 0.960 | 0.928 | 0.994 | 0.020 |
| ACINAR | 0.983 | 0.966 | 1.000 | 0.053 |
| PAPILLARY | 1.019 | 1.003 | 1.036 | 0.022 |
| SOLID | 1.031 | 1.016 | 1.047 | <0.001 |
| Non-Cardioechogram (N = 97) | Cardioechogam (N = 110) | p-Value | ||
|---|---|---|---|---|
| SEX (%) | Male (44.4) | 38 (41.3) | 54 (58.7) | 0.15 |
| Female (55.6) | 59 (51.3) | 56 (48.7) | ||
| AGE (YEAR-OLD) | 60.32 ± 11.18 | 60.71 ± 10.82 | 0.80 | |
| BMI (KG/M2) | 24.20 ± 3.38 | 23.83 ± 4.34 | 0.50 | |
| SURGICAL TYPE (%) | Lobectomy (60.9) | 64 (50.8) | 62 (49.2) | 0.16 |
| Sublobar (39.1) | 33 (40.7) | 48 (59.3) | ||
| TUMOR SIZE (CM) | 1.58 ± 0.66 | 1.54 ± 0.63 | 0.61 | |
| ADENOCARCINOMA SUBTYPE (%) | Lepidic (21.7) | 23(51.1) | 22 (48.9) | 0.52 |
| Acinar (54.6) | 53 (46.9) | 60 (53.1) | 0.98 | |
| Papillary (15.9) | 16 (48.5) | 17 (51.5) | 0.84 | |
| Micropapillary (1.4) | 3 (75) | 1 (25) | 0.25 | |
| Solid (6.4) | 4 (30.8) | 9 (69.2) | 0.23 | |
| PERCENTAGE OF ADENOCARCINOMA SUBTYPE (%) | Lepidic | 28.09 ± 29.60 | 24.95 ± 27.45 | 0.43 |
| Acinar | 46.96 ± 30.14 | 48.64 ± 29.05 | 0.68 | |
| Papillary | 15.52 ± 23.77 | 16.32 ± 21.71 | 0.80 | |
| Micropapillary | 3.20 ± 12.53 | 1.27 ± 4.20 | 0.15 | |
| Solid | 5.10 ± 14.12 | 7.68 ± 19.07 | 0.27 | |
| T STATUS (%) | T1a (31.9) | 37 (56.1) | 29 (43.9) | 0.070 |
| T1b (21.7) | 16 (35.6) | 29 (64.4) | 0.086 | |
| T1c (4.3) | 2 (22.2) | 7 (77.8) | 0.13 | |
| T2a (42.1) | 42 (48.3) | 45 (51.7) | 0.73 | |
| METASTASIS (%) | No (91.8) | 86 (45.3) | 104 (54.7) | 0.12 |
| Yes (8.2) | 11 (64.7) | 6 (35.3) | ||
| BLOOD PRESSURE (MMHG) | Systolic | 134.15 ± 17.85 | 135.27 ± 18.94 | 0.72 |
| Diastolic | 77.68 ± 11.79 | 78.99 ± 11.85 | 0.50 | |
| CARDIOVASCULAR DISEASES (%) | Yes (33.8) | 30 (42.9) | 40 (57.1) | 0.41 |
| No (66.2) | 67 (48.9) | 70 (51.1) |
| No Metastasis (n = 104) | POM (N = 6) | p-Value | ||
|---|---|---|---|---|
| SEX (%) | Male (51.9) | 51 (94.4) | 3 (5.6) | 0.96 |
| Female (48.1) | 53 (94.6) | 3 (5.4) | ||
| AGE (YEAR-OLD) | 60.79 ± 11.04 | 59.33 ± 6.44 | 0.75 | |
| BMI (KG/M2) | 24.23 ± 3.77 | 21.45 ± 2.89 | 0.078 | |
| SURGICAL TYPE (%) | Lobectomy (54.8) | 57 (91.9) | 5 (8.1) | 0.17 |
| Sublobar (45.2) | 47 (97.9) | 1 (2.1) | ||
| TUMOR SIZE (CM) | 1.51 ± 0.63 | 2.05 ± 0.50 | 0.042 | |
| ADENOCARCINOMA SUBTYPE (%) | Lepidic (20) | 22 (100) | 0 (0) | 0.21 |
| Acinar (54.6) | 59 (98.3) | 1 (1.7) | 0.052 | |
| Papillary (15.4) | 15 (88.2) | 2 (11.8) | 0.22 | |
| Micropapillary (0.9) | 1 (100) | 0 (0) | 0.81 | |
| Solid (8.1) | 6 (66.7) | 3 (33.3) | <0.001 | |
| PERCENTAGE OF ADENOCARCINOMA SUBTYPE (%) | Lepidic | 25.96 ± 27.78 | 7.5 ± 11.73 | 0.11 |
| Acinar | 49.81 ± 28.81 | 28.33 ± 27.87 | 0.078 | |
| Papillary | 15.82 ± 21.44 | 25.00 ± 26.65 | 0.32 | |
| Micropapillary | 1.35 ± 4.31 | 0.00 ± 0.00 | 0.45 | |
| Solid | 5.87 ± 15.43 | 39.17 ± 42.00 | <0.001 | |
| T STATUS (%) | T1a (26.4) | 29 (100) | 0 (0) | 0.13 |
| T1b (26.4)) | 27 (93.1) | 2 (6.9) | 0.69 | |
| T1c (6.3) | 7 (100) | 0 (0) | 0.51 | |
| T2a (40.9) | 41 (91.1) | 4 (8.9) | 0.19 |
| NO (N=) | POM (N=) | p-Value | |
|---|---|---|---|
| Aortic opening diameter (cm) | 3.097 ± 0.461 | 3.010 ± 0.288 | 0.65 |
| (102) | (6) | ||
| Left atrial diameter (cm) | 3.442 ± 0.765 | 3.352 ± 0.316 | 0.78 |
| (102) | (6) | ||
| Aortic valve cusp opening (cm) | 1.813 ± 0.292 | 1.860 ± 0.232 | 0.65 |
| (100) | (6) | ||
| Left atrium to aortic root ratio | 1.129 ± 0.279 | 1.118 ± 0.117 | 0.92 |
| (102) | (6) | ||
| Interventricular septal end diastole (cm) | 0.980 ± 0.226 | 0.948 ± 0.229 | 0.75 |
| (104) | (5) | ||
| Left ventricular internal diameter end diastole (cm) | 4.699 ± 0.679 | 4.646 ± 0.971 | 0.87 |
| (104) | (5) | ||
| Left ventricular posterior wall end diastole (cm) | 0.936 ± 0.184 | 0.928 ± 0.198 | 0.93 |
| (104) | (5) | ||
| Interventricular septal end systole (cm) | 1.396 ± 0.314 | 1.418 ± 0.536 | 0.88 |
| (104) | (5) | ||
| Left ventricular internal diameter end systole (cm) | 2.814 ± 0.581 | 2.894 ± 0.729 | 0.77 |
| (104) | (5) | ||
| Left ventricular posterior wall end systole (cm) | 1.458 ± 0.268 | 1.332 ± 0.361 | 0.31 |
| (104) | (6) | ||
| End diastolic volume (mL) | 105.418 ± 34.443 | 101.440 ± 47.644 | 0.79 |
| (104) | (6) | ||
| End systolic volume (mL) | 31.999 ± 15.515 | 33.460 ± 18.822 | 0.83 |
| (104) | (6) | ||
| Stroke volume (mL) | 73.418 ± 23.915 | 67.980 ± 29.746 | 0.59 |
| (104) | (6) | ||
| Ejection fraction (%) | 70.414 ± 8.601 | 68.057 ± 6.185 | 0.51 |
| (104) | (6) | ||
| Fraction shortening (%) | 40.370 ± 7.249 | 38.066 ± 5.347 | 0.49 |
| (104) | (5) | ||
| Mitral valve peak A velocity (MV Peak A Vel) (cm/s) | 77.5 ± 22.7 | 92.7 ± 20.9 | 0.11 |
| (91) | (6) | ||
| Mitral valve peak E velocity (MV Peak E Vel) (cm/s) | 69.9 ± 17.2 | 80.4 ± 17.6 | 0.15 |
| (92) | (6) | ||
| MVPABMI (MV Peak A Vel/BMI) | 3.243 ± 1.094 | 4.380 ± 1.090 | 0.015 |
| (91) | (6) | ||
| MVPEBMI (MV Peak E Vel/BMI) | 2.948 ± 0.901 | 3.806 ± 0.963 | 0.027 |
| (92) | (6) |
| Univariate Regression Analysis | Multiple Regression Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| MVPABMI MVPEBMI | 2.139 2.293 | 1.136 1.043 | 4.027 5.040 | 0.019 0.039 | (reference) | |||
| SOLID (SUBTYPE) | 12.937 | 2.599 | 64.387 | 0.002 | ||||
| SOLID (PERCENTAGE) | 1.043 | 1.019 | 1.068 | <0.001 | 1.044 | 1.019 | 1.069 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, H.-H.; Chang, P.-C.; Chang, T.-W.; Chen, K.-H.; Liu, Y.-W.; Li, H.-P.; Chou, S.-H.; Chang, Y.-T. Diastole/Body Mass Index Ratio Can Predict Post-Thoracoscopic Surgery Metastasis in Stage I Lung Adenocarcinoma. J. Pers. Med. 2023, 13, 497. https://doi.org/10.3390/jpm13030497
Chiang H-H, Chang P-C, Chang T-W, Chen K-H, Liu Y-W, Li H-P, Chou S-H, Chang Y-T. Diastole/Body Mass Index Ratio Can Predict Post-Thoracoscopic Surgery Metastasis in Stage I Lung Adenocarcinoma. Journal of Personalized Medicine. 2023; 13(3):497. https://doi.org/10.3390/jpm13030497
Chicago/Turabian StyleChiang, Hung-Hsing, Po-Chih Chang, Ting-Wei Chang, Kai-Hua Chen, Yu-Wei Liu, Hsien-Pin Li, Shah-Hwa Chou, and Yu-Tang Chang. 2023. "Diastole/Body Mass Index Ratio Can Predict Post-Thoracoscopic Surgery Metastasis in Stage I Lung Adenocarcinoma" Journal of Personalized Medicine 13, no. 3: 497. https://doi.org/10.3390/jpm13030497
APA StyleChiang, H.-H., Chang, P.-C., Chang, T.-W., Chen, K.-H., Liu, Y.-W., Li, H.-P., Chou, S.-H., & Chang, Y.-T. (2023). Diastole/Body Mass Index Ratio Can Predict Post-Thoracoscopic Surgery Metastasis in Stage I Lung Adenocarcinoma. Journal of Personalized Medicine, 13(3), 497. https://doi.org/10.3390/jpm13030497






