Effects of Dezocine on the Reduction of Emergence Delirium after Laparoscopic Surgery: A Retrospective Propensity Score-Matched Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
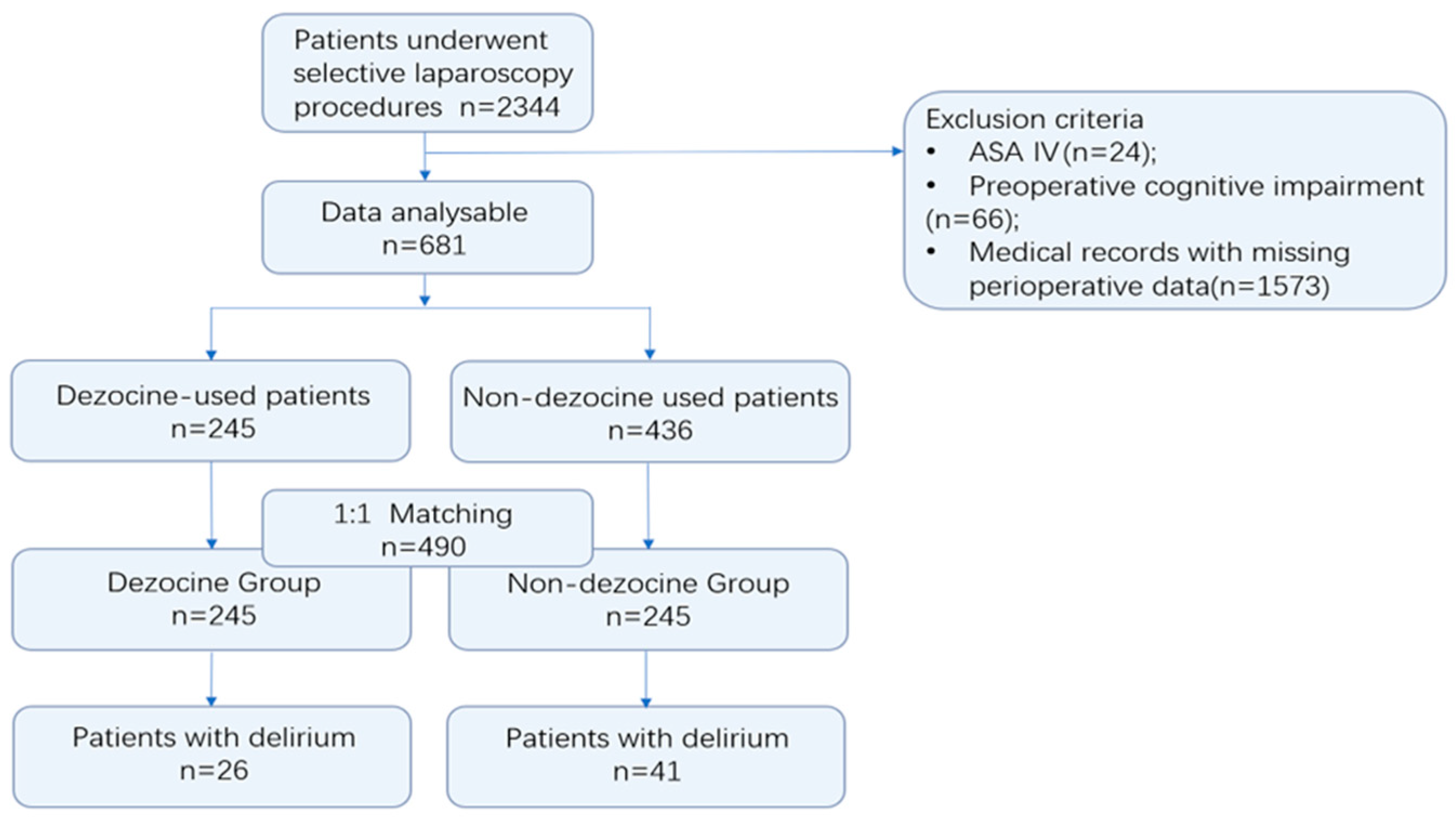
2.3. Participants
2.4. Variables
2.5. Data Sources/Measurement
2.6. Bias
2.7. Study Size
2.8. Quantitative Variables
2.9. Statistical Analysis
3. Results
3.1. Participants
3.2. Descriptive Data
3.3. Emergence Delirium Incidence
3.4. Other Outcomes
4. Discussion
4.1. Key Results
4.2. Interpretation
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desforges, J.F.; Lipowski, Z.J. Delirium in the Elderly Patient. N. Engl. J. Med. 1989, 320, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.I.; Cameron, D.J.; Fahs, M.C. A Prospective Study of Delirium and Prolonged Hospital Stay. Arch. Gen. Psychiatry 1988, 45, 937–940. [Google Scholar] [CrossRef]
- Liu, F.M.; Duan, M.M.; Fu, H.; Zhao, G.; Han, Y.; Lan, F.; Ahmed, Z.B.; Cao, G.; Li, Z.M.; Ma, D.M.; et al. Orthopedic Surgery Causes Gut Microbiome Dysbiosis and Intestinal Barrier Dysfunction in Prodromal Alzheimer Disease Patients. Ann. Surg. 2022, 276, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Makita, T.; Yamashita, H.; Matsunaga, S.; Akiyama, D.; Toba, K.; Hara, K.; Sumikawa, K.; Hara, T. Total intravenous anesthesia with propofol is associated with a lower rate of postoperative delirium in com-parison with sevoflurane anesthesia in elderly patients. J. Clin. Anesth. 2016, 33, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Kapila, A.K.; Watts, H.R.; Wang, T.; Ma, D. The Impact of Surgery and Anesthesia on Post-Operative Cognitive Decline and Alzheimer’s Disease Development: Biomarkers and Preventive Strategies. J. Alzheimer’s Dis. 2014, 41, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-F.; Su, X.; Meng, Z.-T.; Li, H.-L.; Wang, D.-X.; Li, X.-Y.; Maze, M.; Ma, D. Impact of Dexmedetomidine on Long-term Outcomes After Noncardiac Surgery in Elderly: 3-Year Follow-up of a Randomized Controlled Trial. Ann. Surg. 2019, 270, 356–363. [Google Scholar] [CrossRef]
- Vizcaychipi, M.P.; Watts, H.R.; O’Dea, K.P.; Lloyd, D.G.; Penn, J.W.; Wan, Y.; Pac-Soo, C.; Takata, M.; Ma, D. The Therapeutic Potential of Atorvastatin in a Mouse Model of Postoperative Cognitive Decline. Ann. Surg. 2014, 259, 1235–1244. [Google Scholar] [CrossRef]
- Ren, B.; Zong, J.; Tang, J.; Sun, D.; Hui, X.; Li, R.; Zhang, J.; Ji, Y. Effects of intravenous analgesia with combined dezocine and butorphanol on postoperative cognitive function in elderly patients. Genet. Mol. Res. 2015, 14, 5571–5576. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Gao, L.; Ji, X.; Zhai, D.; Song, H.; Wu, X.; Wang, L. Analgesic effect of preoperative dezocine-based local anesthesia in patients undergoing inguinal hernia repair. J. Int. Med. Res. 2018, 46, 4945–4951. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, J.; Xia, S.; Xu, H.; Wang, X. Dezocine Prevents Postoperative Hyperalgesia in Patients Undergoing Open Abdominal Surgery. Evid.-Based Complement. Altern. Med. 2015, 2015, 946194. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, L.; Wu, C.; Yan, L.; Wang, R.; Sun, B.; Wang, J. Efficacy and safety of different doses of dezocine for preemptive analgesia in gynecological laparoscopic surgeries: A prospective, double blind and randomized controlled clinical trial. Int. J. Surg. 2017, 37 (Suppl. S1), 539–545. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Liu, R.-C. Effects of dezocine on postoperative sore throat after maxillofacial procedures: A comparison with flurbiprofen axetil. Beijing Da Xue Xue Bao. Yi Xue Ban 2014, 46, 104–106. [Google Scholar] [PubMed]
- Zhou, L.; Zhang, Y.; Sun, H.; Hu, R.; Wang, J.; Xu, G. Effect of preemptive dezocine before general anesthesia on postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Medicine 2018, 97, e12533. [Google Scholar] [CrossRef] [PubMed]
- An, L.-J.; Zhang, Y.; Su, Z.; Zhang, X.-L.; Liu, H.-L.; Zhang, Z.-J.; Hu, J.-L.; Li, S.-T. A single dose of dezocine suppresses emergence agitation in preschool children anesthetized with sevoflurane-remifentanil. BMC Anesthesiol. 2017, 17, 154. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Sun, Y.; Liu, J. Effects of dezocine on PAED scale and Ramsay sedation scores in patients undergoing NUSS procedure. Am. J. Transl. Res. 2021, 13, 5468–5475. [Google Scholar]
- Ely, E.W.; Truman, B.; Shintani, A.; Thomason, J.W.W.; Wheeler, A.P.; Gordon, S.; Francis, J.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Monitoring Sedation Status Over Time in ICU Patients. JAMA 2003, 289, 2983–2991. [Google Scholar] [CrossRef] [Green Version]
- Lepousé, C.; Lautner, C.A.; Liu, L.; Gomis, P.; Leon, A. Emergence delirium in adults in the post-anaesthesia care unit. Br. J. Anaesth. 2006, 96, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, B.G.; Ingelmo, P.M.; Emre, S.; Meroni, V.; Minardi, C.; Frawley, G.; Benigni, A.; Di Marco, S.; Spotti, A.; Busi, I.; et al. Emergence delirium in children: A comparison of sevoflurane and desflurane anesthesia using the Pae-diatric Anesthesia Emergence Delirium scale. Paediatr. Anaesth. 2013, 23, 301–308. [Google Scholar] [CrossRef]
- Olympio, M.A. Postanesthetic delirium: Historical perspectives. J. Clin. Anesth. 1991, 3, 60–63. [Google Scholar] [CrossRef]
- First, M.B. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv. Ment. Dis. 2013, 201, 727–729. [Google Scholar] [CrossRef]
- Chan, M.T.; Cheng, B.C.; Lee, T.M.; Gin, T. BIS-guided Anesthesia Decreases Postoperative Delirium and Cognitive Decline. J. Neurosurg. Anesthesiol. 2013, 25, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Radtke, F.; Franck, M.; Lendner, J.; Krüger, S.; Wernecke, K.; Spies, C. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br. J. Anaesth. 2013, 110 (Suppl. S1), i98–i105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildes, T.S.; Mickle, A.M.; Abdallah, A.B.; Maybrier, H.R.; Oberhaus, J.; Budelier, T.P.; Kronzer, A.; McKinnon, S.L.; Park, D.; Torres, B.A.; et al. Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium Among Older Adults Undergoing Major Surgery: The ENGAGES Randomized Clinical Trial. JAMA 2019, 321, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Hu, J.; Ma, D. Postoperative delirium: Perioperative assessment, risk reduction, and management. Br. J. Anaesth. 2020, 125, 492–504. [Google Scholar] [CrossRef]
- Carr, G.V.; Bangasser, D.A.; Bethea, T.; Young, M.; Valentino, R.J.; Lucki, I. Antidepressant-Like Effects of κ-Opioid Receptor Antagonists in Wistar Kyoto Rats. Neuropsychopharmacology 2010, 35, 752–763. [Google Scholar] [CrossRef]
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J. Am. Geriatr. Soc. 2015, 63, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Abdellatif, A. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsil-lectomy: A comparison of dexmedetomidine and propofol. Saudi J. Anaesth. 2013, 7, 296–300. [Google Scholar]
- Yao, Y.; Qian, B.; Lin, Y.; Wu, W.; Ye, H.; Chen, Y. Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for lar-yngeal mask airway insertion and emergence delirium in children: A prospective, randomized, double-blind, place-bo-controlled trial. Paediatr. Anaesth. 2015, 25, 492–498. [Google Scholar] [CrossRef]
- Ibacache, M.E.; Muñoz, H.R.; Brandes, V.; Morales, A.L. Single-Dose Dexmedetomidine Reduces Agitation after Sevoflurane Anesthesia in Children. Obstet. Anesth. Dig. 2004, 98, 60–63. [Google Scholar] [CrossRef]
- Bong, C.L.; Lim, E.; Allen, J.C.; Choo, W.L.H.; Siow, Y.N.; Teo, P.B.Y.; Tan, J.S.K. A comparison of single-dose dexmedetomidine or propofol on the incidence of emergence delirium in children undergoing general anaesthesia for magnetic resonance imaging. Anaesthesia 2015, 70, 393–399. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Wang, J.; Sun, Y.; Zhang, G.; Liang, L. Comparison of the efficacy and safety between dezocine injection and morphine injection for persistence of pain in Chinese cancer patients: A meta-analysis. Biosci. Rep. 2017, 37, BSR20170243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childers, W.E.; Abou-Gharbia, M.A. “I’ll Be Back”: The Resurrection of Dezocine. ACS Med. Chem. Lett. 2021, 12, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-S.; Xu, G.-H.; Shen, Q.-Y.; Zhao, Q.; Cheng, X.-Q.; Zhang, J.; Gu, E.-W. Dezocine prevents sufentanil-induced cough during general anesthesia induction: A randomized controlled trial. Pharmacol. Rep. 2015, 67, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Yeh, C.-C.; Lee, M.-S.; Wu, C.-T.; Lin, S.-L.; Wong, C.-S. Prolonged Injection Time and Light Smoking Decrease the Incidence of Fentanyl-Induced Cough. Obstet. Anesth. Dig. 2005, 101, 670–674. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Wang, S.; Ren, Y.; Miao, C. Dezocine attenuates fentanyl-induced cough in a dose-dependent manner-a randomized controlled trial. Int. J. Clin. Exp. Med. 2015, 8, 6091–6096. [Google Scholar]
- Xiong, Z.; Yi, P.; Song, J.; Tan, M. Dezocine prevents sufentanil-induced cough during general anesthesia induction: A meta-analysis of randomised controlled trials. BMC Anesthesiol. 2020, 20, 154. [Google Scholar] [CrossRef]
- Dong, Y.; Chang, X. Comparison of Five Prophylactically Intravenous Drugs in Preventing Opioid-Induced Cough: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 3164. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.R. Pathoetiological Model of Delirium: A Comprehensive Understanding of the Neurobiology of Delirium and an Evidence-Based Approach to Prevention and Treatment. Crit. Care Clin. 2008, 24, 789–856. [Google Scholar] [CrossRef] [PubMed]
- Costi, D.; Cyna, A.M.; Ahmed, S.; Stephens, K.; Strickland, P.; Ellwood, J.; Larsson, J.N.; Chooi, C.; Burgoyne, L.; Middleton, P. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst. Rev. 2014, 9. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Wang, Z.; Zhang, Y.; Cui, Z.; Sun, C. Effect of Dezocine on Hemodynamic Indexes of Postoperative Patients With Traumatic Brain Injury (TBI)—A Pilot Study. Front. Pharmacol. 2022, 13, 665107. [Google Scholar] [CrossRef]
- Liu, M.R.; Huang, X.-P.; Yeliseev, A.; Xi, M.J.; Roth, M.B.L. Novel Molecular Targets of Dezocine and Their Clinical Implications. Anesthesiology 2014, 120, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Gillman, K.W.; Parker, M.F.; Silva, M.; Degnan, A.P.; Tora, G.O.; Lodge, N.J.; Li, Y.-W.; Lelas, S.; Taber, M.; Krause, R.G.; et al. Design, optimization, and in vivo evaluation of a series of pyridine derivatives with dual NK1 antagonism and SERT inhibition for the treatment of depression. Bioorganic Med. Chem. Lett. 2013, 23, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Chartoff, E.; Sawyer, A.; Rachlin, A.; Potter, D.; Pliakas, A.; Carlezon, W.A. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology 2012, 62, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oduyale, O.K.; Eltahir, A.A.; Stem, M.; Prince, E.; Zhang, G.Q.; Safar, B.; Efron, J.E.; Atallah, C. What Does a Diagnosis of Depression Mean for Patients Undergoing Colorectal Surgery? J. Surg. Res. 2021, 260, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Elsamadicy, A.A.; Adogwa, O.; Lydon, E.; Sergesketter, A.; Kaakati, R.; Mehta, A.I.; Vasquez, R.A.; Cheng, J.; Bagley, C.A.; Karikari, I.O. Depression as an independent predictor of postoperative delirium in spine deformity patients undergoing elective spine surgery. J. Neurosurg. Spine 2017, 27, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Falk, A.; Kåhlin, J.; Nymark, C.; Hultgren, R.; Stenman, M. Depression as a predictor of postoperative delirium after cardiac surgery: A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 371–379. [Google Scholar] [CrossRef]
| Categories | Mean ± SD and Median [IQR] and Number of Patients (%) |
|---|---|
| Demographics | |
| Age, year | 60 ± 7.3 |
| Gender (Female) | 371 (54.3%) |
| BMI, kg/m2 | 23.5 ± 3.2 |
| Charlson score | 0 (0–2) |
| Education, year | 9 (6–12) |
| Preoperative MMSE score | 29 (27–30) |
| Type of surgery | |
| Gastrointestinal surgery | 237 (34.8%) |
| Urogenital surgery | 246 (36.1%) |
| Hepatobiliary surgery | 137 (20.1%) |
| Others | 61 (9%) |
| Lesions (malignant) | 382 (56.1%) |
| Operation time a, min | 156 ± 84 |
| Anesthesia time b, min | 226 ± 98 |
| Patient Characteristics | Patient with ED n = 80 | Patient without ED n = 601 | p Value |
|---|---|---|---|
| Age, mean ± SD, Year | 61.4 ± 7.7 | 59.5 ± 7.0 | 0.047 a,* |
| Female, n (%) | 32(40%) | 294 (48.9%) | 0.153 b |
| BMI, mean ± SD, kg/m2 | 22.7 ± 3.1 | 23.8 ± 3.0 | 0.001 a,* |
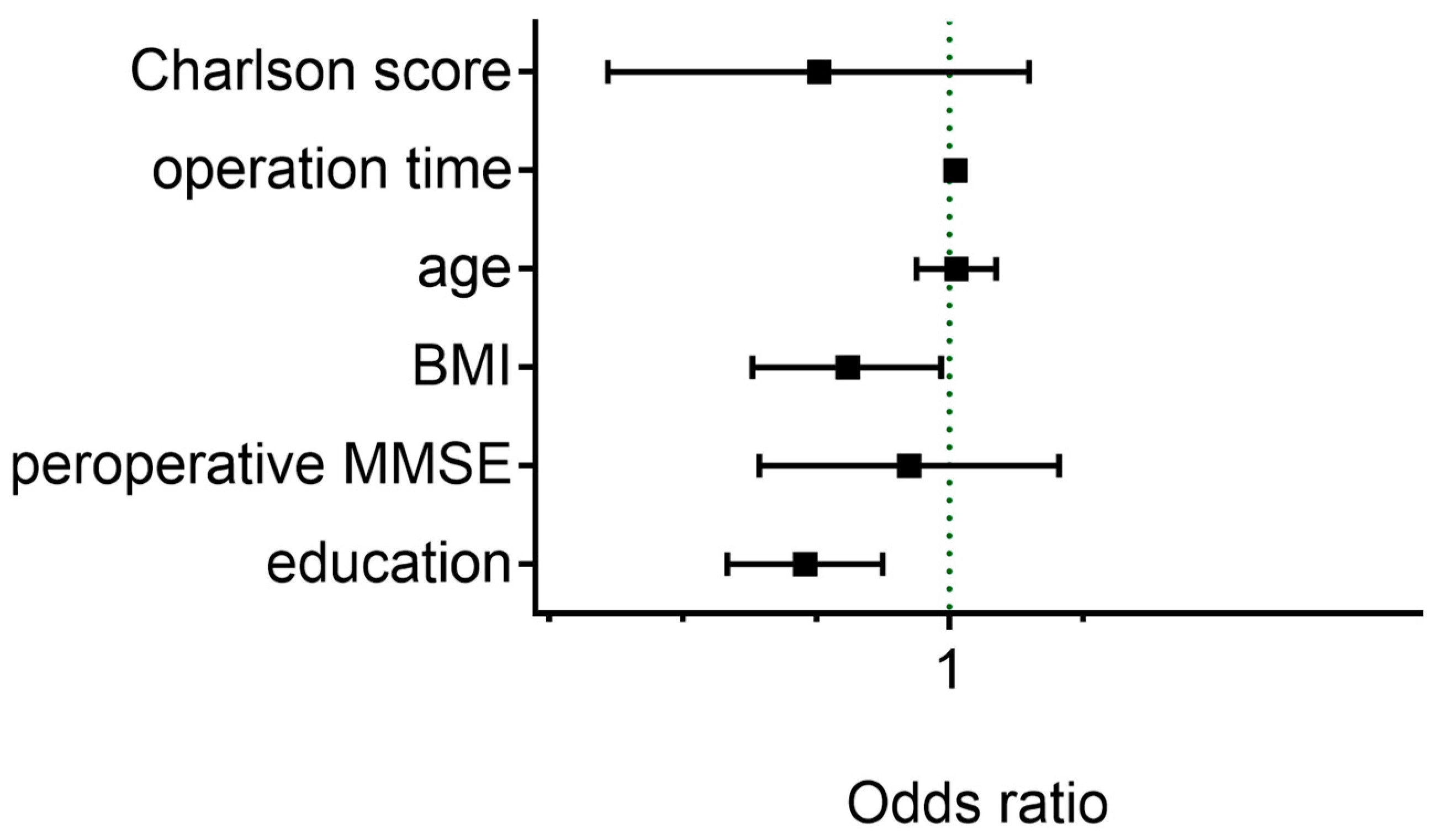
| Charlson score, median (IQR) | 2 (0–2) | 0 (0–2) | 0.039 a,* |
| Education, median (IQR), Year | 8 (5–9) | 9 (7–12) | <0.001 a,* |
| Preoperative MMSE score, median (IQR) | 28 (26–29) | 29 (28–30) | 0.026 a,* |
| Lesion (malignant), n (%) | 55 (68.8%) | 327 (54.4%) | 0.016 b,* |
| Operation time, mean ± SD, min | 197 ± 84 | 151 ± 82 | <0.001 a,* |
| Anesthesia time, mean ± SD, min | 279 ± 96 | 219 ± 96 | <0.001 a,* |
| Variable | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
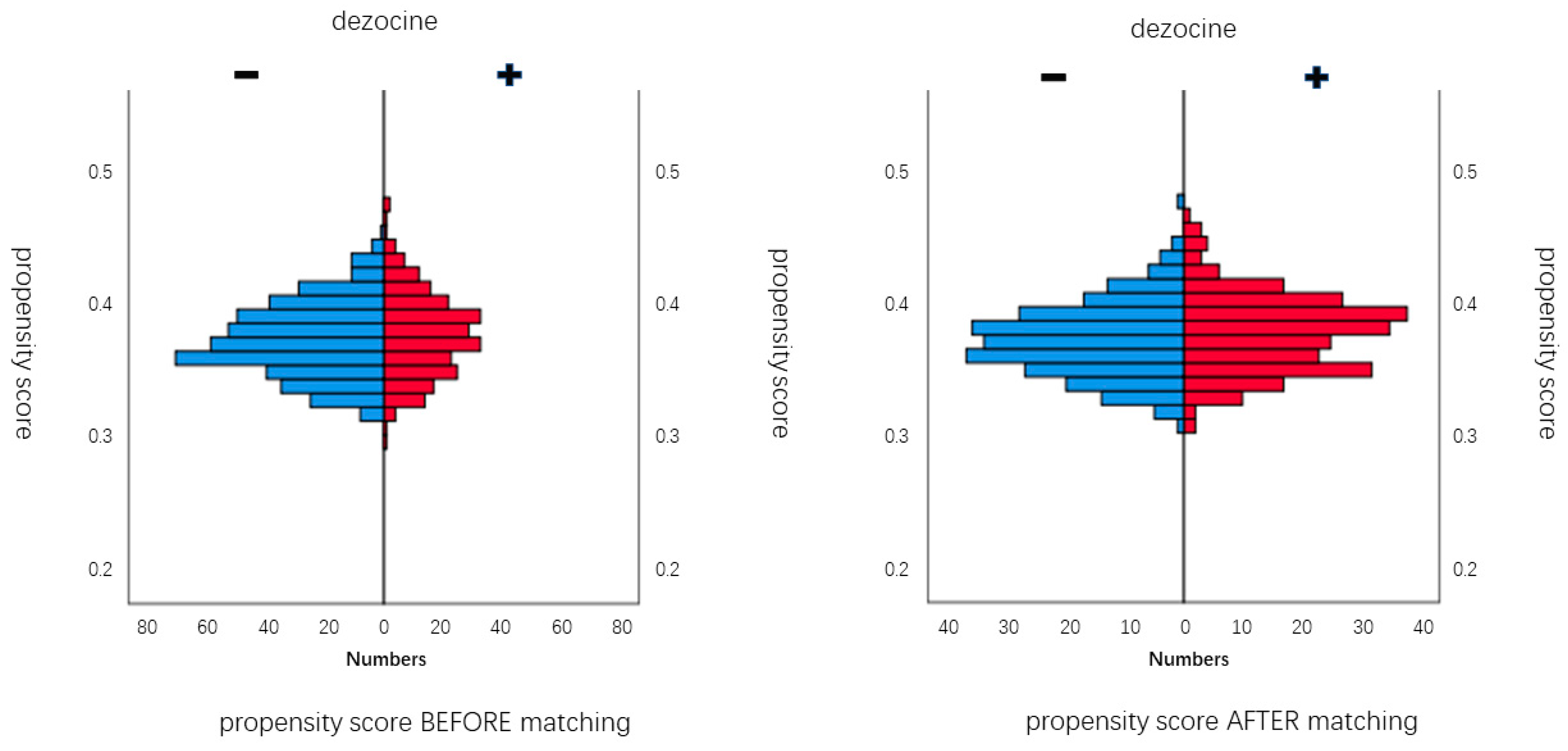
| Dezocine Group (n = 245) | Non-Dezocine Group (n = 436) | SMD a | Dezocine Group (n = 245) | Non-Dezocine Group (n = 245) | SMD | |
| Age, mean ± SD, year | 61.6 ± 7.3 | 59.2 ± 7.1 | 0.237 | 61.6 ± 7.3 | 60.6 ± 7.4 | 0.096 |
| Female, n (%) | 102 (41.6%) | 208 (47.7%) | −0.082 | 102 (41.6%) | 108 (44.1%) | 0.029 |
| BMI, mean ± SD, kg/m2 | 23.5 ± 3.3 | 23.4 ± 3.1 | 0.016 | 23.5 ± 3.3 | 23.0 ± 3.0 | 0.090 |
| Charlson score, median (IQR) | 2 (0–2) | 0 (0–2) | −0.224 | 2 (0–2) | 2 (0–2) | 0.004 |
| Education, median (IQR), Year | 9 (6–12) | 9 (7–12) | 0.045 | 9 (6–12) | 9 (6–12) | 0.061 |
| Preoperative MMSE score, median (IQR) | 28 (26–29) | 29 (28–30) | 0.286 | 28 (26–29) | 28 (27–29) | −0.014 |
| Type of surgery | ||||||
| Gastrointestinal surgery, n (%) | 99 (40.4%) | 138 (31.6%) | 0.038 | 99 (40.4%) | 107 (43.7%) | −0.007 |
| Urogenital surgery, n (%) | 90 (59.6%) | 156 (35.8%) | 90 (59.6%) | 81 (56.3%) | ||
| Hepatobiliary surgery, n (%) | 38 (15.5%) | 99 (22.7%) | 38 (15.5%) | 37 (15.1%) | ||
| Others, n (%) | 18 (0.07%) | 43 (0.1%) | 18 (0.07%) | 20 (0.08%) | ||
| Malignant, n (%) | 163 (66.5%) | 219 (50.2%) | 0.23 | 163 (66.5%) | 159 (64.9%) | 0.02 |
| Operation time b, mean ± SD, min | 172 ± 85 | 148 ± 82 | 0.202 | 172 ± 85 | 182 ± 87 | −0.082 |
| Anesthesia time c, mean ± SD, min | 242 ± 99 | 217 ± 96 | 0.182 | 242 ± 99 | 254 ± 105 | −0.082 |
| Outcomes | Dezocine Group (n = 245) | Non-Dezocine Group (n = 245) | p Value |
|---|---|---|---|
| Primary outcome | |||
| Emergency delirium, n (%) | 26 (10.6%) | 41 (16.7%) | 0.049 |
| Secondary outcome | |||
| VAS a, In PACU, median (IQR) | 3 (0–5) | 2 (0.5–4) | 0.34 |
| VAS a, 24 h after surgery, median (IQR) | 2 (1–4) | 2 (1–4) | 0.35 |
| RASS b, In PACU, median (IQR) | 0 (−1–0) | 0 (−1–0) | 0.226 |
| Nausea and vomiting, n (%) | 59 (24.1%) | 63 (25.7%) | 0.67 |
| Acute kidney injury, n (%) | 14 (5.7%) | 20 (8.2%) | 0.286 |
| MMSE, before discharge, median (IQR) | 28 (26–29) | 28 (26–29) | 0.93 |
| Hospital stay, median (IQR), days | 6 (3–8) | 6 (3–8) | 0.76 |
| Intensive care unit stay, n (%) | 4 (0.8%) | 7 (1.4%) | 0.360 |
| Dezocine Group (n = 245) | Non-Dezocine Group (n = 245) | p Value | |
|---|---|---|---|
| Intraoperative Data | |||
| PACU stay, median (IQR), min | 80 (60–120) | 80 (60–110) | 0.291 |
| Extubation time, median (IQR), min | 35 (25–50) | 35 (25–50) | 0.464 |
| Crystalloid, median (IQR), mL | 1100 (750–1600) | 1100 (750–1600) | 0.514 |
| Colloid, median (IQR), mL | 500 (0–500) | 500 (0–500) | 0.351 |
| Blood transfusion, n (%) | 9 (0.037%) | 18 (0.073%) | 0.075 |
| Intraoperative hemodynamic | |||
| Hypotension, n (%) | 100 (40.8%) | 86 (35%) | 0.456 |
| Vasopressor drugs, n (%) | 71 (30.0%) | 66 (27.0%) | 0.920 |
| Intraoperative Medications | |||
| Benzodiazepines, n (%) | 3 (0.122%) | 3 (0.122%) | 1.000 |
| Atropine, n (%) | 44 (18.0%) | 52 (21.2%) | 0.735 |
| Medication administered for anesthesia maintenance | |||
| Propofol, median (IQR), mg/kg/min | 0.14 (0.1–0.2) | 0.15 (0.9–0.2) | 0.314 |
| Remifentanil, median (IQR), µg/kg/min | 0.18 (0.13–0.26) | 0.19 (0.13–0.29) | 0.318 |
| Sufentanyl, median (IQR), µg/min | 0.39 (0.35–0.43) | 0.43 (0.35–0.43) | 0.883 |
| Sevoflurane, n (%) | 1 (0.004%) | 3 (0.122%) | 0.623 |
| Postoperative analgesia | |||
| PCA pumps, n (%) | 139 (56.7%) | 143 (58.4%) | 0.829 |
| multimodal analgesia, n (%) | 6 (0.024%) | 5 (0.02%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yi, Q.; Ye, C.; Luo, N.; Wang, E. Effects of Dezocine on the Reduction of Emergence Delirium after Laparoscopic Surgery: A Retrospective Propensity Score-Matched Cohort Study. J. Pers. Med. 2023, 13, 590. https://doi.org/10.3390/jpm13040590
Wang L, Yi Q, Ye C, Luo N, Wang E. Effects of Dezocine on the Reduction of Emergence Delirium after Laparoscopic Surgery: A Retrospective Propensity Score-Matched Cohort Study. Journal of Personalized Medicine. 2023; 13(4):590. https://doi.org/10.3390/jpm13040590
Chicago/Turabian StyleWang, Lu, Qiong Yi, Chunyan Ye, Ning Luo, and E Wang. 2023. "Effects of Dezocine on the Reduction of Emergence Delirium after Laparoscopic Surgery: A Retrospective Propensity Score-Matched Cohort Study" Journal of Personalized Medicine 13, no. 4: 590. https://doi.org/10.3390/jpm13040590
APA StyleWang, L., Yi, Q., Ye, C., Luo, N., & Wang, E. (2023). Effects of Dezocine on the Reduction of Emergence Delirium after Laparoscopic Surgery: A Retrospective Propensity Score-Matched Cohort Study. Journal of Personalized Medicine, 13(4), 590. https://doi.org/10.3390/jpm13040590





