Hepatic Venous Occlusion Type of Budd–Chiari Syndrome versus Pyrrolizidine Alkaloid-Induced Hepatic Sinusoidal Obstructive Syndrome: A Multi-Center Retrospective Study
Abstract
1. Introduction
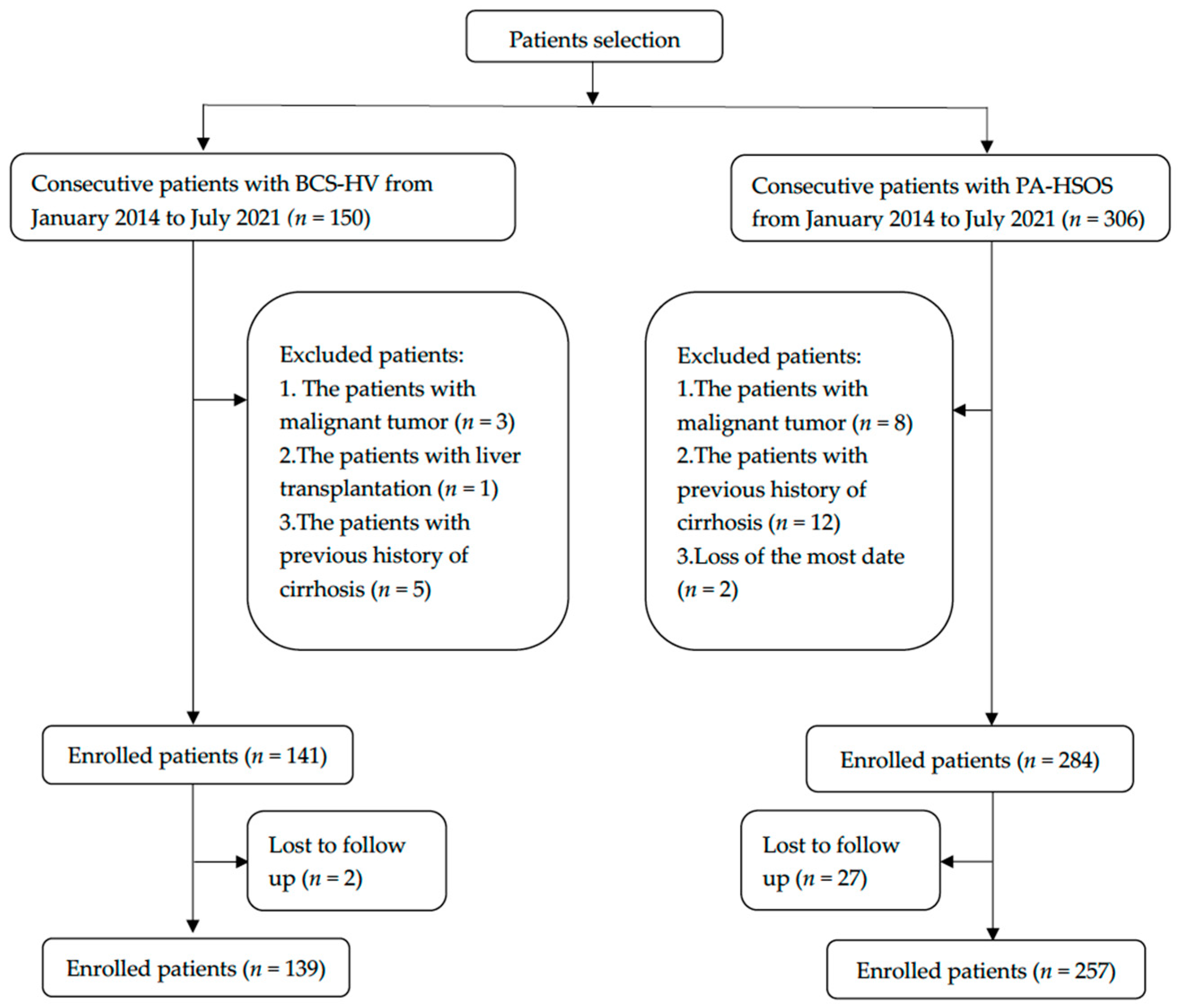
2. Materials and Methods
2.1. Clinical Data
2.2. Enhanced CT and MRI
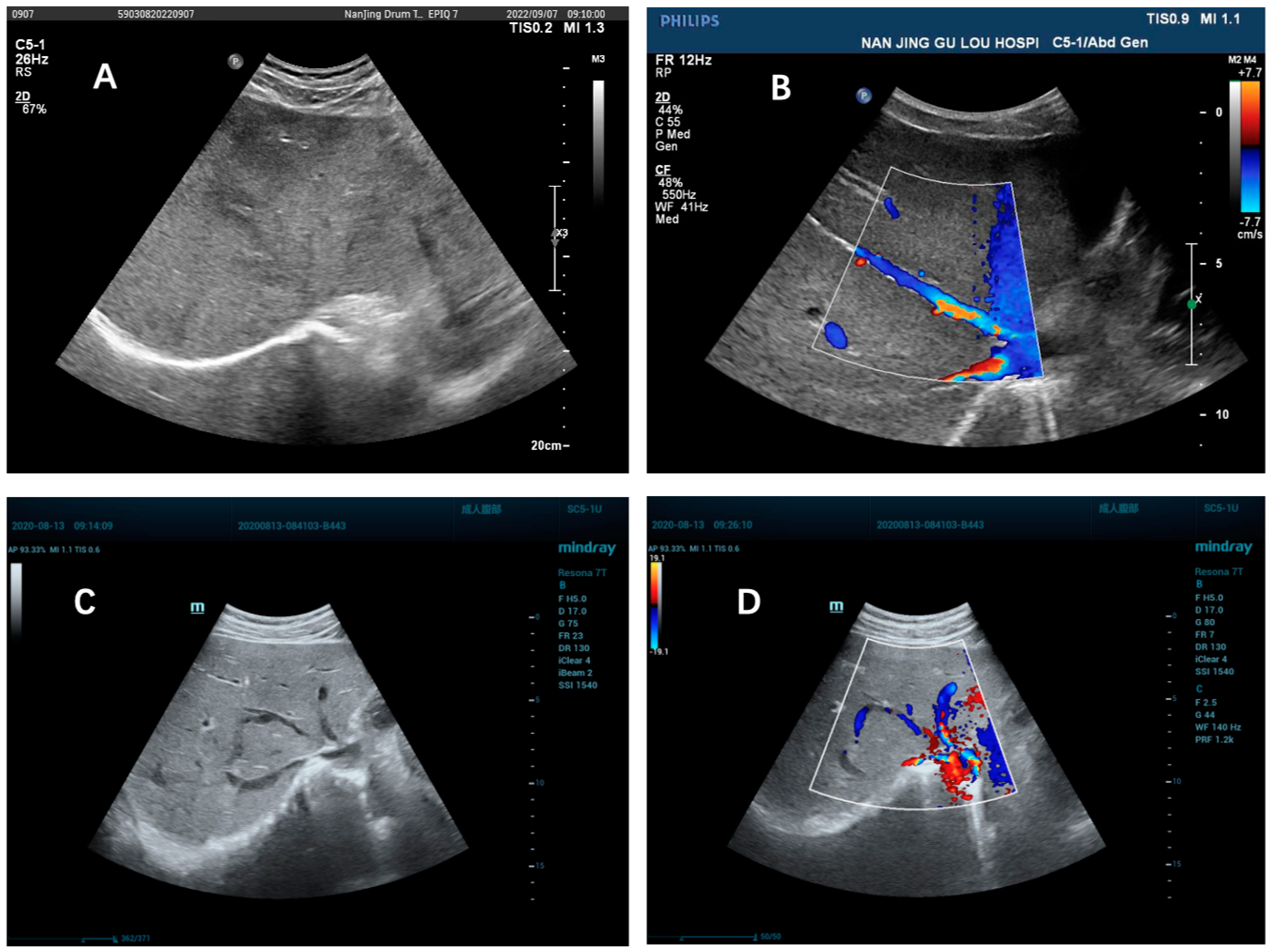
2.3. Doppler Ultrasonography
2.4. Digital Subtraction Angiography
2.5. Statistics
3. Results
3.1. Clinical Presentation
3.2. Laboratory Tests
3.3. Imaging Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J. Hepatol. 2016, 64, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, L.; Zhang, M.; Zhang, F.; Peng, C.; Zhang, B.; Chen, J.; Li, L.; He, J.; Xiao, J.; et al. Validation of the Nanjing Criteria for Diagnosing Pyrrolizidine Alkaloids-induced Hepatic Sinusoidal Obstruction Syndrome. J. Clin. Transl. Hepatol. 2021, 9, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, U.D.; Seren, S.; Bayraktar, Y. Hepatic venous outflow obstruction: Three similar syndromes. World J. Gastroenterol. 2007, 13, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Chojkier, M. Hepatic sinusoidal-obstruction syndrome: Toxicity of pyrrolizidine alkaloids. J. Hepatol. 2003, 39, 437–446. [Google Scholar] [CrossRef]
- Plessier, A.; Rautou, P.E.; Valla, D.C. Management of hepatic vascular diseases. J. Hepatol. 2012, 56 (Suppl. 1), S25–S38. [Google Scholar] [CrossRef]
- Northup, P.G.; Garcia-Pagan, J.C.; Garcia-Tsao, G.; Intagliata, N.M.; Superina, R.A.; Roberts, L.N.; Lisman, T.; Valla, D.C. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 366–413. [Google Scholar] [CrossRef]
- Dai, F.; Qiao, W.; Kang, Z.; Chen, Y.; Li, K.; Shen, W.; Zhang, X. Clinical Features and CT Imaging Analysis of Hepatic Sinuscase-Syndrome and Budd-Chiari Syndrome. Int. J. Gen. Med. 2022, 15, 2389–2396. [Google Scholar] [CrossRef]
- Liu, F.; Rong, X.; Guo, H.; Xu, D.; Liu, C.; Meng, L.; Yang, X.; Guo, T.; Kan, X.; Song, Y. Clinical characteristics, CT signs, and pathological findings of Pyrrolizidine alkaloids-induced sinusoidal obstructive syndrome: A retrospective study. BMC Gastroenterol. 2020, 20, 30. [Google Scholar] [CrossRef]
- Kan, X.; Ye, J.; Rong, X.; Lu, Z.; Li, X.; Wang, Y.; Yang, L.; Xu, K.; Song, Y.; Hou, X. Diagnostic performance of Contrast-enhanced CT in Pyrrolizidine Alkaloids-induced Hepatic Sinusoidal Obstructive Syndrome. Sci. Rep. 2016, 6, 37998. [Google Scholar] [CrossRef]
- Zhuge, Y.Z.; Wang, Y.; Zhang, F.; Zhu, C.K.; Zhang, W.; Zhang, M.; He, Q.; Yang, J.; He, J.; Chen, J.; et al. Clinical characteristics and treatment of pyrrolizidine alkaloid-related hepatic vein occlusive disease. Liver Int. 2018, 38, 1867–1874. [Google Scholar] [CrossRef]
- Sharma, A.; Keshava, S.N.; Eapen, A.; Elias, E.; Eapen, C.E. An Update on the Management of Budd-Chiari Syndrome. Dig. Dis. Sci. 2021, 66, 1780–1790. [Google Scholar] [CrossRef]
- Zu, M.; Xu, K.; Interventional Division of Radiology Society of Chinese Medical Association. Experts Consensus on Chinese Nomenclature of Budd-Chiari Syndrome Interventional Division of Radiology Society of Chinese Medical Association. J. Interv. Med. 2021, 4, 114–116. [Google Scholar] [PubMed]
- Zhuge, Y.; Liu, Y.; Xie, W.; Zou, X.; Xu, J.; Wang, J. Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J. Gastroenterol. Hepatol. 2019, 34, 634–642. [Google Scholar] [CrossRef]
- Adebayo, D.; Neong, S.F.; Wong, F. Refractory Ascites in Liver Cirrhosis. Am. J. Gastroenterol. 2019, 114, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.P.; Palaniyappan, N.; China, L.; Härmälä, S.; Macken, L.; Ryan, J.M.; Wilkes, E.A.; Moore, K.; Leithead, J.A.; Hayes, P.C.; et al. Guidelines on the management of ascites in cirrhosis. Gut 2021, 70, 9–29. [Google Scholar] [CrossRef]
- Shukla, A.; Shreshtha, A.; Mukund, A.; Bihari, C.; Eapen, C.E.; Han, G.; Deshmukh, H.; Cua, I.H.Y.; Lesmana, C.R.A.; Al Meshtab, M.; et al. Budd-Chiari syndrome: Consensus guidance of the Asian Pacific Association for the study of the liver (APASL). Hepatol. Int. 2021, 15, 531–567. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Nevens, F. Budd-Chiari syndrome. United Eur. Gastroenterol. J. 2015, 3, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Van Wettere, M.; Bruno, O.; Rautou, P.E.; Vilgrain, V.; Ronot, M. Diagnosis of Budd-Chiari syndrome. Abdom. Radiol. 2018, 43, 1896–1907. [Google Scholar] [CrossRef]
- Lu, X.; Ma, X.; Xu, K.; Wang, J.; Yang, C. Inferior vena cava obstruction in Budd-Chiari syndrome: A comparative study of rapid quantitative phase-contrast MRI and MRV. Abdom. Radiol. 2020, 45, 1069–1074. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Zheng, Z.; Liu, Y.; Li, Y.; Hu, H.; Wang, W. Budd-Chiari Syndrome: The “inferior vena cava reverse-flow” sign and “jet-blood” sign on CT and MRI. Eur. J. Radiol. 2020, 132, 109288. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016, 51, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Yakushijin, K.; Atsuta, Y.; Doki, N.; Yokota, A.; Kanamori, H.; Miyamoto, T.; Ohwada, C.; Miyamura, K.; Nawa, Y.; Kurokawa, M.; et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant. 2016, 51, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Dignan, F.L.; Wynn, R.F.; Hadzic, N.; Karani, J.; Quaglia, A.; Pagliuca, A.; Veys, P.; Potter, M.N. BCSH/BSBMT guideline: Diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br. J. Haematol. 2013, 163, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, L.; Toma, L.; Mercan-Stanciu, A.; Grumeza, M.; Dodot, M.; Isac, T.; Ioanitescu, S. Budd-Chiari syndrome—Various etiologies and imagistic findings: A pictorial review. Med. Ultrason. 2019, 21, 344–348. [Google Scholar] [CrossRef]
- Yang, X.Q.; Ye, J.; Li, X.; Li, Q.; Song, Y.-H. Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis, treatment, and outcomes. World J. Gastroenterol. 2019, 25, 3753–3763. [Google Scholar] [CrossRef]
- Qi, X.; Wu, F.; Ren, W.; He, C.; Yin, Z.; Niu, J.; Bai, M.; Yang, Z.; Wu, K.; Fan, D.; et al. Thrombotic risk factors in Chinese Budd-Chiari syndrome patients: An observational study with a systematic review of the literature. Thromb. Haemost. 2013, 109, 878–884. [Google Scholar]
- Denninger, M.H.; Chaït, Y.; Casadevall, N.; Hillaire, S.; Guillin, M.-C.; Bezeaud, A.; Erlinger, S.; Briere, J.; Valla, D. Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors. Hepatology 2000, 31, 587–591. [Google Scholar] [CrossRef]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef]
- Larrey, D.; Meunier, L.; Valla, D.; Hillaire, S.; Hernandez-Gea, V.; Dutheil, D.; Plessier, A.; Bureau, C. Drug induced liver injury and vascular liver disease. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 471–479. [Google Scholar] [CrossRef]
- Aydinli, M.; Bayraktar, Y. Budd-Chiari syndrome: Etiology, pathogenesis and diagnosis. World J. Gastroenterol. 2007, 13, 2693–2696. [Google Scholar] [CrossRef]
- Xu, Z.M.; Zhuge, Y.Z.; Xu, T.S. Raise the recognition of hepatic veno- occlusive disease induced by chrysanthemum-like groundsel. Chin. J. Gastroenterol. 2009, 14, 577–579. (In Chinese) [Google Scholar]
- Intagliata, N.M.; Caldwell, S.H.; Tripodi, A. Diagnosis, Development, and Treatment of Portal Vein Thrombosis in Patients with and without Cirrhosis. Gastroenterology 2019, 156, 1582–1599.e1. [Google Scholar] [CrossRef] [PubMed]
- Senzolo, M.; Garcia-Tsao, G.; García-Pagán, J.C. Current knowledge and management of portal vein thrombosis in cirrhosis. J. Hepatol. 2021, 75, 442–453. [Google Scholar] [CrossRef] [PubMed]

| Variables | BCS-HV (n = 139) | PA-HSOS (n = 257) | p Value |
|---|---|---|---|
| Age (year, mean ± SD) | 43.00 ± 13.08 | 62.75 ± 9.68 | <0.001 |
| Male, n (%) | 67 (48.20) | 132 (51.40) | 0.549 |
| Course of disease (months, m (Q1~Q3)) | 3 (1.00–24.00) | 1.00 (0.67–2.00) | <0.001 |
| ≤6 months, n (%) | 85 (61.20) | 249 (96.90) | <0.001 |
| Abdominal distension, n (%) | 89 (64.00) | 257 (100) | <0.001 |
| Abdominal pain, n (%) | 24 (17.30) | 68 (26.10) | 0.047 |
| Ascites | 97 (69.78) | 256 (99.60) | <0.001 |
| None, n (%) | 43 (31.20) | 1 (0.40) | <0.001 |
| Mild, n (%) | 37 (26.80) | 11 (4.30) | <0.001 |
| Moderate to severe, n (%) | 58 (42.00) | 245 (95.30) | <0.001 |
| Varices of abdominal wall, n (%) | 13 (9.40) | 11 (4.30) | 0.044 |
| Jaundice, n (%) | 37 (26.60) | 115 (44.70) | <0.001 |
| Lower extremity edema, n (%) | 28 (20.10) | 66 (25.70) | 0.217 |
| History of PA intake, n (%) | 0 | 210(81.71) | <0.001 |
| Variables | BCS-HV (n = 139) | PA-HSOS (n = 257) | p Value |
|---|---|---|---|
| WBC count, (×109/L, m (Q1~Q3)) | 4.70 (3.20–6.32) | 6.00 (4.80–7.90) | <0.001 |
| HB (g/L, mean ± SD) | 120.14 ± 29.92 | 143.78 ± 20.13 | <0.001 |
| PLT count, (×1012/L, m (Q1~Q3)) | 117.00 (78.00–185.00) | 101.00 (72.00–134.50) | 0.001 |
| ALT (U/L, m (Q1~Q3)) | 23.00 (16.00–32.00) | 48.80 (25.15–101.85) | <0.001 |
| AST (U/L, m (Q1~Q3)) | 28.00 (21.00–40.00) | 64.10 (40.00–107.45) | <0.001 |
| ALP (U/L, m (Q1~Q3)) | 111.00 (78.00–149.00) | 128.80 (97.40–174.05) | 0.001 |
| Albumin (g/L, mean ± SD) | 36.45 ± 5.64 | 32.70 ± 3.52 | <0.001 |
| TBil (μmoI/L, m (Q1~Q3)) | 26.20 (16.06–45.33) | 37.60 (25.40–55.65) | <0.001 |
| SCr (μmoI/L, m (Q1~Q3)) | 57.00 (50.50–68.60) | 72.00 (59.00–88.50) | <0.001 |
| PT (s, m (Q1~Q3)) | 13.90 (12.50–15.90) | 15.20 (13.70–17.55) | <0.001 |
| APTT (s, m (Q1~Q3)) | 38.70 (31.50–62.00) | 35.00 (30.15–43.95) | 0.001 |
| INR (m (Q1~Q3)) | 1.21 (1.09–1.37) | 1.31 (1.19–1.52) | <0.001 |
| Fib (g/L, m (Q1~Q3)) | 2.42 (2.00–2.86) | 2.10 (1.70–2.70) | <0.001 |
| Child–Pugh scores (mean ± SD) | 7.43 ± 1.98 | 9.15 ± 1.48 | <0.001 |
| Child–Pugh class | |||
| A, n (%) | 51 (36.70%) | 2 (0.80%) | |
| B, n (%) | 65 (46.80%) | 152 (59.10%) | |
| C, n (%) | 23 (16.50%) | 103 (40.10%) |
| Variables | BCS-HV | PA-HSOS | p Value |
|---|---|---|---|
| Portal vein thrombosis, % (n/N) | 4.4% (6/136) | 11.70%(30/257) | 0.018 |
| Localized stenosis or occlusion of the hepatic veins, % (n/N) | 100.00% (130/130) | 1.20% (3/256) | <0.001 |
| Imaging manifestations of cirrhosis, % (n/N) | 47.10% (65/138) | 2.00% (5/256) | <0.001 |
| Hepatomegaly, % (n/N) | 56.20% (73/130) | 67.10% (163/243) | 0.037 |
| Splenomegaly, % (n/N) | 63.80% (78/130) | 16.10% (39/242) | <0.001 |
| Collateral circulation of hepatic veins, % (n/N) | 73.90% (99/134) | 0 | <0.001 |
| Diameter of left hepatic vein, (cm, mean ± SD, n/N) | 0.70 (0.50~1.09) (78/134) | 0.39 (0.30~0.45) (156/160) | <0.001 |
| Diameter of middle hepatic vein, (cm, mean ± SD, n/N) | 0.70 (0.50~1.10) (70/134) | 0.42 (0.35~0.49) (160/160) | <0.001 |
| Diameter of right hepatic vein, (cm, mean ± SD, n/N) | 0.80 (0.50~1.10) (54/134) | 0.42 (0.36~0.49) (159/160) | <0.001 |
| Diameter of portal vein, (cm, mean ± SD, n/N) | 1.19 ± 0.27 (112/136) | 1.00 ± 0.16 (243/257) | <0.001 |
| Portal vein blood flow velocity, (cm/s, mean ± SD, n/N) | 21.84 ± 9.22 (54/136) | 15.74 ± 7.99 (238/238) | <0.001 |
| Diameter of splenic vein, (cm, mean ± SD, n/N) | 0.89 ± 0.29 (72/136) | 0.60 ± 0.12 (230/230) | 0.002 |
| Splenic vein blood flow velocity, (cm/s, mean ± SD, n/N) | 18.78 ± 9.42 (30/136) | 13.47 ± 6.26 (230/230) | 0.001 |
| Patchy liver enhancement, % (n/N) | 78.46% (102/130) | 92.66% (164/177) | <0.001 |
| Enlarged caudate lobe of the liver, n/N (%) | 47.70% (62/130) | 0 | <0.001 |
| Early strengthening nodules in the liver, n/N (%) | 8.50% (11/129) | 0 | <0.001 |
| Narrowed inferior vena cava, n/N (%) | 55.20% (69/125) | 44.20% (80/177) | 0.087 |
| Esophageal varicose veins, n/N (%) | 64.00% (80/125) | 21.20% (38/179) | <0.001 |
| Variables | BCS-HV | PA-HSOS | ||||
|---|---|---|---|---|---|---|
| DUS | CT/MRI | p Value | DUS | CT/MRI | p Value | |
| Hepatic venous stenosis or occlusion, % (n/N) | 86.29% (107/124) | 4.55% (5/110) | <0.001 | 1.88% (3/160) | 2.83% (5/177) | 0.726 |
| Unclear display of hepatic vein, % (n/N) | 12.90% (16/124) | 94.55% (104/110) | <0.001 | 0.62% (1/160) | 97.18% (172/177) | <0.001 |
| Collateral circulation of hepatic veins, % (n/N) | 70.97% (88/124) | 4.55% (5/110) | <0.001 | 0 | 0 | / |
| Enlarged caudate lobe, % (n/N) | / | 47.27% (52/110) | / | / | 0 | / |
| Early strengthening nodules, % (n/N) | / | 8.18% (9/110) | / | / | 0 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Zhang, M.; Qi, Z.; Wu, W.; Chen, J.; He, F.; Han, H.; Ding, P.; Wang, G.; Zhuge, Y. Hepatic Venous Occlusion Type of Budd–Chiari Syndrome versus Pyrrolizidine Alkaloid-Induced Hepatic Sinusoidal Obstructive Syndrome: A Multi-Center Retrospective Study. J. Pers. Med. 2023, 13, 603. https://doi.org/10.3390/jpm13040603
Tong Y, Zhang M, Qi Z, Wu W, Chen J, He F, Han H, Ding P, Wang G, Zhuge Y. Hepatic Venous Occlusion Type of Budd–Chiari Syndrome versus Pyrrolizidine Alkaloid-Induced Hepatic Sinusoidal Obstructive Syndrome: A Multi-Center Retrospective Study. Journal of Personalized Medicine. 2023; 13(4):603. https://doi.org/10.3390/jpm13040603
Chicago/Turabian StyleTong, Yaru, Ming Zhang, Zexue Qi, Wei Wu, Jinjun Chen, Fuliang He, Hao Han, Pengxu Ding, Guangchuan Wang, and Yuzheng Zhuge. 2023. "Hepatic Venous Occlusion Type of Budd–Chiari Syndrome versus Pyrrolizidine Alkaloid-Induced Hepatic Sinusoidal Obstructive Syndrome: A Multi-Center Retrospective Study" Journal of Personalized Medicine 13, no. 4: 603. https://doi.org/10.3390/jpm13040603
APA StyleTong, Y., Zhang, M., Qi, Z., Wu, W., Chen, J., He, F., Han, H., Ding, P., Wang, G., & Zhuge, Y. (2023). Hepatic Venous Occlusion Type of Budd–Chiari Syndrome versus Pyrrolizidine Alkaloid-Induced Hepatic Sinusoidal Obstructive Syndrome: A Multi-Center Retrospective Study. Journal of Personalized Medicine, 13(4), 603. https://doi.org/10.3390/jpm13040603







