Highly Selective Targeting of Pancreatic Cancer in the Liver with a Near-Infrared Anti-MUC5AC Probe in a PDOX Mouse Model: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
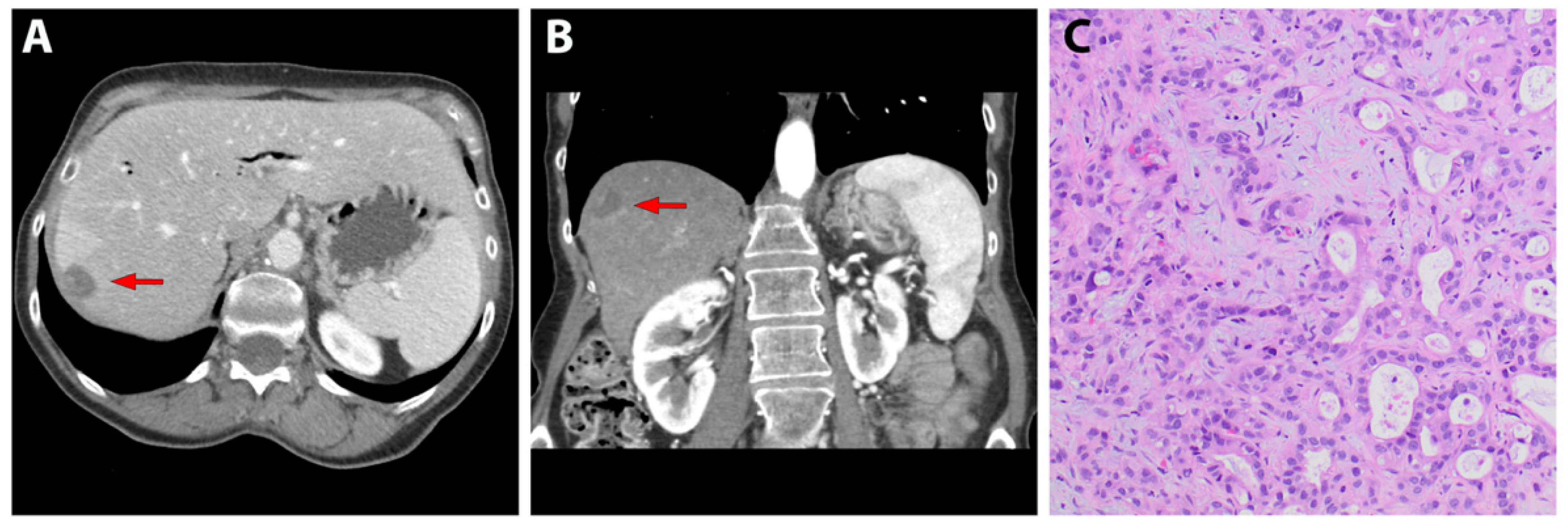
3.1. Patient-Derived Liver Metastasis of Pancreatic Cancer
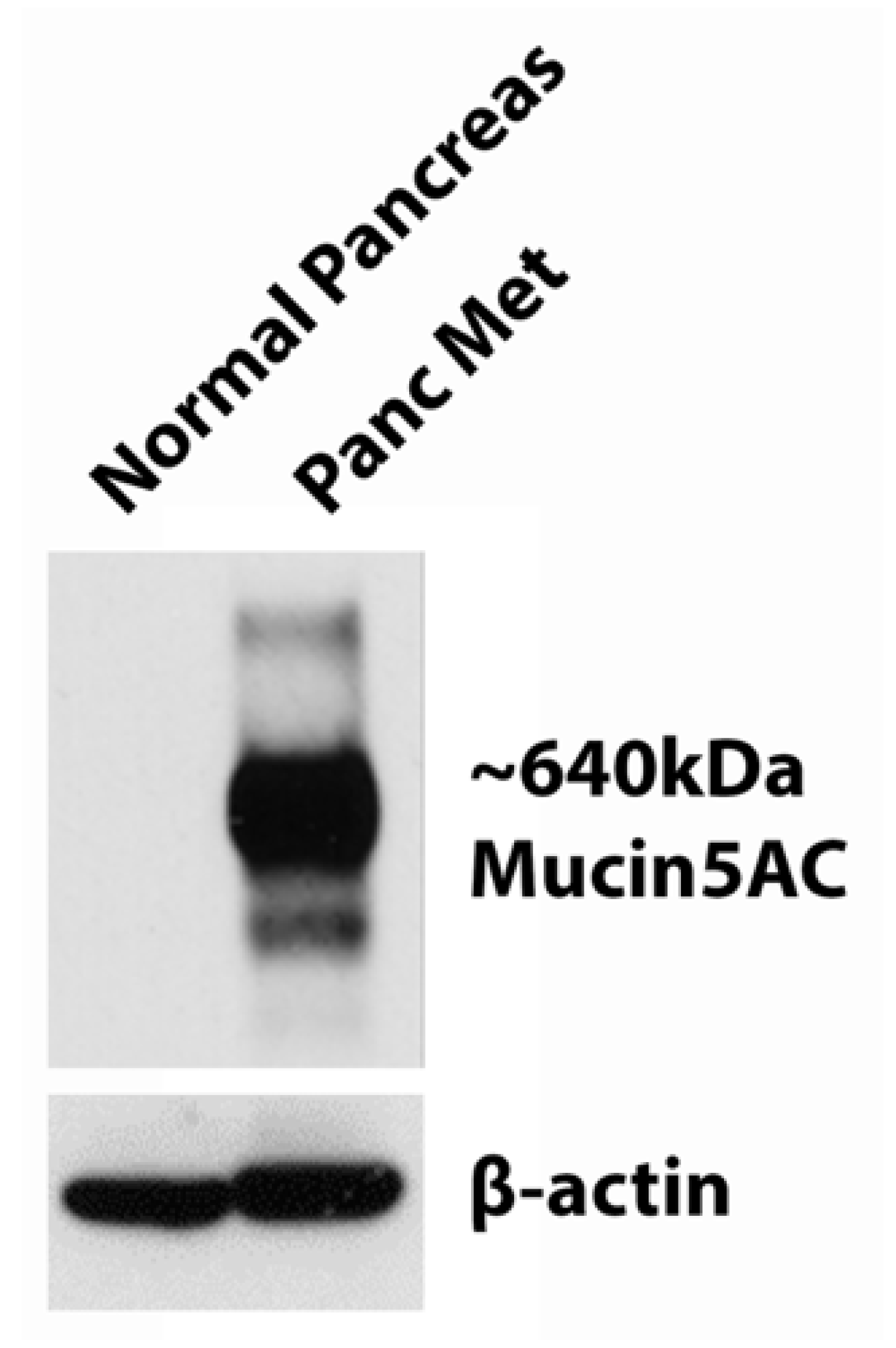
3.2. Western Blot of MUC5AC Expression
3.3. Labeling Subcutaneous Tumors with MUC5AC-IR800
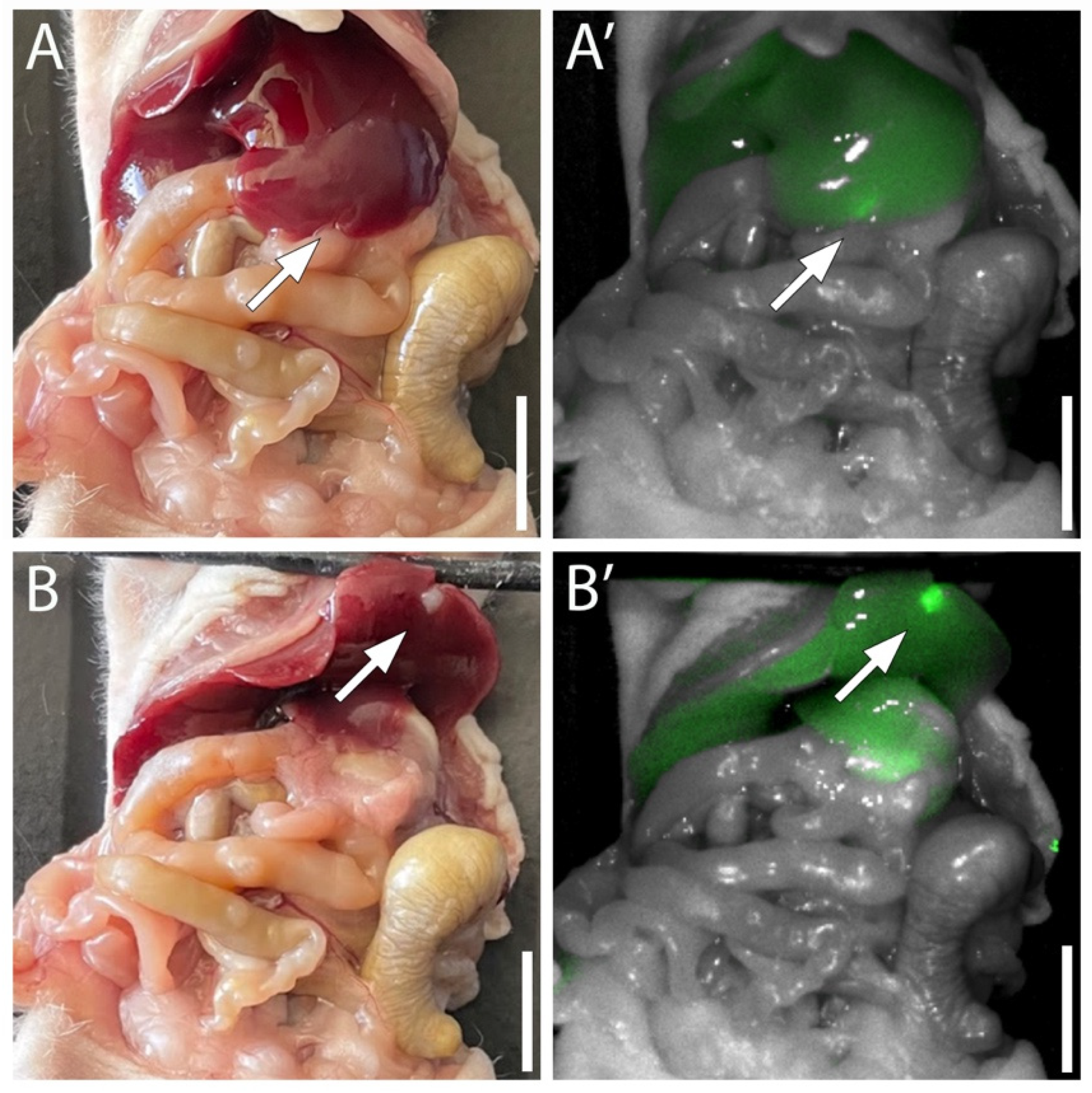
3.4. Targeting Pancreatic Cancer in the Liver with MUC5AC-IR800
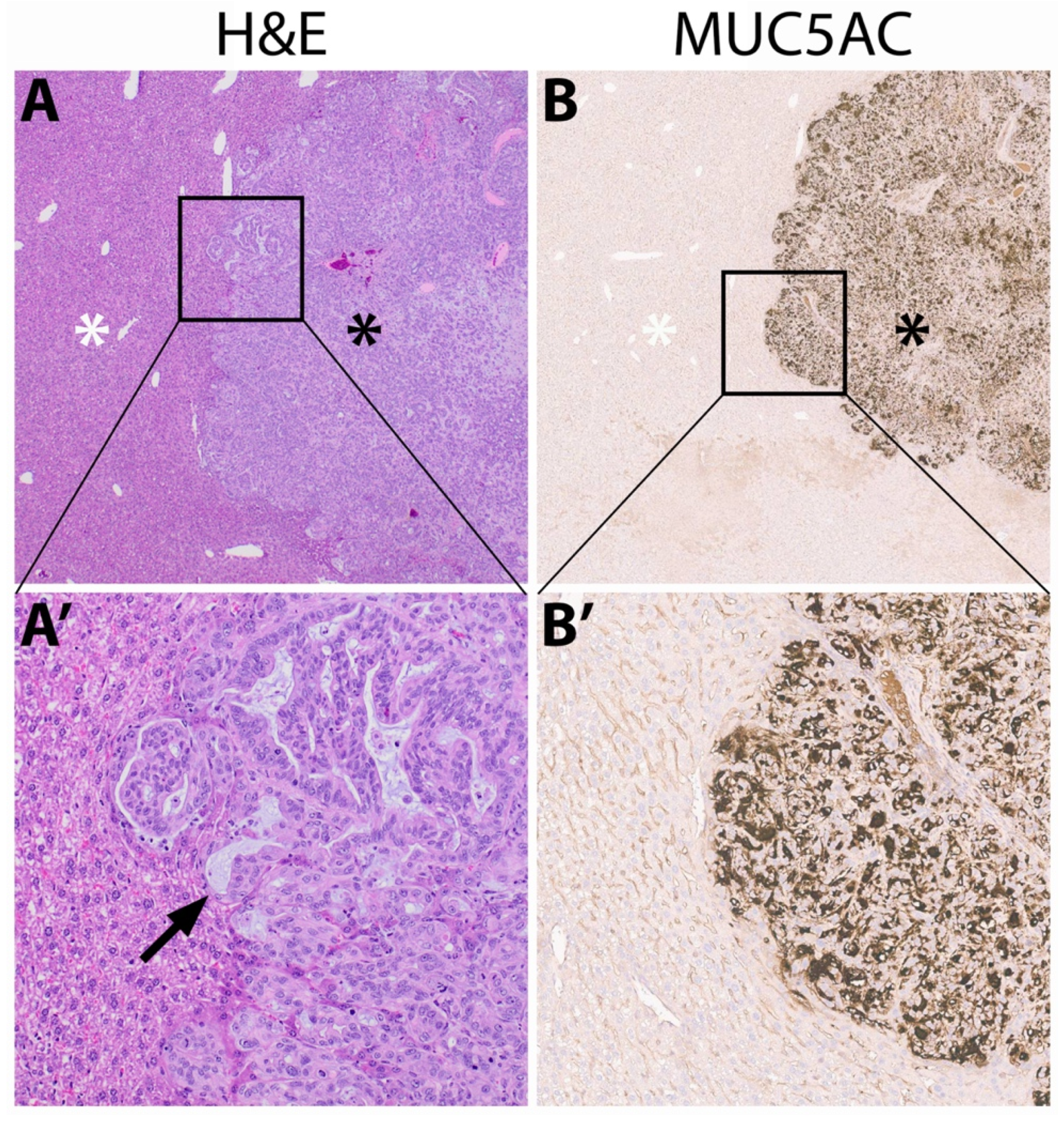
3.5. Immunohistochemistry of Mucin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bellon, E.; Gebauer, F.; Tachezy, M.; Izbicki, J.R.; Bockhorn, M. Pancreatic cancer and liver metastases: State of the art. Updat. Surg. 2016, 68, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Yamashita, Y.I.; Yamao, T.; Kuroda, D.; Eto, T.; Kitano, Y.; Arima, K.; Miyata, T.; Okabe, H.; Nitta, H.; et al. Efficacy of Staging Laparoscopy for Pancreatic Cancer. Anticancer Res. 2020, 40, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Takadate, T.; Morikawa, T.; Ishida, M.; Aoki, S.; Hata, T.; Iseki, M.; Miura, T.; Ariake, K.; Maeda, S.; Kawaguchi, K.; et al. Staging laparoscopy is mandatory for the treatment of pancreatic cancer to avoid missing radiologically negative metastases. Surg. Today 2021, 51, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Hollandsworth, H.M.; Schmitt, V.; Amirfakhri, S.; Filemoni, F.; Schmidt, A.; Landström, M.; Lyndin, M.; Backert, S.; Gerhard, M.; Wennemuth, G.; et al. Fluorophore-conjugated Helicobacter pylori recombinant membrane protein (HopQ) labels primary colon cancer and metastases in orthotopic mouse models by binding CEA-related cell adhesion molecules. Transl. Oncol. 2020, 13, 100857. [Google Scholar] [CrossRef] [PubMed]
- Hollandsworth, H.M.; Lwin, T.M.; Amirfakhri, S.; Filemoni, F.; Batra, S.K.; Hoffman, R.M.; Dhawan, P.; Bouvet, M. Anti-Claudin-1 Conjugated to a Near-Infrared Fluorophore Targets Colon Cancer in PDOX Mouse Models. J. Surg. Res. 2019, 242, 145–150. [Google Scholar] [CrossRef]
- Turner, M.A.; Hollandsworth, H.M.; Amirfakhri, S.; Lwin, T.M.; Nishino, H.; Neel, N.C.; Natarajan, G.; Kaur, S.; Mallya, K.; Hoffman, R.M.; et al. Anti-mucin 4 fluorescent antibody brightly targets colon cancer in patient-derived orthotopic xenograft mouse models: A proof-of-concept study for future clinical applications. Am. J. Surg. 2022, 224, 1081–1085. [Google Scholar] [CrossRef]
- Turner, M.A.; Amirfakhri, S.; Nishino, H.; Lwin, T.M.; Savides, T.J.; Reid, T.R.; Singer, B.B.; Hoffman, R.M.; Bouvet, M. A Patient-Derived Orthotopic Xenograft Model of Gastroesophageal-Junction Adenocarcinoma Translated to the Clinic by Tumor-Targeting Fluorescent Antibodies to Carcinoembryonic-Antigen-Related Cell-Adhesion Molecules. Vivo Athens Greece 2021, 35, 1959–1963. [Google Scholar] [CrossRef]
- Lwin, T.M.; Murakami, T.; Miyake, K.; Yazaki, P.J.; Shivley, J.E.; Hoffman, R.M.; Bouvet, M. Tumor-Specific Labeling of Pancreatic Cancer Using a Humanized Anti-CEA Antibody Conjugated to a Near-Infrared Fluorophore. Ann. Surg. Oncol. 2018, 25, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.A.; Hollandsworth, H.M.; Nishino, H.; Amirfakhri, S.; Lwin, T.M.; Lowy, A.M.; Kaur, S.; Natarajan, G.; Mallya, K.; Hoffman, R.M.; et al. Fluorescent Anti-MUC5AC Brightly Targets Pancreatic Cancer in a Patient-derived Orthotopic Xenograft. In Vivo 2022, 36, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; You, L.; Dai, M.; Zhao, Y. Mucins in pancreatic cancer: A well-established but promising family for diagnosis, prognosis and therapy. J. Cell. Mol. Med. 2020, 24, 10279–10289. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.E.; Bae, H.; Park, H.; Kuan, S.; Crawley, S.C.; Ho, J.J.; Kim, Y.S. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 2002, 123, 1052–1060. [Google Scholar] [CrossRef]
- Nishino, H.; Hollandsworth, H.M.; Sugisawa, N.; Yamamoto, J.; Tashiro, Y.; Inubushi, S.; Hamada, K.; Sun, Y.U.; Lim, H.; Amirfakhri, S.; et al. Sutureless Surgical Orthotopic Implantation Technique of Primary and Metastatic Cancer in the Liver of Mouse Models. In Vivo 2020, 34, 3153–3157. [Google Scholar] [CrossRef]
- DeLong, J.C.; Murakami, T.; Yazaki, P.J.; Hoffman, R.M.; Bouvet, M. Near-infrared-conjugated humanized anti-carcinoembryonic antigen antibody targets colon cancer in an orthotopic nude-mouse model. J. Surg. Res. 2017, 218, 139–143. [Google Scholar] [CrossRef]
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Luiken, G.A.; Hoffman, R.M.; Bouvet, M. Polyethylene glycol (PEG) linked to near infrared (NIR) dyes conjugated to chimeric anti-carcinoembryonic antigen (CEA) antibody enhances imaging of liver metastases in a nude-mouse model of human colon cancer. PLoS ONE 2014, 9, e97965. [Google Scholar] [CrossRef]
- Yazaki, P.J.; Lwin, T.M.; Minnix, M.; Li, L.; Sherman, A.; Molnar, J.; Miller, A.; Frankel, P.; Chea, J.; Poku, E.; et al. Improved antibody-guided surgery with a near-infrared dye on a pegylated linker for CEA-positive tumors. J. Biomed. Opt. 2019, 24, 1–9. [Google Scholar] [CrossRef]
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Luiken, G.A.; Hoffman, R.M.; Bouvet, M. Specific tumor labeling enhanced by polyethylene glycol linkage of near infrared dyes conjugated to a chimeric anti-carcinoembryonic antigen antibody in a nude mouse model of human pancreatic cancer. J. Biomed. Opt. 2014, 19, 101504. [Google Scholar] [CrossRef]
- Hoogstins, C.E.S.; Boogerd, L.S.F.; Mulder, B.G.S.; Mieog, J.S.D.; Swijnenburg, R.J.; Van De Velde, C.J.H.; Farina Sarasqueta, A.; Bonsing, B.A.; Framery, B.; Pèlegrin, A.; et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann. Surg. Oncol. 2018, 25, 3350–3357. [Google Scholar] [CrossRef]
- Meng, Q.; Shi, S.; Liang, C.; Liang, D.; Xu, W.; Ji, S.; Zhang, B.; Ni, Q.; Xu, J.; Yu, X. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: A systematic review and meta-analysis. OncoTargets Ther. 2017, 10, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, N.; Skrypek, N.; Van Seuningen, I. Mucins and pancreatic cancer. Cancers 2010, 2, 1794–1812. [Google Scholar] [CrossRef]
- Jonckheere, N.; Vincent, A.; Veve, B.; Van Seuningen, I. Mucin expression, epigenetic regulation and patient survival: A toolkit of prognostic biomarkers in epithelial cancers. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188538. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Smith, L.M.; Patel, A.; Menning, M.; Watley, D.C.; Malik, S.S.; Krishn, S.R.; Mallya, K.; Aithal, A.; Sasson, A.R.; et al. A Combination of MUC5AC and CA19-9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study. Am. J. Gastroenterol. 2017, 112, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.E.; Shaffer, T.M.; Mack, K.N.; Ring, J.; Ogirala, A.; Klein-Scory, S.; Eilert-Micus, C.; Schmiegel, W.; Bracht, T.; Sitek, B.; et al. Exploiting the MUC5AC Antigen for Noninvasive Identification of Pancreatic Cancer. J. Nucl. Med. 2021, 62, 1384–1390. [Google Scholar] [CrossRef]
- Nakata, N.; Kobashi, N.; Okumura, Y.; eSato, M.; Matono, M.; Otsuki, K.; Tanaka, A.; Hayashi, A. Radiation dosimetry and efficacy of an 89Zr/225Ac-labeled humanized anti-MUC5AC antibody. Nucl. Med. Biol. 2022, 108–109, 33–43. [Google Scholar] [CrossRef]
- Chen, X.; Liu, F.; Xue, Q.; Weng, X.; Xu, F. Metastatic pancreatic cancer: Mechanisms and detection. Oncol. Rep. 2021, 46, 231. [Google Scholar] [CrossRef]
- Mihaljevic, A.L.; Michalski, C.W.; Friess, H.; Kleeff, J. Molecular mechanism of pancreatic cancer—Understanding proliferation, invasion, and metastasis. Langenbecks Arch. Surg. 2010, 395, 295–308. [Google Scholar] [CrossRef]
- Hollandsworth, H.M.; Amirfakhri, S.; Filemoni, F.; Schmitt, V.; Wennemuth, G.; Schmidt, A.; Hoffman, R.M.; Singer, B.B.; Bouvet, M. Anti-carcinoembryonic antigen-related cell adhesion molecule antibody for fluorescence visualization of primary colon cancer and metastases in patient-derived orthotopic xenograft mouse models. Oncotarget 2020, 11, 429–439. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Asbun, H.; Bain, A.; Behrman, S.W.; Benson, A.B., III; Binder, E.; Cardin, D.B.; Cha, C.; et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1028–1061. [Google Scholar] [CrossRef]
- Su, B.-B.; Bai, D.-S.; Yu, J.-Q.; Zhang, C.; Jin, S.-J.; Zhou, B.-H.; Jiang, G.-Q. Can Patients with Pancreatic Cancer and Liver Metastases Obtain Survival Benefit from Surgery? A Population-Based Study. J. Cancer 2021, 12, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Grassi, E.; Palloni, A.; Carloni, R.; Casadei, R.; Ricci, C.; Serra, C.; Ercolani, G.; Brandi, G.; Di Marco, M. Searching for novel multimodal treatments in oligometastatic pancreatic cancer. BMC Cancer 2020, 20, 271. [Google Scholar] [CrossRef] [PubMed]
- Kandel, P.; Wallace, M.B.; Stauffer, J.; Bolan, C.; Raimondo, M.; Woodward, T.A.; Gomez, V.; Ritter, A.W.; Asbun, H.; Mody, K. Survival of Patients with Oligometastatic Pancreatic Ductal Adenocarcinoma Treated with Combined Modality Treatment Including Surgical Resection: A Pilot Study. J. Pancreat. Cancer 2018, 4, 88–94. [Google Scholar] [CrossRef]
- Tachezy, M.; Gebauer, F.; Janot, M.; Uhl, W.; Zerbi, A.; Montorsi, M.; Perinel, J.; Adham, M.; Dervenis, C.; Agalianos, C.; et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 2016, 160, 136–144. [Google Scholar] [CrossRef]
- Gebauer, F.; Damanakis, A.I.; Popp, F.; Quaas, A.; Kütting, F.; Lutz, K.; Held, S.; Deuß, B.; Göser, T.; Waldschmidt, D.; et al. Study protocol of an open-label, single arm phase II trial investigating the efficacy, safety and quality of life of neoadjuvant chemotherapy with liposomal irinotecan combined with Oxaliplatin and 5-fluorouracil/Folinic acid followed by curative surgical resection in patients with hepatic Oligometastatic adenocarcinoma of the pancreas (HOLIPANC). BMC Cancer 2021, 21, 1239. [Google Scholar] [CrossRef]
- Oura, K.; Aoki, T.; Tashiro, Y.; Matsuda, K.; Koizumi, T.; Kusano, T.; Wada, Y.; Shibata, H.; Yamashita, T.; Date, H.; et al. Indocyanine green fluorescence image-guided laparoscopic hepatectomy enabled resection of a tumor invisible with ultrasonography. Anticancer Res. 2021, 41, 3867–3869. [Google Scholar] [CrossRef]
- Tashiro, Y.; Aoki, T.; Hirai, T.; Koizumi, T.; Mansou, D.A.; Kusano, T.; Matsuda, K.; Yamada, K.; Nogaki, K.; Hakozaki, T.; et al. Pathological Validity of Using Near-infrared Fluorescence Imaging for Securing Surgical Margins During Liver Resection. Anticancer Res. 2020, 40, 3873–3882. [Google Scholar] [CrossRef]
- Tashiro, Y.; Aoki, T.; Hirai, T.; Koizumi, T.; Kusano, T.; Matsuda, K.; Yamada, K.; Nogaki, K.; Hakozaki, T.; Wada, Y.; et al. Indocyanine Green Labeling of Tumors in the Liver Recurring After Radiofrequency Ablation Enables Complete Resection by Fluorescence-guided Surgery. Anticancer Res. 2022, 42, 1345–1350. [Google Scholar] [CrossRef]
- Maawy, A.A.; Hiroshima, Y.; Kaushal, S.; Luiken, G.A.; Hoffman, R.M.; Bouvet, M. Comparison of a chimeric anti-carcinoembryonic antigen antibody conjugated with visible or near-infrared fluorescent dyes for imaging pancreatic cancer in orthotopic nude mouse models. J. Biomed. Opt. 2013, 18, 126016. [Google Scholar] [CrossRef]
| 24 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|
| Tumor mFI | 0.140 | 0.144 | 0.143 | 0.131 |
| Background mFI | 0.044 | 0.031 | 0.022 | 0.019 |
| Average TBR (SD) | 3.332 (0.884) | 4.913 (1.682) | 7.034 (3.285) | 6.915 (1.880) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, M.A.; Cox, K.E.; Neel, N.; Amirfakhri, S.; Nishino, H.; Clary, B.M.; Hosseini, M.; Natarajan, G.; Mallya, K.; Mohs, A.M.; et al. Highly Selective Targeting of Pancreatic Cancer in the Liver with a Near-Infrared Anti-MUC5AC Probe in a PDOX Mouse Model: A Proof-of-Concept Study. J. Pers. Med. 2023, 13, 857. https://doi.org/10.3390/jpm13050857
Turner MA, Cox KE, Neel N, Amirfakhri S, Nishino H, Clary BM, Hosseini M, Natarajan G, Mallya K, Mohs AM, et al. Highly Selective Targeting of Pancreatic Cancer in the Liver with a Near-Infrared Anti-MUC5AC Probe in a PDOX Mouse Model: A Proof-of-Concept Study. Journal of Personalized Medicine. 2023; 13(5):857. https://doi.org/10.3390/jpm13050857
Chicago/Turabian StyleTurner, Michael A., Kristin E. Cox, Nicholas Neel, Siamak Amirfakhri, Hiroto Nishino, Bryan M. Clary, Mojgan Hosseini, Gopalakrishnan Natarajan, Kavita Mallya, Aaron M. Mohs, and et al. 2023. "Highly Selective Targeting of Pancreatic Cancer in the Liver with a Near-Infrared Anti-MUC5AC Probe in a PDOX Mouse Model: A Proof-of-Concept Study" Journal of Personalized Medicine 13, no. 5: 857. https://doi.org/10.3390/jpm13050857
APA StyleTurner, M. A., Cox, K. E., Neel, N., Amirfakhri, S., Nishino, H., Clary, B. M., Hosseini, M., Natarajan, G., Mallya, K., Mohs, A. M., Hoffman, R. M., Batra, S. K., & Bouvet, M. (2023). Highly Selective Targeting of Pancreatic Cancer in the Liver with a Near-Infrared Anti-MUC5AC Probe in a PDOX Mouse Model: A Proof-of-Concept Study. Journal of Personalized Medicine, 13(5), 857. https://doi.org/10.3390/jpm13050857






