Nomogram for Predicting Recurrence-Free Survival of Primary Localized Gastrointestinal Stromal Tumor
Abstract
1. Introduction
2. Materials and Methods
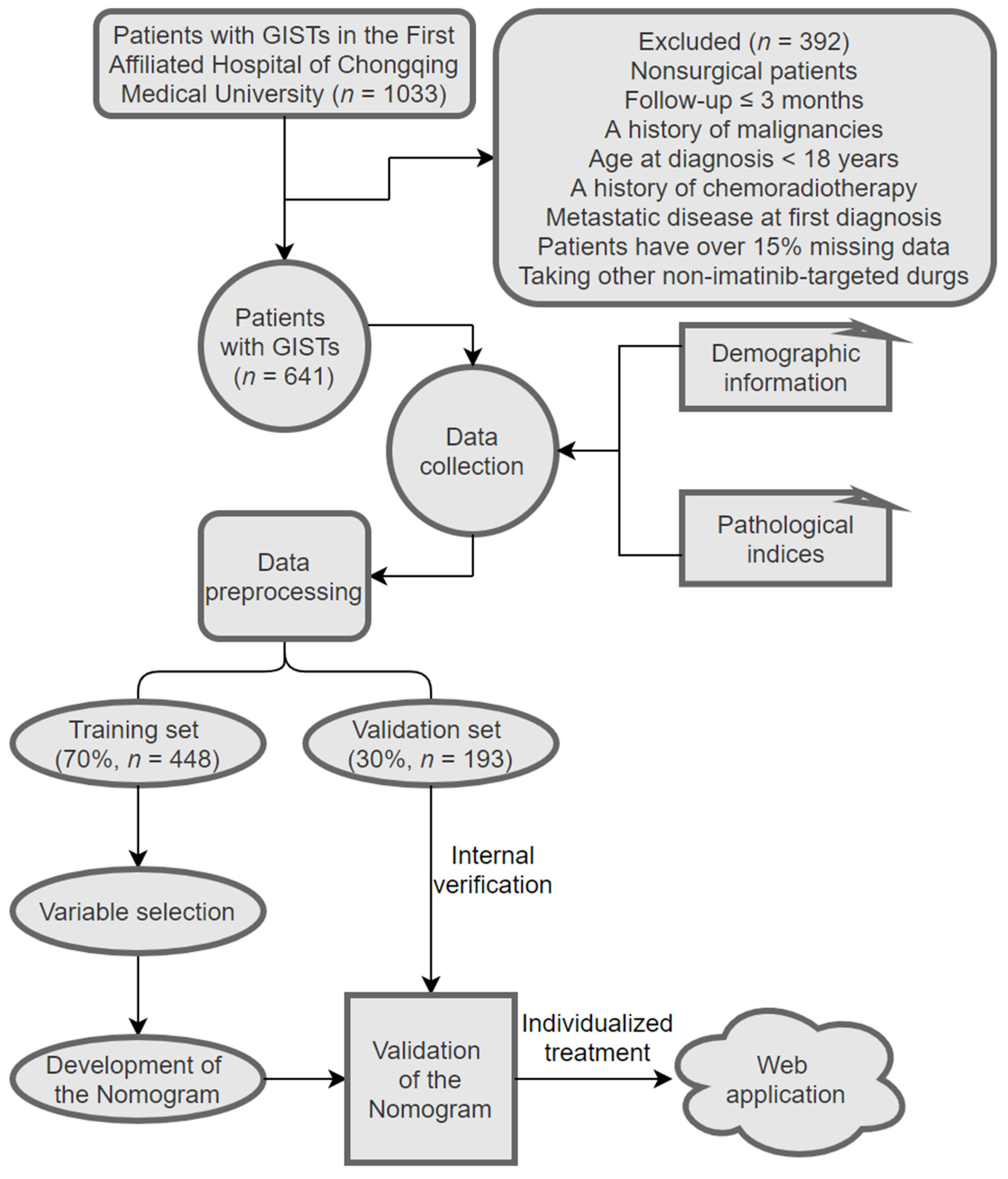
2.1. Patients
2.2. Collection of Demographic, Pathological, and Follow-Up Data
2.3. Statistical Analysis
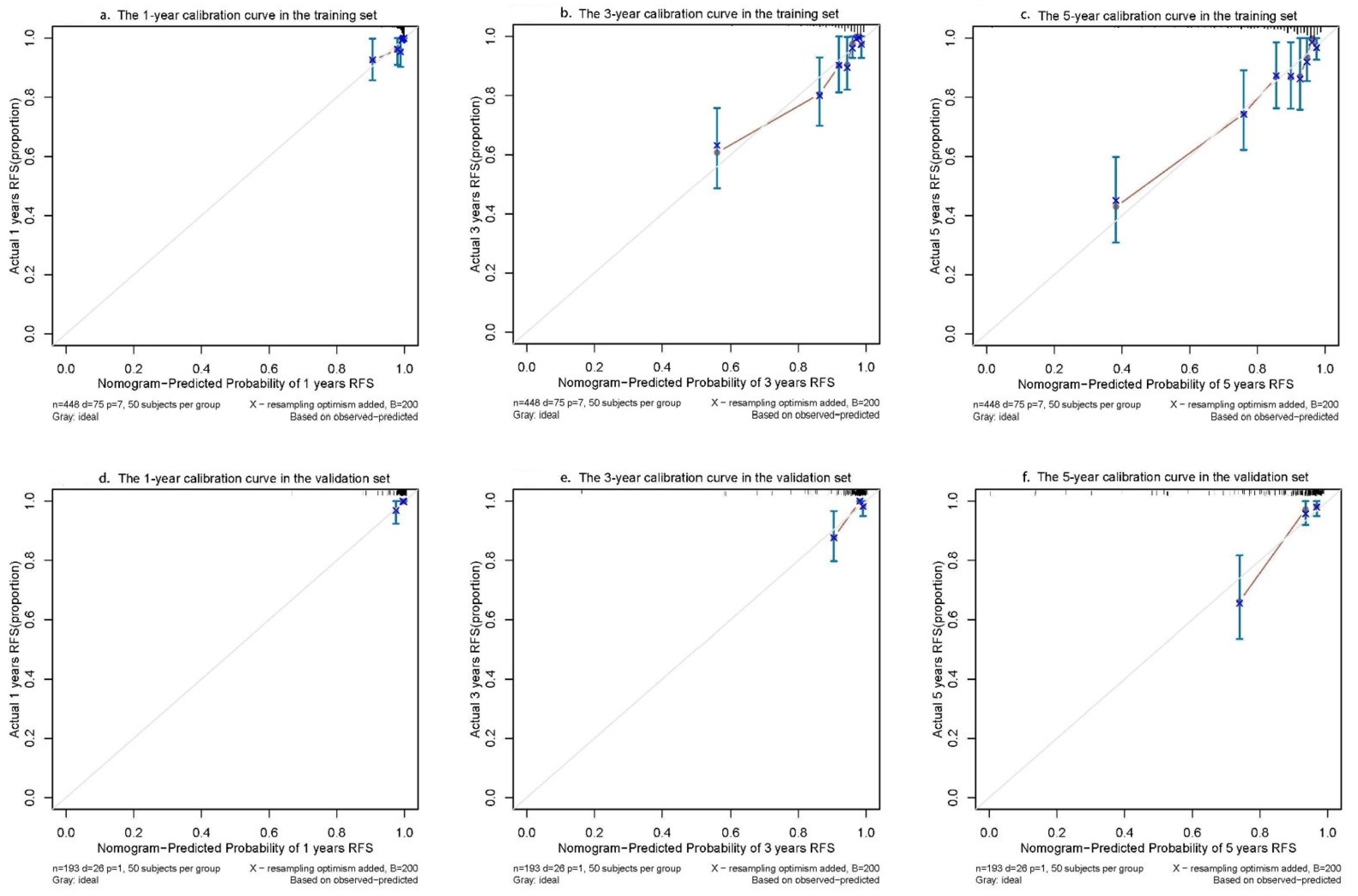
2.4. Evaluation of the Nomogram Model’s Performance
3. Results
3.1. Patient Characteristics
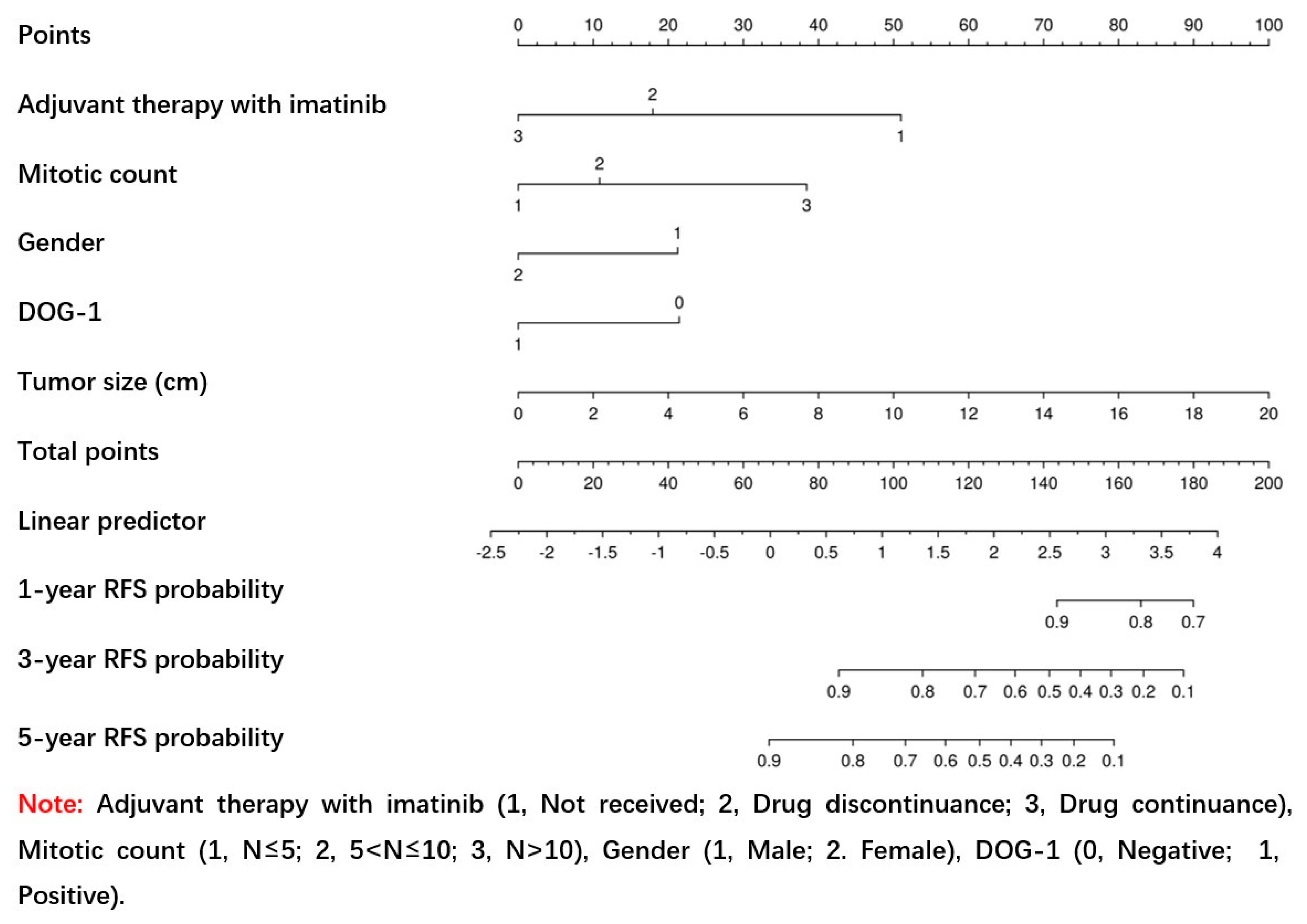
3.2. Creation of the Nomogram
3.3. Evaluation of the Accuracy and Clinical Utility of Nomogram
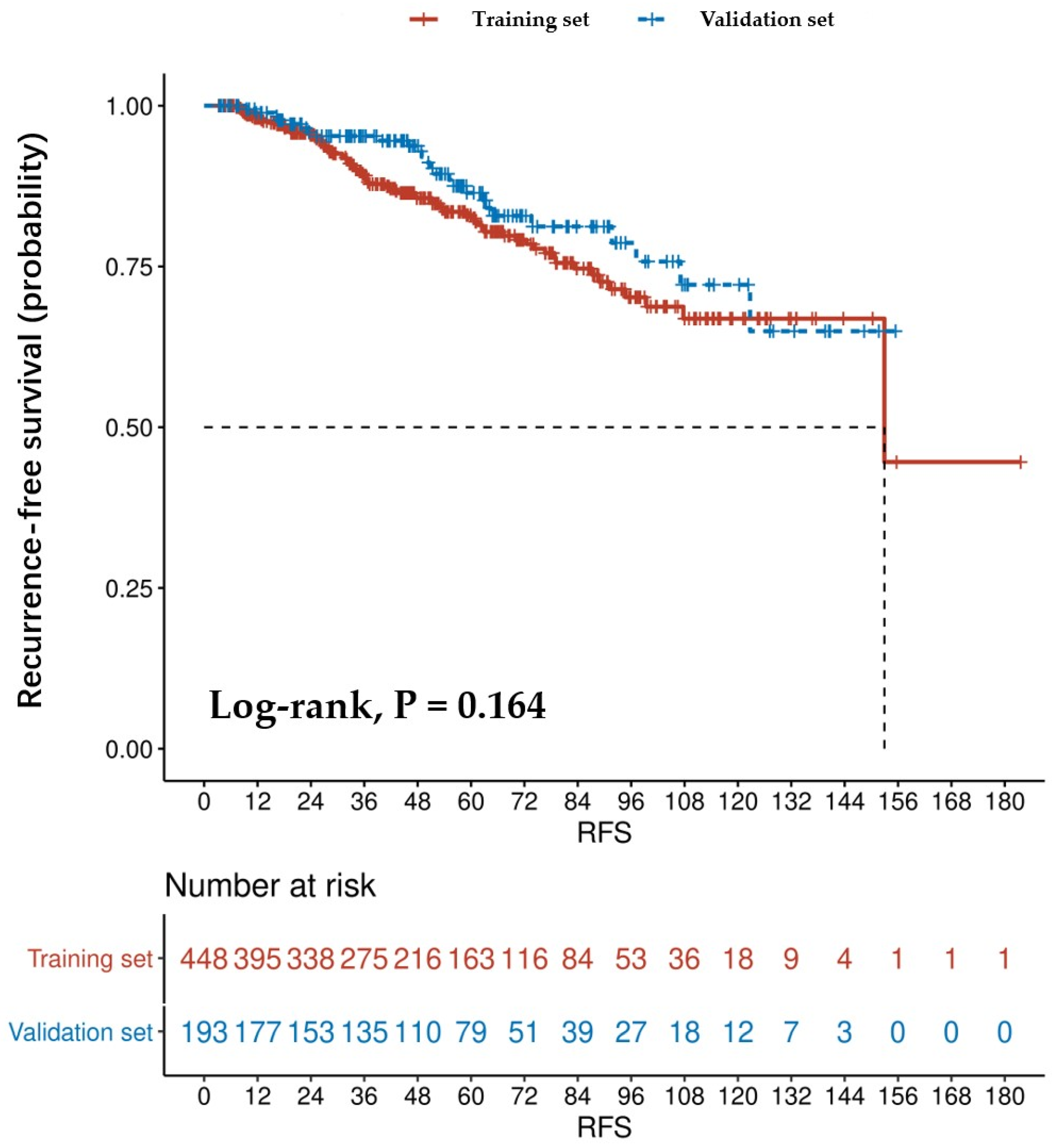
3.4. Confirmation of the Nomogram
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors—Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001, 438, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M.; Pezzica, E.; Tamborini, E.; Guerra, U.; Lagonigro, S.M.; Forloni, B.; Barni, S. Neoadjuvant imatinib in a locally advanced gastrointestinal stromal tumour (GIST) of the rectum: A rare case of two GISTs within a family without a familial GIST syndrome. Eur. J. Gastroenterol. Hepatol. 2007, 19, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors (GISTs): Definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol. J. Pathol. 2003, 54, 3–24. [Google Scholar] [PubMed]
- Dumont, A.G.; Rink, L.; Godwin, A.K.; Miettinen, M.; Joensuu, H.; Strosberg, J.R.; Gronchi, A.; Corless, C.L.; Goldstein, D.; Rubin, B.P.; et al. A nonrandom association of gastrointestinal stromal tumor (GIST) and desmoid tumor (deep fibromatosis): Case series of 28 patients. Ann. Oncol. 2011, 23, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Nowecki, Z.I.; Michej, W.; Dębiec-Rychter, M.; Woźniak, A.; Limon, J.; Siedlecki, J.; Grzesiakowska, U.; Kąkol, M.; Osuch, C.; et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann. Surg. Oncol. 2007, 14, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- DeMatteo, R.P.; Ballman, K.V.; Antonescu, C.R.; Maki, R.G.; Pisters, P.W.; Demetri, G.D.; Blackstein, M.E.; Blanke, C.D.; von Mehren, M.; Brennan, M.F.; et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet 2009, 373, 1097–1104. [Google Scholar] [CrossRef]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.; Miettinen, M.; O'Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002, 33, 459–465. [Google Scholar] [CrossRef]
- Gold, J.S.; Gönen, M.; Gutiérrez, A.; Broto, J.M.; García-Del-Muro, X.; Smyrk, T.C.; Maki, R.G.; Singer, S.; Brennan, M.F.; Antonescu, C.R.; et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 2009, 10, 1045–1052. [Google Scholar] [CrossRef]
- Chen, T.; Xu, L.; Ye, L.; Qiu, H.; Hu, Y.; Liu, H.; Zhou, Z.; Li, G.; Yu, J. A new nomogram for recurrence-free survival prediction of gastrointestinal stromal tumors: Comparison with current risk classification methods. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 1109–1114. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Nazzicari, N.; Li, X.; Wei, Y.; Pecetti, L.; Brummer, E.C. Accuracy of genomic selection for alfalfa biomass yield in different reference populations. BMC Genom. 2015, 16, 1020. [Google Scholar] [CrossRef]
- He, S.; Zhao, Y.; Mette, M.F.; Bothe, R.; Ebmeyer, E.; Sharbel, T.F.; Reif, J.C.; Jiang, Y. Prospects and limits of marker imputation in quantitative genetic studies in European elite wheat (Triticum aestivum L.). BMC Genom. 2015, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Belfiori, G.; Sartelli, M.; Cardinali, L.; Tranà, C.; Bracci, R.; Gesuita, R.; Marmorale, C. Risk stratification systems for surgically treated localized primary Gastrointestinal Stromal Tumors (GIST). Review of literature and comparison of the three prognostic criteria: MSKCC nomogramm, NIH-fletcher and AFIP-miettinen. Ann. Ital. Di. Chir. 2015, 86, 219–227. [Google Scholar]
- Racz, J.M.; Brar, S.S.; Msc, M.C.C.; Jimenez, M.C.; Azin, A.; Atenafu, E.G.; Jackson, T.D.; Okrainec, A.; Quereshy, F.A. The accuracy of three predictive models in the evaluation of recurrence rates for gastrointestinal stromal tumors. J. Surg. Oncol. 2015, 111, 371–376. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, D.; Li, S. Novel prognostic nomogram for recurrence-free survival of patients with primary gastrointestinal stromal tumors after surgical resection: Combination of prognostic nutritional index and basic variables. Front. Oncol. 2020, 10, 581855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ning, L.; Hu, Y.; Zhao, S.; Li, Z.; Li, L.; Dai, Y.; Jiang, L.; Wang, A.; Chu, X.; et al. Prognostic factors for primary localized gastrointestinal stromal tumors after radical resection: Shandong gastrointestinal surgery study group, study 1201. Ann. Surg. Oncol. 2020, 27, 2812–2821. [Google Scholar] [CrossRef]
- Yen, C.-C.; Yeh, C.-N.; Cheng, C.-T.; Jung, S.-M.; Huang, S.-C.; Chang, T.-W.; Jan, Y.-Y.; Tzeng, C.-H.; Chao, T.-C.; Chen, Y.-Y.; et al. Integrating bioinformatics and clinicopathological research of gastrointestinal stromal tumors: Identification of aurora kinase a as a poor risk marker. Ann. Surg. Oncol. 2012, 19, 3491–3499. [Google Scholar] [CrossRef]
- Cavnar, M.J.; Seier, K.; Curtin, C.; Balachandran, V.P.; Coit, D.G.; Yoon, S.S.; Crago, A.M.; Strong, V.E.; Tap, W.D.; Gönen, M.; et al. Outcome of 1000 patients with Gastrointestinal Stromal Tumor (GIST) treated by surgery in the pre- and post-imatinib eras. Ann. Surg. 2021, 273, 128–138. [Google Scholar] [CrossRef]
- Rong, J.; Chen, S.; Song, C.; Wang, H.; Zhao, Q.; Zhao, R.; He, Y.; Yan, L.; Song, Y.; Wang, F.; et al. The prognostic value of gender in gastric gastrointestinal stromal tumors: A propensity score matching analysis. Biol. Sex Differ. 2020, 11, 43. [Google Scholar] [CrossRef]
- Na Kang, Y.; Jung, H.R.; Hwang, I. Clinicopathological and immunohistochemical features of gastointestinal stromal tumors. Cancer Res. Treat. 2010, 42, 135–143. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, W.; Wang, Z.; Li, T.; Meng, X. Tumor sites and microscopic indicators are independent prognosis predictors of gastrointestinal stromal tumors. Tohoku J. Exp. Med. 2014, 233, 65–72. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Feng, G.-W.; Liu, Z.-F.; Qiao, L.; Zhang, T.; Gao, C.; Xu, Y. A young man with primary prostatic extra-gastrointestinal stromal tumor: A rare case report and review of the literature. Int. J. Clin. Exp. Pathol. 2014, 7, 1764–1770. [Google Scholar] [PubMed]
- Sun, X.F.; Gao, X.D.; Yuan, W. Clinicopathological features and prognosis of 59 patients with platelet-derived growth factor al-pha-mutant gastrointestinal stromal tumor. Zhonghua Wei Chang. Wai Ke Za Zhi 2020, 23, 880–887. [Google Scholar] [PubMed]
- Lv, M.; Wu, C.; Zheng, Y.; Zhao, N. Incidence and survival analysis of gastrointestinal stromal tumors in Shanghai: A population-based study from 2001 to 2010. Gastroenterol. Res. Pract. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Eriksson, M.; Hall, K.S.; Hartmann, J.T.; Pink, D.; Schütte, J.; Ramadori, G.; Hohenberger, P.; Duyster, J.; Al-Batran, S.; et al. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer 2014, 120, 2325–2333. [Google Scholar] [CrossRef]
- Gronchi, A. Risk stratification models and mutational analysis: Keys to optimising adjuvant therapy in patients with gastrointestinal stromal tumour. Eur. J. Cancer 2013, 49, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, E.; Sun, Y. Postoperative adjuvant imatinib therapy-associated nomogram to predict overall survival of gastroin-testinal stromal tumor. Front Med. 2022, 9, 777181. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kim, T.S.; Bauer, S.; Sicklick, J.K. Gastrointestinal stromal tumor: New insights for a multimodal approach. Surg. Oncol. Clin. N. Am. 2022, 31, 431–446. [Google Scholar] [CrossRef]
- Lin, J.X.; Chen, Q.F.; Zheng, C.H. Is 3 years duration of adjuvant imatinib mesylate treatment sufficient for patients with high-risk gastrointestinal stromal tumor? A study based on long-term follow-up. J. Cancer Res. Clin. Oncol. 2017, 143, 727–734. [Google Scholar] [CrossRef]
- Raut, C.P.; Espat, N.J.; Maki, R.G. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 clinical trial. JAMA Oncol. 2018, 4, e184060. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, M.; Jia, J.; Wan, W.; Wang, T.; Yang, W.; Li, C.; Chen, X.; Cao, H.; Zhang, P.; et al. Development and validation of a prognostic nomogram to predict recurrence in high-risk gastrointestinal stromal tumour: A retrospective analysis of two independent cohorts. Ebiomedicine 2020, 60, 103016. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cui, J.; Yu, T.; Li, Z.; Zhao, G. Fibrinogen/albumin ratio index is an independent prognosis predictor of recurrence-free survival in patients after surgical resection of gastrointestinal stromal tumors. Front. Oncol. 2020, 10, 1459. [Google Scholar] [CrossRef]
- Li, R.; Sun, Z.; Song, S.; He, X.; Shi, X.; Li, Z.; Song, J. NARFIB: A novel prognostic score based on the neutrophil-to-albumin ratio and fibrinogen can predict the prognosis of gastrointestinal stromal tumors. Cancer Manag. Res. 2020, 12, 11183–11190. [Google Scholar] [CrossRef] [PubMed]
- Racz, J.M.; Cleghorn, M.C.; Jimenez, M.C.; Atenafu, E.G.; Jackson, T.D.; Okrainec, A.; Venkat Raghavan, L.; Quereshy, F.A. Pre-dictive ability of blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in gastrointestinal stromal tumors. Ann. Surg. Oncol. 2015, 22, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-X.; Li, X.; Wang, S.-F. Prognostic value of fibrinogen and D-dimer-fibrinogen ratio in resectable gastrointestinal stromal tumors. World J. Gastroenterol. 2018, 24, 5046–5056. [Google Scholar] [CrossRef] [PubMed]


| Variable | Total | Training Set | Validation Set | p Value |
|---|---|---|---|---|
| (n = 641) | (n = 448) | (n = 193) | ||
| Age at diagnosis (years) | 55.60 ± 11.91 | 55.67 ± 11.89 | 55.44 ± 12.00 | 0.827 a |
| Tumor size (cm) | 6 (4–9) | 6 (4–9) | 6 (4–9) | 0.757 b |
| Ki-67 Li (%) | 5 (3–10) | 5 (3–10) | 5 (3–10) | 0.953 b |
| Gender | 0.556 c | |||
| Male | 281 (43.84%) | 193 (43.08%) | 88 (45.60%) | |
| Female | 360 (56.16%) | 255 (56.92%) | 105 (54.40%) | |
| Residence | 0.776 c | |||
| City | 450 (70.20%) | 313 (69.87%) | 137 (70.98%) | |
| Rural township | 191 (29.80%) | 135 (30.13%) | 56 (29.02%) | |
| Initial symptom | 0.333 d | |||
| Asymptomatic | 199 (31.05%) | 139 (31.03%) | 60 (31.09%) | |
| Hematemesis | 15 (2.34%) | 9 (2.01%) | 6 (3.11%) | |
| Hematochezia | 141 (22.00%) | 92 (20.54%) | 49 (25.39%) | |
| Abdominal discomfort | 263 (41.03%) | 194 (43.30%) | 69 (35.75%) | |
| Sour regurgitation | 12 (1.87%) | 8 (1.79%) | 4 (2.07%) | |
| Eating obstruction | 11 (1.72%) | 6 (1.34%) | 5 (2.59%) | |
| Mitotic count (per 50 HPFs) | 0.278 c | |||
| N ≤ 5 | 443 (69.11%) | 315 (70.31%) | 128 (66.32%) | |
| 5 < N ≤ 10 | 138 (21.53%) | 89 (19.87%) | 49 (25.39%) | |
| N > 10 | 60 (9.36%) | 44 (9.82%) | 16 (8.29%) | |
| Tumor site | 0.290 c | |||
| Stomach | 323 (50.39%) | 222 (49.55%) | 101 (52.33%) | |
| Small intestine | 249 (38.85%) | 183 (40.85%) | 66 (34.20%) | |
| Colorectum/rectal | 31 (4.84%) | 20 (4.46%) | 11 (5.70%) | |
| Other | 38 (5.93%) | 23 (5.13%) | 15 (7.77%) | |
| CD34 | 0.091 c | |||
| Negative | 74 (11.54%) | 58 (12.95%) | 16 (8.29%) | |
| Positive | 567 (88.46%) | 390 (87.05%) | 177 (91.71%) | |
| CD117 | 0.972 c | |||
| Negative | 23 (3.59%) | 16 (3.57%) | 7 (3.63%) | |
| Positive | 618 (96.41%) | 432 (96.43%) | 186 (96.37%) | |
| DOG-1 | 0.994 c | |||
| Negative | 59 (9.20%) | 41 (9.15%) | 18 (9.33%) | |
| Positive | 582 (90.80%) | 407 (90.85%) | 175 (90.67%) | |
| Gene mutation | 0.159 d | |||
| KIT exon 11 | 446 (69.58%) | 301 (67.19%) | 145 (75.13%) | |
| KIT exon 9 | 110 (17.16%) | 87 (19.42%) | 23 (11.92%) | |
| PDGFR-α | 21 (3.28%) | 16 (3.57%) | 5 (2.59%) | |
| Wild type | 56 (8.74%) | 39 (8.71%) | 17 (8.81%) | |
| Other | 8 (1.25%) | 5 (1.12%) | 3 (1.55%) | |
| Neoadjuvant therapy with imatinib | 0.735 c | |||
| Not received | 591 (92.20%) | 412 (91.96%) | 179 (92.75%) | |
| Received | 50 (7.80%) | 36 (8.04%) | 14(7.25%) | |
| Adjuvant therapy with imatinib | 0.929 c | |||
| Not received | 171 (26.68%) | 118 (26.34%) | 53 (27.46%) | |
| Drug discontinuance | 104 (16.22%) | 72 (16.07%) | 32 (16.58%) | |
| Drug continuance | 366 (57.10%) | 258 (57.59%) | 108 (55.96%) | |
| Variable | RFS | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age at diagnosis (years) | 0.99 (0.98–1.01) | 0.575 | - | |
| Tumor size (cm) | 1.19 (1.13–1.25) | <0.001 | 1.16 (1.09–1.24) | <0.001 |
| Ki-67 Li (%) | 1.04 (1.02–1.07) | <0.001 | 1.01 (0.97–1.05) | 0.519 |
| Gender | 0.002 | 0.003 | ||
| Male | 1.0 (ref) | 1.0 (ref) | ||
| Female | 0.47 (0.30–0.75) | 0.49 (0.30–0.78) | ||
| Residence | 0.521 | - | ||
| City | 1.0 (ref) | |||
| Rural township | 1.18 (0.71–1.94) | |||
| Initial symptom | 0.380 | - | ||
| Asymptomatic | 1.0 (ref) | |||
| Hematemesis | 0.71 (0.10–5.36) | |||
| Hematochezia | 0.90 (0.42–1.90) | |||
| Abdominal discomfort | 1.60 (0.93–2.78) | |||
| Sour regurgitation | 0.00 (0.00–1.94) | |||
| Eating obstruction | 1.93 (0.45–8.32) | |||
| Mitotic counts (per 50 HPFs) | <0.001 | 0.016 | ||
| N ≤ 5 | 1.0 (ref) | 1.0 (ref) | ||
| 5 < N ≤ 10 | 1.49 (0.84–2.64) | 1.30 (0.68–2.49) | ||
| N > 10 | 4.79 (2.76–8.33) | 3.00 (1.39–6.50) | ||
| Tumor site | 0.002 | 0.608 | ||
| Stomach | 1.0 (ref) | 1.0 (ref) | ||
| Small intestine | 2.54 (1.53–4.20) | 1.43 (0.82–2.50) | ||
| Colorectum/rectal Other | 0.54 (0.07–3.98) | 0.82 (0.11–6.22) | ||
| 2.60 (1.05–6.39) | 1.49 (0.58–3.83) | |||
| CD34 | 0.362 | - | ||
| Negative | 1.0 (ref) | |||
| Positive | 0.76 (0.43–1.37) | |||
| CD117 | 0.238 | - | ||
| Negative | 1.0 (ref) | |||
| Positive | 0.55 (0.20–1.49) | |||
| DOG-1 | <0.001 | 0.018 | ||
| Negative | 1.0 (ref) | 1.0 (ref) | ||
| Positive | 0.29 (0.18–0.49) | 0.52 (0.30–0.89) | ||
| Gene mutation | 0.990 | |||
| KIT exon 11 | 1.0 (ref) | |||
| KIT exon 9 | 1.06 (0.61–1.82) | |||
| PDGFR-α | 1.14 (0.28–4.72) | |||
| Wild type | 1.22 (0.58–2.59) | |||
| Other | 0.00 (0.00–5.60) | |||
| Neoadjuvant therapy with imatinib | 0.280 | - | ||
| Not received | 1.0 (ref) | |||
| Received | 0.46 (0.11–1.88) | |||
| Adjuvant therapy with imatinib | <0.001 | <0.001 | ||
| Not received | 1.0 (ref) | 1.0 (ref) | ||
| Drug discontinuance | 0.28 (0.15–0.53) | 0.33 (0.17–0.64) | ||
| Drug continuance | 0.22 (0.13–0.37) | 0.19 (0.11–0.32) | ||
| Criteria | Training Set | Validation Set | ||||||
|---|---|---|---|---|---|---|---|---|
| 1-Year AUC | 3-Year AUC | 5-Year AUC | C-Index | 1-Year AUC | 3-Year AUC | 5-Year AUC | C-Index | |
| Nomogram | 0.868 | 0.838 | 0.816 | 0.830 | 0.977 | 0.845 | 0.869 | 0.849 |
| MSKCC | 0.749 | 0.760 | 0.762 | 0.747 | 0.893 | 0.803 | 0.728 | 0.745 |
| NIH–Fletcher | 0.848 | 0.770 | 0.781 | 0.763 | 0.973 | 0.803 | 0.755 | 0.766 |
| Chen’s nomogram | 0.562 | 0.700 | 0.729 | 0.702 | 0.955 | 0.859 | 0.737 | 0.768 |
| AFIP–Miettinen | 0.812 | 0.777 | 0.799 | 0.763 | 0.888 | 0.796 | 0.712 | 0.751 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, P.; Tan, T.; Zhou, H.; Li, J.; Yang, H.; Li, J.; Zhang, J. Nomogram for Predicting Recurrence-Free Survival of Primary Localized Gastrointestinal Stromal Tumor. J. Pers. Med. 2023, 13, 498. https://doi.org/10.3390/jpm13030498
Ran P, Tan T, Zhou H, Li J, Yang H, Li J, Zhang J. Nomogram for Predicting Recurrence-Free Survival of Primary Localized Gastrointestinal Stromal Tumor. Journal of Personalized Medicine. 2023; 13(3):498. https://doi.org/10.3390/jpm13030498
Chicago/Turabian StyleRan, Pan, Tao Tan, Hui Zhou, Jinjin Li, Hao Yang, Juan Li, and Jun Zhang. 2023. "Nomogram for Predicting Recurrence-Free Survival of Primary Localized Gastrointestinal Stromal Tumor" Journal of Personalized Medicine 13, no. 3: 498. https://doi.org/10.3390/jpm13030498
APA StyleRan, P., Tan, T., Zhou, H., Li, J., Yang, H., Li, J., & Zhang, J. (2023). Nomogram for Predicting Recurrence-Free Survival of Primary Localized Gastrointestinal Stromal Tumor. Journal of Personalized Medicine, 13(3), 498. https://doi.org/10.3390/jpm13030498






