Abstract
Asthma and COPD have characteristic symptoms, yet patients with both are prevalent. Despite this, there is currently no globally accepted definition for the overlap between asthma and COPD, commonly referred to as asthma–COPD overlap (ACO). Generally, ACO is not considered a distinct disease or symptom from either clinical or mechanistic perspectives. However, identifying patients who present with both conditions is crucial for guiding clinical therapy. Similar to asthma and COPD, ACO patients are heterogeneous and presumably have multiple underlying disease processes. The variability of ACO patients led to the establishment of multiple definitions describing the condition’s essential clinical, physiological, and molecular characteristics. ACO comprises numerous phenotypes, which affects the optimal medication choice and can serve as a predictor of disease prognosis. Various phenotypes of ACO have been suggested based on host factors including but not limited to demographics, symptoms, spirometric findings, smoking history, and underlying airway inflammation. This review provides a comprehensive clinical guide for ACO patients to be used in clinical practice based on the available limited data. Future longitudinal studies must evaluate the stability of ACO phenotypes over time and explore their predictive powers to facilitate a more precise and effective management approach.
1. Introduction
Asthma and chronic obstructive pulmonary disease (COPD) have unique clinical features. Nevertheless, these clinical features can coexist, making it difficult to distinguish between the two conditions in a clinical setting. As a result, the term “asthma–COPD overlap” (ACO) was introduced to describe a group of clinical characteristics rather than a single entity [1,2]. Based on clinical and molecular perspectives, ACO is unlikely to be a single disease or syndrome, but rather a complex interaction and combination of different pathophysiological mechanisms. Identifying patients with ACO is crucial to guiding clinical care. Given the multiple phenotypes associated with ACO, it is challenging to create a single definition. Various societies and researchers have attempted to define ACO by utilizing clinical characteristics [3,4], spirometry [5], or both [6]. Nonetheless, there is currently no globally accepted definition of ACO.
Most proposed definitions of ACO require the patient to be over 40, have airflow limitation, and have asthma or partial bronchodilator (BD) reversibility [7]. The term ACO is favored over ACOS (ACO syndrome) since there is no singular disease or syndrome.
In 2015, the Global Initiative for Asthma (GINA) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) issued a collaborative statement defining what was formerly known as ACOS. They defined ACOS as the presence of persistent airflow limitation with characteristics commonly associated with both asthma and COPD [8]. Hence, in clinical practice, ACOS is identified by the overlapping features it shares with both asthma and COPD.
Then in 2017, the American Thoracic Society (ATS) and the National Heart, Lung, and Blood Institute (NHLBI) collaborated on an ACO workshop report. The panel concluded that ACO, like asthma and COPD, does not represent a singular unique disease entity [1]. Instead, ACO should be utilized to describe patients with a prolonged history of asthma, a modest smoking history, and fixed-airflow obstruction. Concurrently, those who exhibit asthma-like symptoms, such as peripheral eosinophilia and bronchodilator responsiveness, may have ACO. While both patient groups are clinically and likely pathologically unique, they are both within the purview of ACO.
Other entities and groups have suggested further major and minor characteristics that may aid in identifying patients with ACO, as detailed in Table 1 [8,9,10,11,12,13,14]. At the same time, the 2020 GOLD Strategy update dismissed the term “asthma–COPD overlap” because asthma and COPD are distinct illnesses that may have overlapping characteristics, such as eosinophilia or a degree of reversibility [3]. Furthermore, GOLD indorses that concurrent diagnoses of asthma and COPD may happen in a single patient, and in such cases, that treatment should generally adhere to asthma guidelines. However, COPD-specific therapeutic methods may be necessary for some individuals.

Table 1.
Clinical features of asthma, COPD, and ACO.
This review provides a comprehensive clinical guide for ACO patients to be used in clinical practice based on the available limited data. This review covers an overview of the epidemiology, pathogenesis, and diagnosis, followed by non-pharmacological and pharmacological treatment options and future directions.
2. Epidemiology
The lack of a standardized definition for ACO makes it challenging to estimate its disease burden accurately. According to self-reported physician diagnosis or a combination of both spirometry and symptom reporting, the prevalence of ACO in the overall population ranges from 2 to 3 percent, whereas the prevalence of asthma and COPD ranges from 5 to 17 percent and 2 to 12 percent, respectively [7]. However, in COPD patients, the prevalence of ACO may be as high as 25 percent [15], and in asthma patients, the estimated prevalence of ACO ranges from 10 to 31 percent [7].
Studies indicate that individuals with ACO are more likely to be female, have a higher body mass index, and possess lower education and socioeconomic levels than those with COPD. However, there is considerable variety within this patient population [16,17,18,19]. The data concerning the outcomes of patients with ACO compared to those with either asthma or COPD without overlap are varied, possibly due to the heterogeneity of patients included in the ACO umbrella term. For example, such variation can be observed in a population-based cohort study that followed patients for a median of nine years. In this study, patients with ACO and patients with COPD had comparable higher risks of exacerbations and all-cause mortality than those of symptomatic smokers without COPD [20]. In contrast, other studies suggest that disease control in ACO individuals may be worse than in those with asthma or COPD alone, especially regarding lung function, exacerbation rates, and symptoms [21]. Furthermore, patients with ACO have a similar risk of developing lung cancer as those with COPD, and a higher risk than other groups of smokers [22].
3. Pathogenesis
Whether ACO is caused by a unique pathogenic mechanism or by asthma and COPD coexisting in the same patient is a common question. Certainly, even the pathophysiology of COPD is contested. Likewise, the pathogenic processes that underlie asthma are diverse.
The Dutch hypothesis proposes that asthma and COPD are caused by a single disease entity, however the clinical phenotype is influenced by various factors such as genetics and environmental exposures [23,24]. Instead, the British hypothesis proposes that asthma and COPD have independent origins, each with its own characteristic inflammatory causes, such as allergic inflammation in asthma and persistent bacterial infection in COPD [25]. According to the Dutch hypothesis, ACO would fall on the same continuous spectrum as asthma and COPD. However, according to the British hypothesis, different causes would drive distinctive inflammation, distinguishing ACO from asthma and COPD.
Hypothetically, it is easy to recognize how smoking tobacco can increase neutrophilic inflammation, cause fixed-airflow limitation, and ultimately lead to COPD in patients with asthma [26]. Conversely, a patient without asthma who is atopic “sensitive to allergens” may initially develop COPD, but eventually exhibit airway hyperresponsiveness and type 2-mediated airway inflammation. Allergen sensitivity has also been reported in older patients with COPD [27]. Given the variability in the clinical characteristics of ACO patients, it is likely that the primary inflammatory pathway differs among individuals.
The risk factors that cause ACO are crucial. Smoking, age, and airway inflammation are risk factors for greater lung function declines and permanent airway obstruction in asthma patients later in life. Similarly, COPD patients may exhibit eosinophilia or elevated BD reactivity.
The interaction between an organism’s genome and its environment produces its phenotype [28,29]. Phenotypes are categorized based on factors such as the age of onset, triggers, and therapeutic or inflammatory responses such as eosinophilic or neutrophilic [29]. However, phenotypes alone are insufficient for identifying the underlying pathway of diseases. In addition, disease mechanisms that are functional or pathobiological are referred to as endotypes [28,29]. Molecular phenotyping can help identify causal pathways and improve patient outcomes [28]. Ultimately, endotypes are established using molecular phenotyping.
4. Respiratory Microbiome and ACO during Stable and Exacerbation States
Different respiratory pathogens play essential roles in the exacerbations of asthma and COPD [30,31,32,33]. ACO patients’ outcomes differ from those of asthma or COPD patients without overlap, possibly due to this group’s wide diversity of individuals. ACO patients are often excluded from research studies for asthma and COPD. Consequently, large-scale trials that analyze critical patient outcomes in well-defined ACO populations are required. The respiratory microbiome could be a promising biomarker in ACO, facilitating personalized medicine implementation.
In 2023, we published the first investigation in the literature which characterized ACO patients’ bacterial respiratory microbiome in both stable and exacerbation states [34]. The study utilized Next Generation Sequencing (NGS) to reveal the composition of the pulmonary microbiome and detect bacteria that may have been missed by traditional antimicrobial therapy due to cultural challenges. In this study, the lower airways of clinically stable ACO patients were found to have bacterial colonization, which makes it challenging to establish the role of bacteria in exacerbation. The examination also revealed significant differences between the study groups [34].
In the exacerbation condition, the taxonomic richness of bacteria decreased, and the evenness of microbiota increased compared to stable states in ACO patients. This shift in the airway bacterial population suggests that significant pathogens may replace most of the airway microbiome during an exacerbation, decreasing microbial richness [34]. The Prevotella genus was found to be substantially more abundant in exacerbation samples than in stable samples, suggesting that some species of Prevotella may contribute to ACO pathogenicity [34].
Moreover, high-throughput Illumina MiSeq, used in this recent work [34], revealed a large amount of bacterial variety in sputum. Similar to earlier COPD investigations utilizing culture techniques, this study found substantial abundances of the bacterial aerobic genera Streptococcus, Haemophilus, Pseudomonas, and Moraxella [35,36]. The investigation also detected high frequencies of Prevotella, Veillonella, and Actinomyces anaerobes, suggesting a rich spectrum of taxa. The composition of microbial communities in the airways differed from one patient to another. It is worth mentioning that the community structures specific to each patient, which showed a predominance of one or a few microbial genera, were in line with previous studies on COPD patients [32,37,38]. These studies showed that a particular signature persists throughout time.
An improved understanding of this association could lead to the development of more precise antibiotic therapies that can effectively target the increased number and activity of harmful bacteria, especially during exacerbations. This would enhance clinical effectiveness and improve patient outcomes.
The bacterial microbiome is influenced by various host and pathogen factors [32,39]. Further research is needed to understand the ACO bacterial microbiome, including its development, exacerbations, and treatments such as antibiotics and inhaled corticosteroids, which can alter bacteria in the stable airways of ACO patients. Bacteria can impact the progression of ACO disease and exacerbations. Moreover, antimicrobial intervention studies may be required if bacteria are found to cause unfavorable outcomes in stable ACO patients.
5. Clinical Presentation and Diagnosis
ACO is a condition characterized by substantial clinical variations amongst diverse subgroups or phenotypes [40]. Clinical manifestations that have been described for ACO are included in Table 1 and Table 2. The clinical term ACO has been employed for patients who display symptoms of both asthma and COPD. Nevertheless, a clear set of features that establishes a definitive diagnosis of ACO has not been universally agreed upon [1]. Despite the lack of a widely accepted definition, GINA acknowledges several characteristics that support an ACO diagnosis.

Table 2.
Proposed ACO diagnostic criteria according to previous ten years’ studies.
Additional airway illnesses, for example bronchiectasis, obliterative bronchiolitis, and central airway obstruction, are included in the differential diagnosis of ACO [2,4]. Table 3 represents the concluded proposed data collection for ACO diagnostic.

Table 3.
The concluded proposed data collection for ACO diagnostic.
6. Proposed Treatment Guide for ACO
6.1. Non-Pharmacologic Treatment
Patients with ACO can benefit from general nonpharmacologic measures derived from effective therapies in managing asthma and/or COPD [3,4]. Table 4 shows the most appropriate options.

Table 4.
The recommended non-pharmacological therapy for patients with ACO.
6.2. Pharmacologic Treatment
Agreement over the effective management of ACO is in its infancy, and there is limited consensus among medical professionals on the most effective approach. The initial pharmacological options indicated in the joint GINA and GOLD statement on ACO depend on the expert opinion of medical professionals [3]. Unfortunately, formal data on the management of ACO are rare, primarily because clinical studies of asthma and COPD medicines have usually excluded ACO patients specifically. Despite this, some practitioners take a similar approach to ACO as they do with asthma, which includes the use of inhaled corticosteroids (ICS) and adjusting the medication depending on patient responsiveness. The use of LABA and/or LAMA treatment is given nearly similar weight and is recommended as the starting point for maintenance treatment in COPD [3,4]. However, the findings of the Salmeterol Multicenter Asthma Research Trial (SMART) have shown that LABA monotherapy is not recommended in asthma [41]. This 28-week trial compared metered-dose inhaler (MDI) salmeterol to a placebo. In a preliminary study of 26,355 participants, respiratory and asthma-related mortality increased, particularly among African Americans, when using LABA therapy alone to maintain asthma control, raising safety concerns. Since then, the safety of asthma patients utilizing combined ICS-LABA inhalers has increased. Therefore, ICS-LABA combination inhalers are safe for ACO, unlike LABA monotherapy.
A randomized, open-label crossover study was conducted with 16 ACO patients as participants (determined as the combination of fixed-airflow obstruction with airway hyperresponsiveness by methacholine inhalation challenge). This trial found that 4 weeks of once-daily fluticasone furoate with vilanterol improved FEV1 more significantly than a run-in phase of twice-daily fluticasone propionate and salmeterol [42].
Despite treatment with ICS, some ACO patients may continue to experience exercise limits or frequent exacerbations. There is no evidence to guide subsequent modifications in medicine; a stepwise approach depending on symptoms, exacerbations, and therapy response is feasible, similar to the strategy employed in asthma [3,43].
For patients who experience persistent symptoms and/or exacerbations despite ICS/LABA or ICS/LAMA treatment, triple therapy (LAMA-LABA-ICS) is recommended.
In randomized, open-label crossover study with 17 ACO patients, adding umeclidinium (LAMA) to fluticasone furoate/vilanterol (ICS/LABA) resulted in a higher improvement in FEV1 after four weeks compared to continuing fluticasone furoate/vilanterol alone [44]. Patients with severe, persistent asthma or COPD with frequent exacerbations have had positive outcomes after undergoing triple therapy [3,43].
It is crucial to identify various phenotypes of ACO to develop comprehensive treatment approaches and apply targeted medicines for more precise management [40].
Based on the evidence presented in this review and previous research [5,14,40,45,46,47,48], the following recommendations are encouraged (Figure 1):
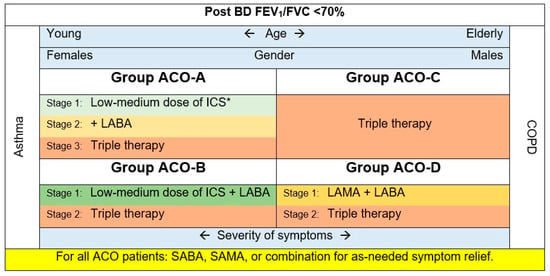
Figure 1.
The treatment scheme for ACO patients based on the four phenotypes. * This stage can be skipped for patients with frequent exacerbations.
- Group ACO-A: Asthmatics, non-smokers, most probably females. These patients may exhibit eosinophilic inflammation signs.
All patients with ACO should have easy access to a rapid onset of an inhaled BD (e.g., short-acting beta 2 agonists (SABA), a short-acting muscarinic antagonist (SAMA), or combination) for as-needed symptom relief.
Inhaled corticosteroids (ICS) are appropriate in this group of ACO, as ICS are a mainstay of asthma treatment [4]. ICS will be essential in treating patients with asthma-predominant phenotypes exhibiting eosinophilic inflammation. Following the GINA strategy, this guide suggests regular low doses of ICS.
Adding a long-acting beta-agonist (LABA), long-acting muscarinic antagonist (LAMA), or both may be essential for symptom control. Nevertheless, as in asthma, LABA monotherapy should be avoided. Leukotriene receptor antagonists can be considered add-on treatments.
- Group ACO-B: Asthmatics, smokers, patients having a fixed airway obstruction. These patients are typically younger females with a higher prevalence of atopic characteristics [46,47]. Their utilization of health care services is usually greater than that of groups ACO-C and D.
All ACO patients should have convenient availability to an inhaled BD with rapid onset (e.g., SABA, SAMA, or combination) for symptom alleviation as needed.
Given the limited data, we recommend starting with ICS plus LABA, then moving to the triple therapy for patients non-responsive to the first stage.
- Group ACO-C: COPD accompanied by eosinophilia. Patients in this group tend to be older men with a higher eosinophil count than smokers (>300 cells/µL) and T helper cell type 2–related indicators [49].
All ACO patients should have convenient availability to an inhaled BD with rapid onset (e.g., SABA, SAMA, or combination) for symptom alleviation as needed.
They usually use LAMA [49]. However, accumulating evidence in COPD suggests an association between higher levels of blood eosinophils and ICS response [50,51]. In contrast, at the beginning of COPD treatment in group B COPD patients, LAMAs and LABAs are administered alone or in combination; ICS are not used as monotherapy for COPD [3]. However, the 2023 GOLD recommends starting LABA and LAMA combination for group B COPD patients and triple therapy for group E (previously known as group C and D) [52,53]. Taking these into consideration, this ACO_CP 2023 guide recommends starting with the triple therapy for this ACO phenotype.
- Group ACO-D: COPD with a substantial BD response (FEV1 ≥ 15% and ≥400 mL), as defined by previous publications [14,48,49]. These patients have numerous characteristics of COPD with eosinophilia phenotype, as they are usually older males with comparable baseline lung function [49,54]. Their blood eosinophil level is typically <300 cells/µL.
All ACO patients should have convenient availability to an inhaled BD with rapid onset (e.g., SABA, SAMA, or combination) for symptom alleviation as needed.
Individuals with COPD-predominant ACO and low eosinophil levels were usually treated with LAMA. Other treatments include LABA (prescribed alone or in combination with LAMA), ICS, macrolides, and roflumilast [5]. ICS are not used as monotherapy for COPD [3]. However, in the 2023 GOLD, they recommend starting LABA and LAMA combination for group B COPD; considering that, this ACO_CP 2023 guide recommends starting with LAMA plus LABA as initial therapy for this ACO phenotype. Adding ICS (triple therapy) can be considered for unresponsive cases.
7. Biologic Agents
Patients who continue to have symptoms or have exacerbations despite triple therapy should be investigated for indications of one or more of the biological medicines identified for asthma. These indications include sensitivity to perennial allergens, peripheral blood eosinophilia, and/or high total serum IgE. While waiting for data in ACO patients, some clinicians often apply the same criteria as in severe persistent asthma.
Evidence on the usage of biologics in patients with ACO is infrequent and unreliable. In Australia, Omalizumab enhanced asthmatic patients’ control and quality of life [55]. Likewise, a post hoc study of PROSPERO revealed that ACO patients treated with Omalizumab had similar outcomes as asthma patients treated with the same medication [56]. Nonetheless, a study published in 2022 compared the response to biologics in asthmatics and ACO patients in the real world found that just 16% of ACO patients gained clinical control, compared to 40% of asthmatics [57]. Several monoclonal anti–IL-5 and anti–IL- 5Ra antibodies are licensed to treat eosinophilic asthma. Only mepolizumab and benralizumab have been demonstrated to reduce the rate of exacerbation in a highly chosen sample of COPD patients with elevated eosinophil levels [58]. Dupilumab, an anti–IL-4Ra antibody, has been demonstrated to enhance lung function and exacerbations in individuals with severe asthma, with a more remarkable improvement in patients with greater eosinophil levels [59]. However, its efficacy in individuals with COPD and ACO has not been confirmed. Although biologic medicines are not currently the usual care for ACO, selecting a medication with demonstrated effectiveness in asthma and eosinophilic COPD may be prudent.
Table 5 describes the limited evidence on using biological agents in COPD that may notify therapy selection in patients with ACO features.

Table 5.
The use of biologic agents in patients with ACO features.
7.1. Anti-IgE Therapy
Omalizumab’s forty-eight weeks observational study analyzed patients with asthma who were not excluded because they had concomitant COPD or previous smoking history [56]. Using different ACO definitions, patients in this study who met the criteria for ACO showed the same improvement in exacerbation rates and symptoms as patients who did not meet the ACO criteria, compared to rates before omalizumab use.
The Australian Xolair Registry found that patients with a COPD diagnosis based on physician assessment or fixed-airflow obstruction had better asthma control and health-related quality of life ratings but no significant change in FEV1 [62].
A case series of ten ACO patients found that the IL-4 levels were lower and respiratory symptoms were improved [63]. In small trials such as this, it is a challenge to ascertain whether the patients investigated had ACO or severe asthma that was not reversible post-BD.
7.2. Anti-IL-5/IL-5 Receptor Alpha (IL-5Ra) Therapies
In eosinophil development, maturation, and tissue migration, IL-5 is a crucial mediator. Several anti-IL-5 and anti-IL-5Ra monoclonal antibodies are licensed as maintenance treatments for severe asthma and eosinophilia [66,67,68].
While previous phase 2 research with benralizumab indicated a trend toward exacerbation reductions in COPD patients with blood eosinophil concentrations of ≥200 cells/µL [72], two phase 3 COPD research findings were undesirable. Mepolizumab outcomes for COPD are similarly variable. A phase 3 study of COPD patients with blood eosinophil levels of 150 cells/µL at screening or 300 cells/L the year prior revealed a clinically and statistically significant decrease in mild or severe exacerbations. The parallel investigation was unsuccessful [73]. Consequently, additional evidence will be required to determine whether ACO patients may benefit.
7.3. IL-4 Receptor Alpha (IL-4Ra) and IL-13 Therapies
IL-4 and IL-13 contribute to allergic inflammation by attracting eosinophils and mast cells to locations of allergic inflammation and inducing goblet cell metaplasia [74].
Lebrikizumab and tralokinab, two anti-IL-13 antibodies, failed to prevent asthma exacerbations in phase 3 trials and are not now licensed for clinical usage [75,76].
8. Future Directions
To better understand ACO and its treatment, further investigation is needed to identify the types of biomarkers and phenotypes that can identify patients who are most sensitive to particular therapies. For instance, in certain patients, incompatibility of illness course and clinical presentation with some pulmonary diseases such as asthma or COPD results in the patient’s status becoming more complicated in the face of optimal therapeutic protocol.
Current research on ACO traits has not utilized all possible classification methods. Therefore, such contributing and provoking factors as spirometry findings; patient record history; the prognosis of patient status, including disease exacerbating conditions; and intrinsic atopy-related markers should be considered in future clinical trials.
Longitudinal studies are required to enable a deeper understanding of these phenotypes and how they influence management decisions. In addition, performing detailed omics research, including the respiratory microbiome, could considerably improve phenotyping.
9. Conclusions and Clinical Care Points
Asthma and COPD have characteristic symptoms, yet patients with both are prevalent. However, there is no globally accepted definition of ACO, and it may not be considered a distinct disease or clinical entity. Recognizing patients with both disorders helps guide clinical therapy. As with asthma and COPD, ACO patients are heterogeneous and presumably have multiple underlying disease processes.
Besides the age and smoking history of the patient, recurrent pulmonary infections and inflammation have been considered as provoking factors contributing to the worsening of pulmonary function in elders.
The examination of suspected ACO is similar to asthma and COPD, focusing on airflow limitation and BD reversibility. A chest radiograph can help rule out other causes of dyspnea besides asthma, COPD, and ACO. Laboratory findings that monitor elevated total serum IgE, eosinophilia, intrinsic or extrinsic atopic hypersensitivity, and efficient CT scan might be useful for clarifying mysterious diagnostic cases.
Although the diagnosis of ACO depends on a mix of clinical features, not all are required: age 40; non-reversible airflow limitation; improvement in FEV1 by 10% of the predicted value after BD; a history of asthma, atopy or allergies; and exposure to agents such as smoke. Eosinophil count >300 cells/µL supports ACO or asthma diagnosis.
No single biomarker can be used to identify and classify ACO phenotypes. However, the number of eosinophils in the blood and BD reversibility can aid in phenotyping ACO. While total IgE, allergen-specific IgE, FeNO, chest imaging, and metabolic markers may assist in diagnosing ACO, their role in phenotyping ACO is unclear.
Nonpharmacologic treatment options for ACO patients include smoking cessation, annual influenza and pneumococcus vaccinations, inhaler technique education, avoidance of provoking allergy factors for people susceptible to known allergens, and pulmonary rehabilitation.
Free and easy access to BDs inhalers should be available for any patient with ACO to alleviate the symptoms rapidly. Similar to asthma, the treatment of ACO tends to be escalated to counteract clinical manifestations and prevent further worsening. This may involve increasing ICS doses or adding LAMA, ICS, and LABA (triple therapy). However, despite triple inhaled treatment in patients with persistent symptoms or exacerbations, they should be assessed for manifestations that indicate a potential advantages from monoclonal based pharmacotherapy such as Omalizumab, mepolizumab, or benralizumab developed for asthma. When selecting biologic drugs for ACO patients, we utilize the same criteria for severe persistent asthma.
It is essential to conduct large-scale trials to evaluate clinically relevant outcomes in patients with well-defined ACO.
Author Contributions
A.R.A. was responsible and involved in all of the parts of this review. M.S.A.-S. and M.A. were responsible for writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Woodruff, P.G.; van den Berge, M.; Boucher, R.C.; Brightling, C.; Burchard, E.G.; Christenson, S.A.; Han, M.K.; Holtzman, M.J.; Kraft, M.; Lynch, D.A.; et al. American Thoracic Society/National Heart, Lung, and Blood Institute Asthma-Chronic Obstructive Pulmonary Disease Overlap Workshop Report. Am. J. Respir. Crit. Care Med. 2017, 196, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respirat. J. 2022, 59, 17–35. [Google Scholar] [CrossRef] [PubMed]
- GOLD. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2022. Available online: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf (accessed on 1 January 2022).
- GINA. Global Initiative for Asthma (GINA): Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/reports/ (accessed on 1 October 2022).
- Rhee, C.K. Phenotype of asthma-chronic obstructive pulmonary disease overlap syndrome. Korean J. Int. Med. 2015, 30, 443–449. [Google Scholar] [CrossRef]
- Fujino, N.; Sugiura, H. Aco (Asthma–copd overlap) is independent from copd, a case in favor: A systematic review. Diagnostics 2021, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Sin, D.D. Asthma-COPD overlap syndrome: Pathogenesis, clinical features, and therapeutic targets. BMJ 2017, 358, j3772. [Google Scholar] [CrossRef]
- Barrecheguren, M.; Esquinas, C.; Miravitlles, M. The asthma–chronic obstructive pulmo-nary disease overlap syndrome (ACOS): Opportunities and challenges. Curr. Opin. Pulm. Med. 2015, 21, 74–79. [Google Scholar] [CrossRef]
- Cosio, B.G.; Soriano, J.B.; López-Campos, J.L.; Calle-Rubio, M.; Soler-Cataluna, J.J.; de-Torres, J.P.; Marín, J.M.; Martínez-Gonzalez, C.; de Lucas, P.; Mir, I.; et al. Defining the Asthma-COPD Overlap Syndrome in a COPD Cohort. Chest 2016, 149, 45–52. [Google Scholar] [CrossRef]
- Sin, D.D.; Miravitlles, M.; Mannino, D.M.; Soriano, J.B.; Price, D.; Celli, B.R.; Leung, J.M.; Nakano, Y.; Park, H.Y.; Wark, P.A.; et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur. Respir. J. 2016, 48, 664–673. [Google Scholar] [CrossRef]
- Cataldo, D.; Corhay, J.L.; Derom, E.; Louis, R.; Marchand, E.; Michils, A.; Ninane, V.; Peché, R.; Pilette, C.; Vincken, W.; et al. A Belgian survey on the diagnosis of asthma-COPD overlap syndrome. Int. J. Chron. Obstruct. Pulmon Dis. 2017, 12, 601–613. [Google Scholar] [CrossRef]
- Koblizek, V.; Chlumsky, J.; Zindr, V.; Neumannova, K.; Zatloukal, J.; Zak, J.; Sedlak, V.; Kocianova, J.; Zatloukal, J.; Hejduk, K.; et al. Chronic Obstructive Pulmonary Disease: Official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2013, 157, 189–201. [Google Scholar] [CrossRef]
- Soler-Cataluña, J.J.; Cosío, B.; Izquierdo, J.L.; López-Campos, J.L.; Marín, J.M.; Agüero, R.; Baloira, A.; Carrizo, S.; Esteban, C.; Galdiz, J.B.; et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch. Bronconeumol. 2012, 48, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Alvarez-Gutierrez, F.J.; Calle, M.; Casanova, C.; Cosio, B.G.; López-Viña, A.; Pérez de Llano, L.; Quirce, S.; Roman-Rodríguez, M.; Soler-Cataluña, J.J.; et al. Algorithm for identification of asthma-COPD overlap: Consensus between the Spanish COPD and asthma guidelines. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Alshabanat, A.; Zafari, Z.; Albanyan, O.; Dairi, M.; FitzGerald, J.M. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PLoS ONE 2015, 10, e0136065. [Google Scholar] [CrossRef] [PubMed]
- van Boven, J.F.; Román-Rodríguez, M.; Palmer, J.F.; Toledo-Pons, N.; Cosío, B.G.; Soriano, J.B. Comorbidome, Pattern, and Impact of Asthma-COPD Overlap Syndrome in Real Life. Chest 2016, 149, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, J.; Zhao, H.; Hardin, M.; Hersh, C.P.; Crapo, J.; Kim, V.; Criner, G.J. Analysis of Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome Defined on the Basis of Bronchodilator Response and Degree of Emphysema. Ann. Am. Thorac. Soc. 2016, 13, 1483–1489. [Google Scholar] [CrossRef]
- Kumbhare, S.; Pleasants, R.; Ohar, J.A.; Strange, C. Characteristics and Prevalence of Asthma/Chronic Obstructive Pulmonary Disease Overlap in the United States. Ann. Am. Thorac. Soc. 2016, 13, 803–810. [Google Scholar] [CrossRef]
- Dodd, K.E.; Wood, J.; Mazurek, J.M. Mortality Among Persons with Both Asthma and Chronic Obstructive Pulmonary Disease Aged ≥25 Years, by Industry and Occupation—United States, 1999–2016. MMWR Morb. Mort. Wkly Rep. 2020, 69, 670–679. [Google Scholar] [CrossRef]
- Çolak, Y.; Nordestgaard, B.G.; Lange, P.; Vestbo, J.; Afzal, S. Prognosis of Patients with Chronic Obstructive Pulmonary Disease Not Eligible for Major Clinical Trials. Am. J. Respir. Crit. Care Med. 2022, 206, 271–280. [Google Scholar] [CrossRef]
- Vaz Fragoso, C.A.; Murphy, T.E.; Agogo, G.O.; Allore, H.G.; McAvay, G.J. Asthma-COPD overlap syndrome in the US: A prospective population-based analysis of patient-reported outcomes and health care utilization. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 517–527. [Google Scholar] [CrossRef]
- Charokopos, A.; Braman, S.S.; Brown, S.A.W.; Mhango, G.; de-Torres, J.P.; Zulueta, J.J.; Sharma, S.; Holguin, F.; Sigel, K.M.; Powell, C.A.; et al. Lung Cancer Risk among Patients with Asthma-Chronic Obstructive Pulmonary Disease Overlap. Ann. Am. Thorac. Soc. 2021, 18, 1894–1900. [Google Scholar] [CrossRef]
- Orie, N.G. Correlations of emphysema and asthmatic constitution. Acta Allergol. 1961, 16, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Orie, N. The host factor in bronchitis. In Proceedings of the Bronchitis an International Symposium, Groningen, The Netherlands, 9–11 June 1961. [Google Scholar]
- Barnes, P.J. Against the Dutch hypothesis: Asthma and chronic obstructive pulmonary disease are distinct diseases. Am. J. Respir. Crit. Care Med. 2006, 174, 240–243, discussion 243–244. [Google Scholar] [CrossRef] [PubMed]
- Siew, L.Q.C.; Wu, S.Y.; Ying, S.; Corrigan, C.J. Cigarette smoking increases bronchial mucosal IL-17A expression in asthmatics, which acts in concert with environmental aeroallergens to engender neutrophilic inflammation. Clin. Exp. Allerg. 2017, 47, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, S.; Fukushima, T.; Aikawa, T.; Yanai, M.; Sekizawa, K.; Sasaki, H.; Takishima, T. Allergic sensitization in elderly patients with chronic obstructive pulmonary disease. Respiration 1990, 57, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Camiolo, M.; Fitzpatrick, A.; Gauthier, M.; Wenzel, S.E. Are we meeting the promise of endotypes and precision medicine in asthma? Physiol. Rev. 2020, 100, 983–1017. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Talib, W.; Al-Dulaimi, A.; Daoud, S.; Al Maqbali, M. The first detection of Pneumocystis jirovecii in asthmatic patients post-COVID-19 in Jordan. Bosn. J. Basic Med. Sci. 2022, 22, 784–790. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Al-Dulaimi, A.; Alkhatib, M.; Al Maqbali, M.; Al-Najjar, M.A.A.; Al-Rshaidat, M.M.D. A comprehensive clinical guide for Pneumocystis jirovecii pneumonia: A missing therapeutic target in HIV-uninfected patients. Expert Rev. Respir. Med. 2022, 16, 1167–1190. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Abed, A.; Khader, H.A.; Al-Shdifat, L.M.H.; Hasoun, L.; Al-Rshaidat, M.M.D.; Alkhatib, M.; Zihlif, M. Molecular Accounting and Profiling of Human Respiratory Microbial Communities: Toward Precision Medicine by Targeting the Respiratory Microbiome for Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 4086. [Google Scholar] [CrossRef]
- Al-Dulaimi, A.; Alsayed, A.R.; Maqbali, M.A.; Zihlif, M. Investigating the human rhinovirus co-infection in patients with asthma exacerbations and COVID-19. Pharm. Pract. (Granada) 2022, 20, 2665. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Abed, A.; Jarrar, Y.B.; Alshammari, F.; Alshammari, B.; Basheti, I.A.; Zihlif, M. Alteration of the Respiratory Microbiome in Hospitalized Patients with Asthma–COPD Overlap during and after an Exacerbation. J. Clin. Med. 2023, 12, 2118. [Google Scholar] [CrossRef]
- Sethi, S.; Evans, N.; Grant, B.J.B.; Murphy, T.F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 2002, 347, 465–471. [Google Scholar] [CrossRef]
- Murphy, T.F.; Brauer, A.L.; Eschberger, K.; Lobbins, P.; Grove, L.; Cai, X.; Sethi, S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 853–860. [Google Scholar] [CrossRef]
- Sze, M.A.; Dimitriu, P.A.; Hayashi, S.; Elliott, W.M.; McDonough, J.E.; Gosselink, J.V.; Cooper, J.; Sin, D.D.; Mohn, W.W.; Hogg, J.C. The lung tissue microbiome in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Erb-Downward, J.R.; Thompson, D.L.; Han, M.K.; Freeman, C.M.; McCloskey, L.; Schmidt, L.A.; Young, V.B.; Toews, G.B.; Curtis, J.L.; Sundaram, B. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE 2011, 6, e16384. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A.; Al-Doori, A.; Al-Dulaimi, A.; Alnaseri, A.; Abuhashish, J.; Aliasin, K.; Alfayoumi, I. Influences of bovine colostrum on nasal swab microbiome and viral upper respiratory tract infections—A case report. Respir. Med. Case Rep. 2020, 31, 101189. [Google Scholar] [CrossRef]
- Adrish, M.; Anand, M.P.; Hanania, N.A. Phenotypes of Asthma-Chronic Obstructive Pulmonary Disease Overlap. Immunol. Allerg. Clin. N. Am. 2022, 42, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.S.; Weiss, S.T.; Bleecker, E.R.; Yancey, S.W.; Dorinsky, P.M. The Salmeterol Multicenter Asthma Research Trial: A comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006, 129, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ishiura, Y.; Fujimura, M.; Shiba, Y.; Ohkura, N.; Hara, J.; Kasahara, K. A comparison of the efficacy of once-daily fluticasone furoate/vilanterole with twice-daily fluticasone propionate/salmeterol in asthma-COPD overlap syndrome. Pulm. Pharmacol. Ther. 2015, 35, 28–33. [Google Scholar] [CrossRef] [PubMed]
- GINA. Global Strategy for Asthma Management and Prevention. 2022. Available online: https://ginasthma.org (accessed on 1 July 2022).
- Ishiura, Y.; Fujimura, M.; Ohkura, N.; Hara, J.; Kasahara, K.; Ishii, N.; Tamaki, T.; Shimizu, T.; Nomura, S. Effect of triple therapy in patients with asthma-COPD overlap. Int. J. Clin. Pharmacol. Ther. 2019, 57, 384–392. [Google Scholar] [CrossRef]
- Joo, H.; Han, D.; Lee, J.H.; Rhee, C.K. Heterogeneity of asthma–COPD overlap syndrome. Int. J. Chron. Obstruct. Pulm. Dis. 2017, 12, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Llano, L.; Cosio, B.G. Asthma-COPD overlap is not a homogeneous disorder: Further supporting data. Respir. Res. 2017, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.-P.; Boulay, M.-È.; Dérival, J.-L.; Milot, J.; Lepage, J.; Bilodeau, L.; Maltais, F. Asthma-COPD overlap phenotypes and smoking: Comparative features of asthma in smoking or non-smoking patients with an incomplete reversibility of airway obstruction. COPD J. Chron. Obstruct. Pulm. Dis. 2018, 15, 130–138. [Google Scholar] [CrossRef]
- Plaza, V.; Álvarez, F.; Calle, M.; Casanova, C.; Cosío, B.G.; López-Viña, A.; de Llano, L.P.; Quirce, S.; Román-Rodríguez, M.; Soler-Cataluña, J.J. Consensus on the asthma–COPD overlap (ACO) between the Spanish COPD guidelines (GesEPOC) and the Spanish guidelines on the management of asthma (GEMA). Arch. Bronconeumol. 2017, 53, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Pons, N.; Van Boven, J.F.M.; Román-Rodríguez, M.; Pérez, N.; Felices, J.L.V.; Soriano, J.B.; Cosío, B.G. ACO: Time to move from the description of different phenotypes to the treatable traits. PLoS ONE 2019, 14, 0210915. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, S.; Barnes, N.; Brusselle, G.; Compton, C.; Criner, G.J.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Lange, P.; et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: Analysis of the IMPACT trial. Lancet Respir. Med. 2019, 7, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; Peterson, S.; De Blas, M.A.; Calverley, P.M.; Rennard, S.I.; Richter, K.; Fagerås, M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: A post-hoc analysis of three randomised trials. Lancet Respir. Med. 2018, 6, 117–126. [Google Scholar] [CrossRef]
- GOLD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available online: https://goldcopd.org/2023-gold-report-2/ (accessed on 1 January 2023).
- Venkatesan, P. GOLD COPD report: 2023 update. Lancet Respir. Med. 2023, 11, 18. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, Y.S.; Min, K.H.; Hur, G.Y.; Lee, S.Y.; Kang, K.H.; Rhee, C.K.; Park, S.J.; Khan, A.; Na, J.; et al. Increased urinary l-histidine in patients with asthma-COPD overlap: A pilot study. Int. J. COPD 2018, 13, 1809–1818. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the Asthma Control Test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Hanania, N.A.; Chipps, B.E.; Griffin, N.M.; Yoo, B.; Iqbal, A.; Casale, T.B. Omalizumab effectiveness in asthma-COPD overlap: Post hoc analysis of PROSPERO. J. Allerg. Clin. Immunol. 2019, 143, 1629–1633.e1622. [Google Scholar] [CrossRef]
- de Llano, L.P.; Rivas, D.D.; Malanda, N.M.; Moral, V.P.; Blanco, J.A.G.; Muñoz-Esquerre, M.; García-Moguel, I.; Campos, R.M.D.; Martínez-Moragón, E.; Mena, A.H.; et al. The Response to Biologics is Better in Patients with Severe Asthma Than in Patients with Asthma–COPD Overlap Syndrome. J. Asthma Allerg. 2022, 15, 363–369. [Google Scholar] [CrossRef]
- Donovan, T.; Crossingham, I.; Milan, S.J.; Wang, R.; Bradley, P. Anti-IL5 therapies for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019, 2019, 14651858. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Cioppa, G.D.; van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allerg. Clin. Immunol. 2001, 108, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lemanske, R.F., Jr.; Nayak, A.; McAlary, M.; Everhard, F.; Fowler-Taylor, A.; Gupta, N. Omalizumab improves asthma-related quality of life in children with allergic asthma. Pediatrics 2002, 110, e55. [Google Scholar] [CrossRef]
- Maltby, S.; Gibson, P.G.; Powell, H.; McDonald, V.M. Omalizumab Treatment Response in a Population with Severe Allergic Asthma and Overlapping COPD. Chest 2017, 151, 78–89. [Google Scholar] [CrossRef]
- Yalcin, A.D.; Celik, B.; Yalcin, A.N. Omalizumab (anti-IgE) therapy in the asthma-COPD overlap syndrome (ACOS) and its effects on circulating cytokine levels. Immunopharmacol. Immunotoxicol. 2016, 38, 253–256. [Google Scholar] [CrossRef]
- Tat, T.S.; Cilli, A. Omalizumab treatment in asthma-COPD overlap syndrome. J. Asthm. 2016, 53, 1048–1050. [Google Scholar] [CrossRef]
- Kupryś-Lipińska, I.; Pałczyński, C.; Molinska, J.; Kuna, P. Omalizumab therapy in a patient with severe asthma and co-existing chronic obstructive pulmonary disease. Postepy Dermatol. Alergol. 2019, 36, 239–241. [Google Scholar] [CrossRef]
- Castro, M.; Wenzel, S.E.; Bleecker, E.R.; Pizzichini, E.; Kuna, P.; Busse, W.W.; Gossage, D.L.; Ward, C.K.; Wu, Y.; Wang, B.; et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: A phase 2b randomised dose-ranging study. Lancet Respir. Med. 2014, 2, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Ambrose, C.S.; Sałapa, K.; Roseti, S.L.; Griffiths, J.M.; Parnes, J.R.; Colice, G. Efficacy of Tezepelumab in Patients with Severe, Uncontrolled Asthma and Perennial Allergy. J. Allerg. Clin. Immunol. Pract. 2021, 9, 4334–4342.e4336. [Google Scholar] [CrossRef]
- Corren, J.; Chen, S.; Callan, L.; Gil, E.G. The effect of tezepelumab on hospitalizations and emergency department visits in patients with severe asthma. Ann. Allerg. Asthm. Immunol. 2020, 125, 211–214. [Google Scholar] [CrossRef]
- Brightling, C.E.; Bleecker, E.R.; Panettieri, R.A., Jr.; Bafadhel, M.; She, D.; Ward, C.K.; Xu, X.; Birrell, C.; van der Merwe, R. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir. Med. 2014, 2, 891–901. [Google Scholar] [CrossRef]
- Pavord, I.D.; Chanez, P.; Criner, G.J.; Kerstjens, H.A.M.; Korn, S.; Lugogo, N.; Martinot, J.B.; Sagara, H.; Albers, F.C.; Bradford, E.S.; et al. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2017, 377, 1613–1629. [Google Scholar] [CrossRef]
- Kau, A.L.; Korenblat, P.E. Anti-interleukin 4 and 13 for asthma treatment in the era of endotypes. Curr. Opin. Allerg. Clin. Immunol. 2014, 14, 570–575. [Google Scholar] [CrossRef]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.; et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Panettieri, R.A., Jr.; Sjöbring, U.; Péterffy, A.; Wessman, P.; Bowen, K.; Piper, E.; Colice, G.; Brightling, C.E. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): Two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir. Med. 2018, 6, 511–525. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).