Awareness and Compliance with the Recommendations of Primary and Secondary Prevention of Cancer in Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Statistical Methods
3. Results
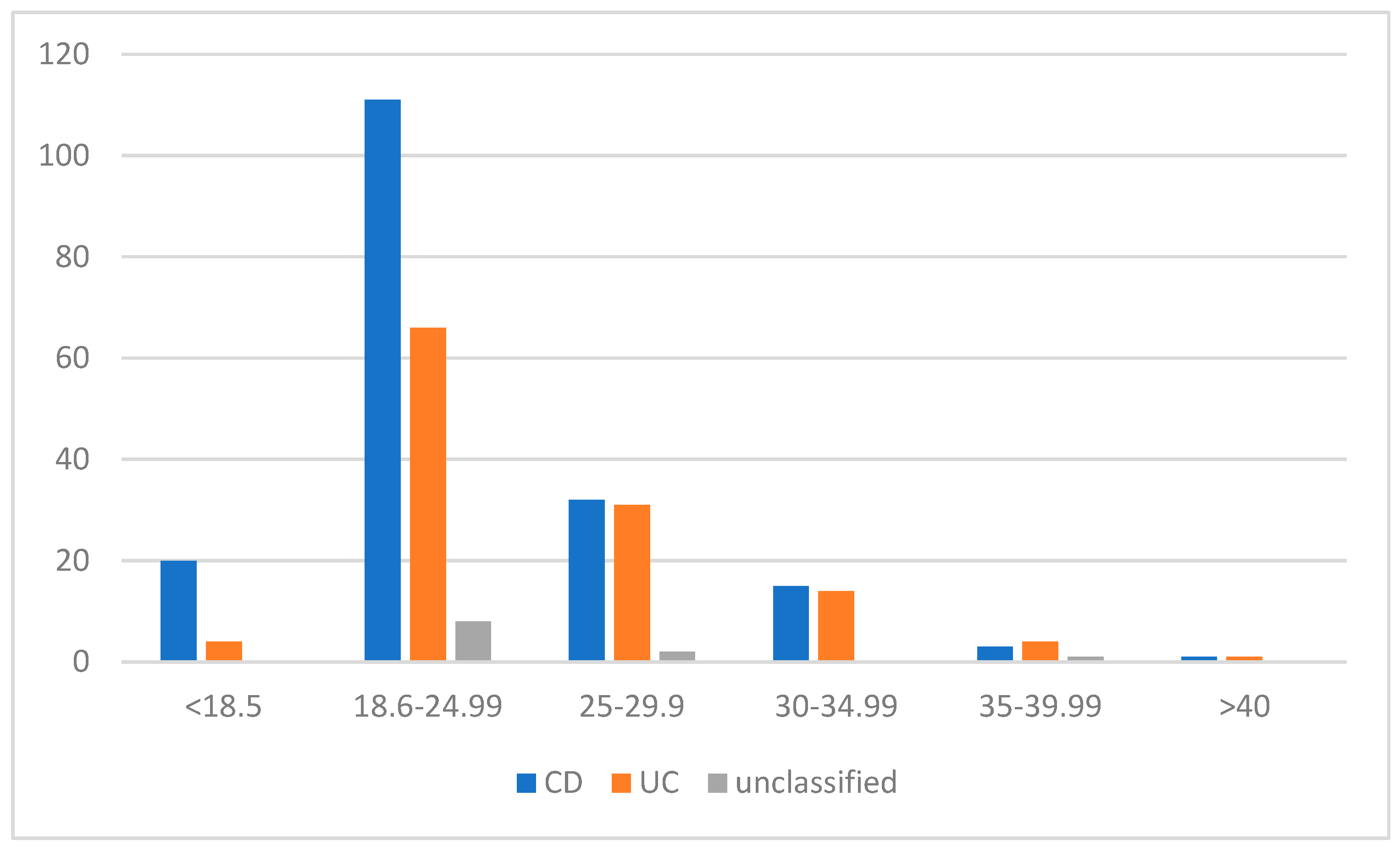
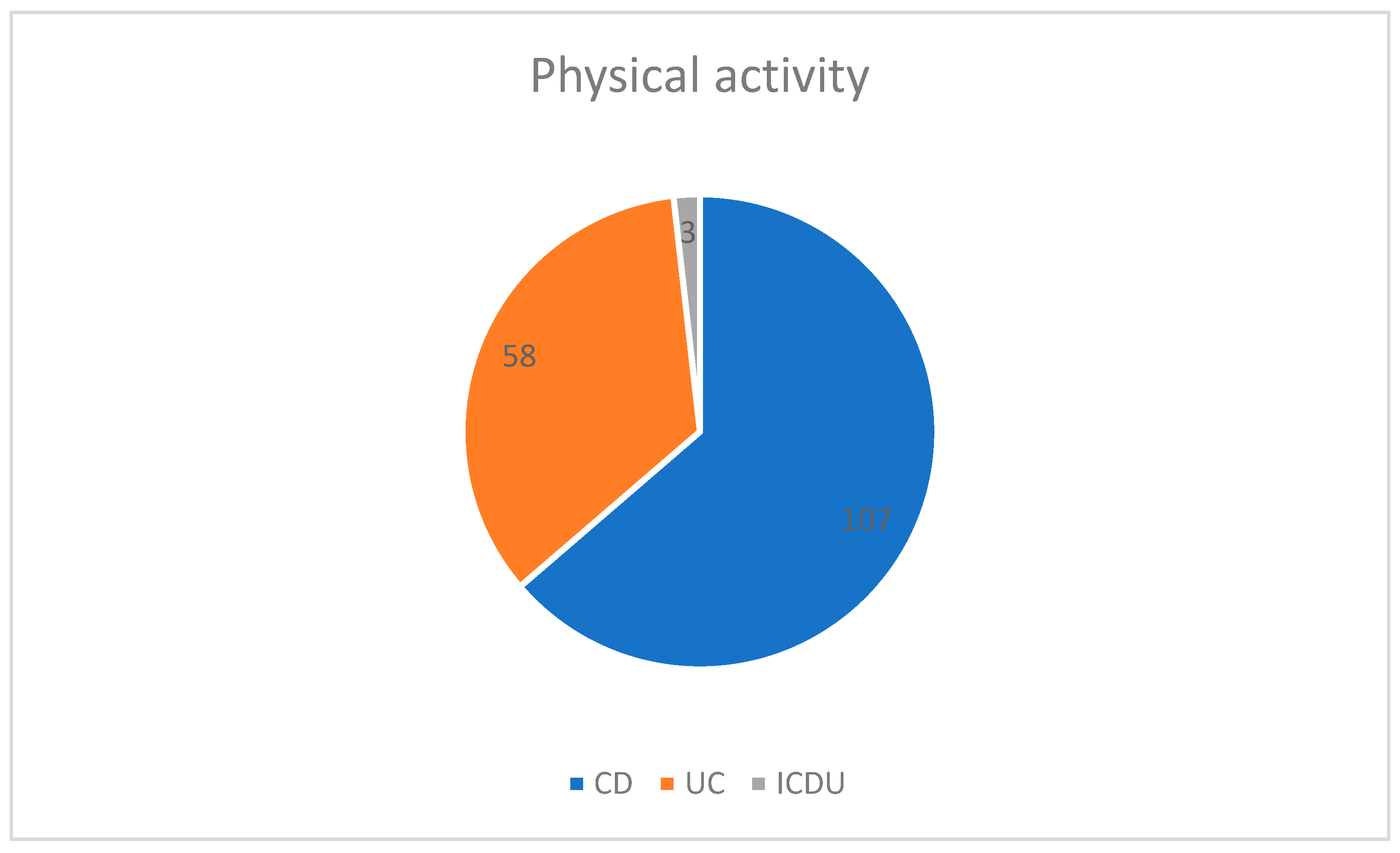
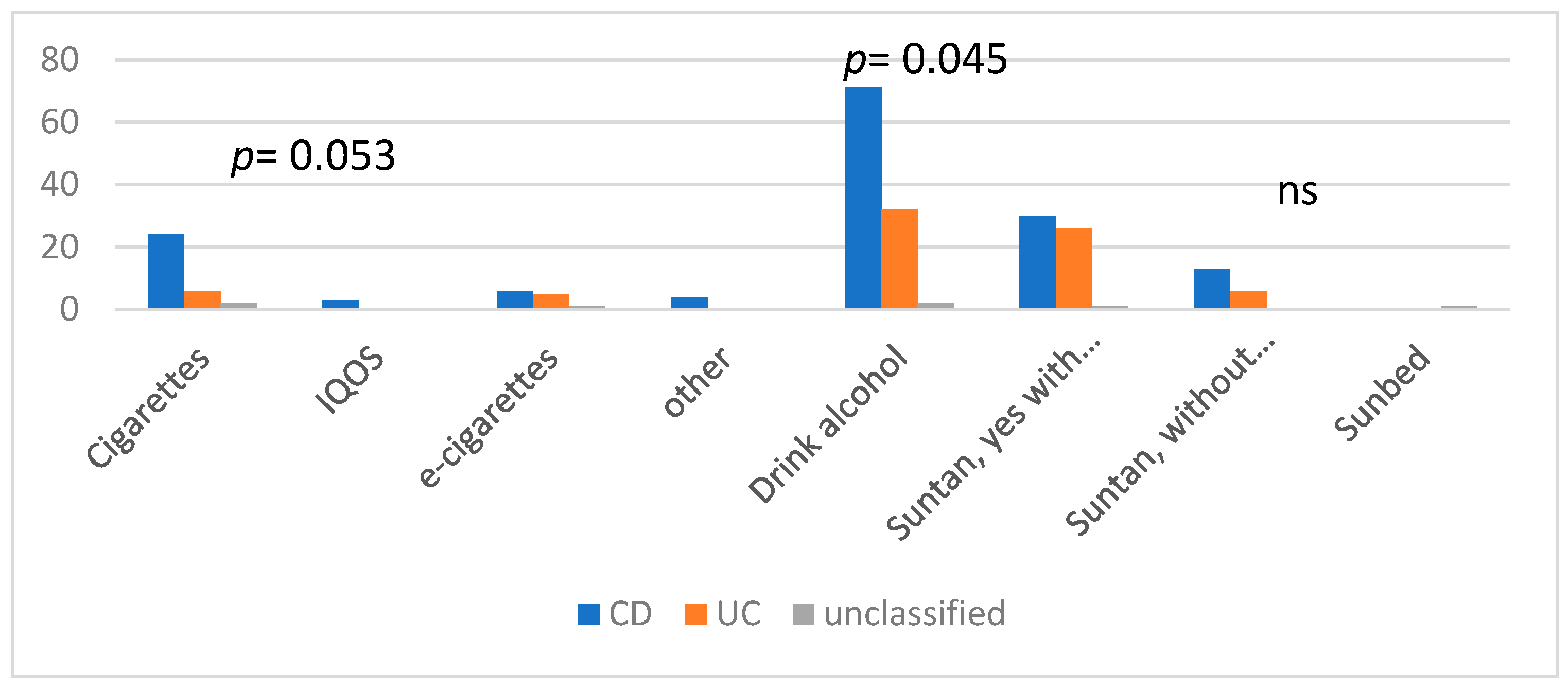
3.1. Physical Activity Status, BMI, Smoking, and Alcohol Intake
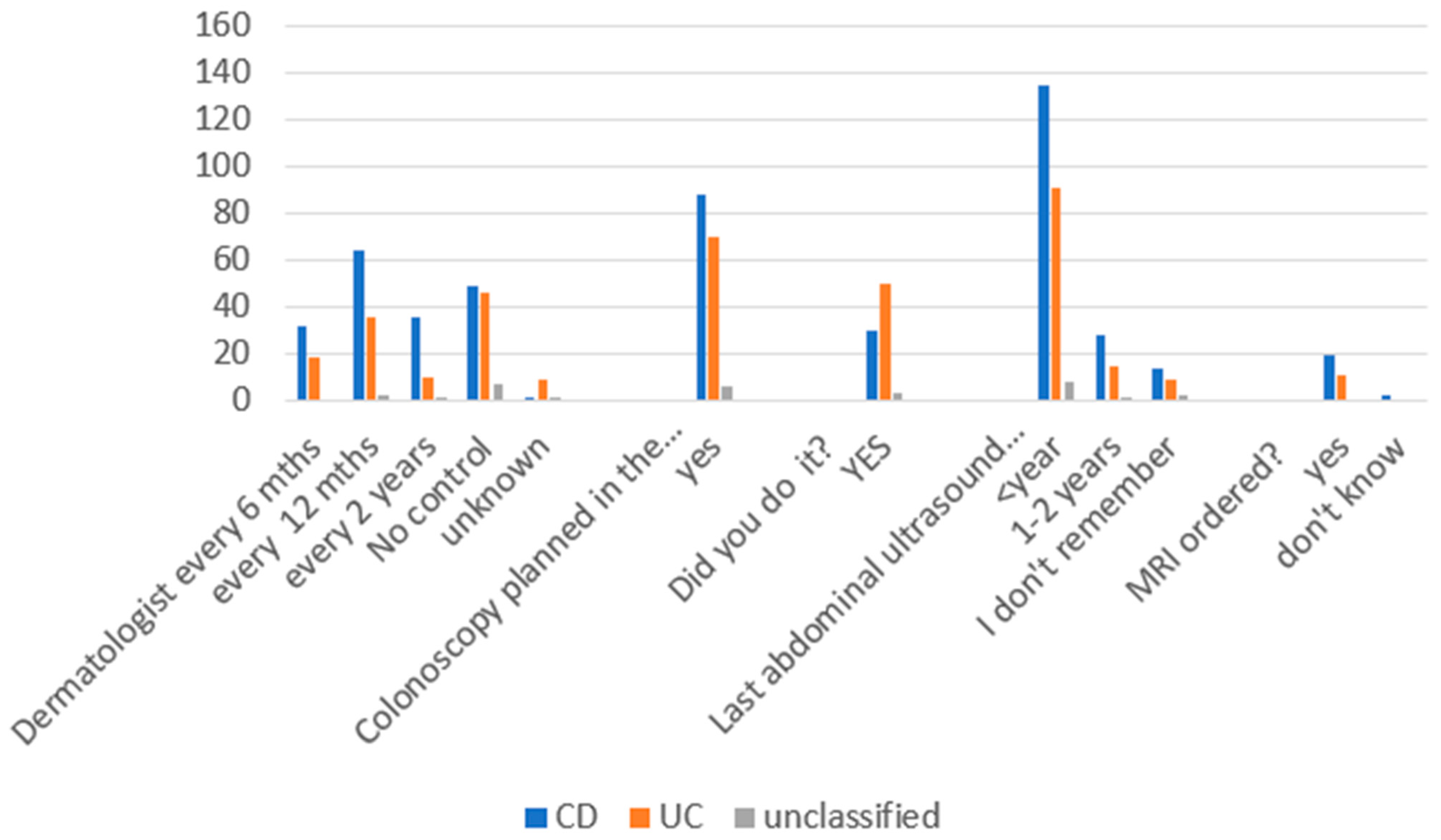
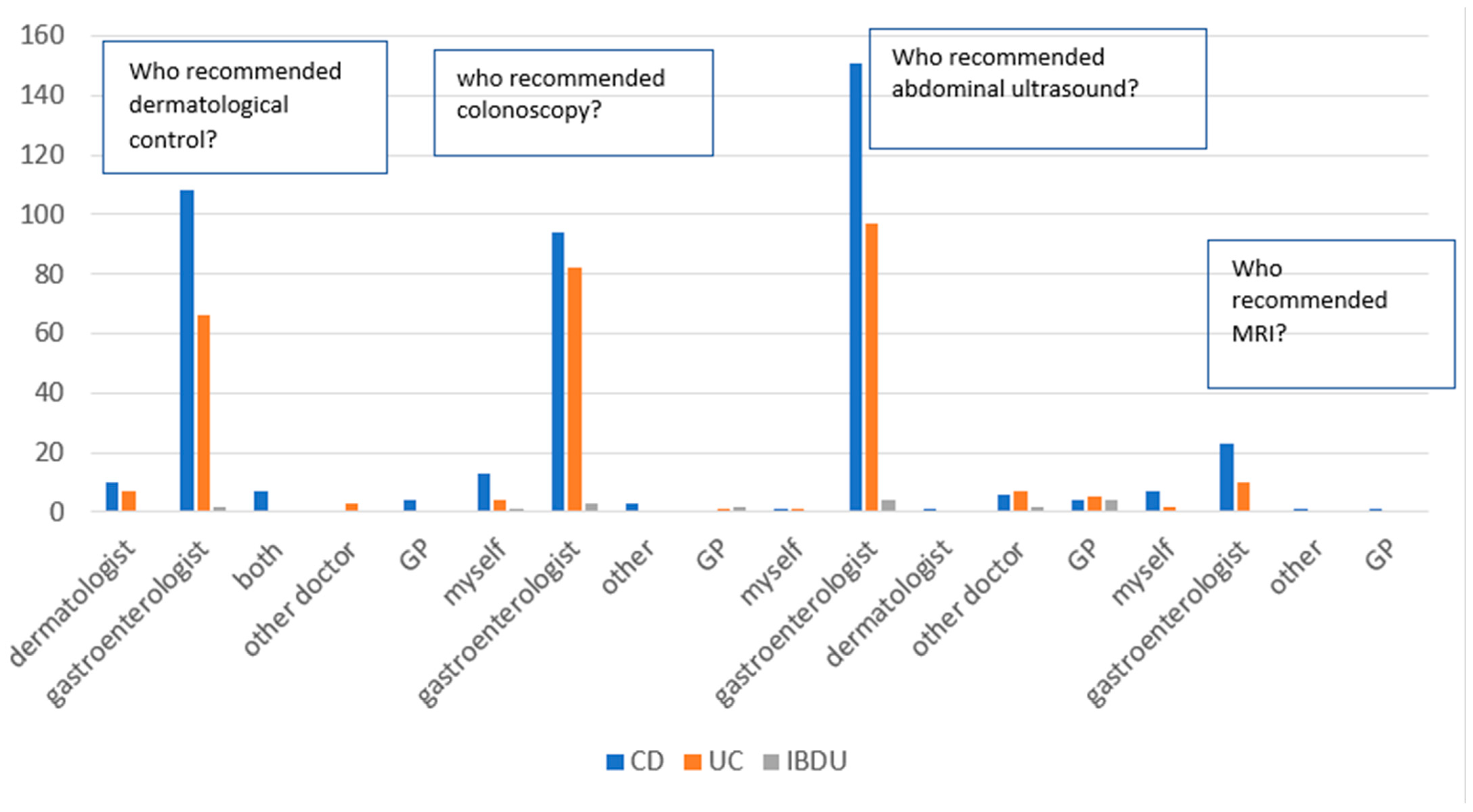
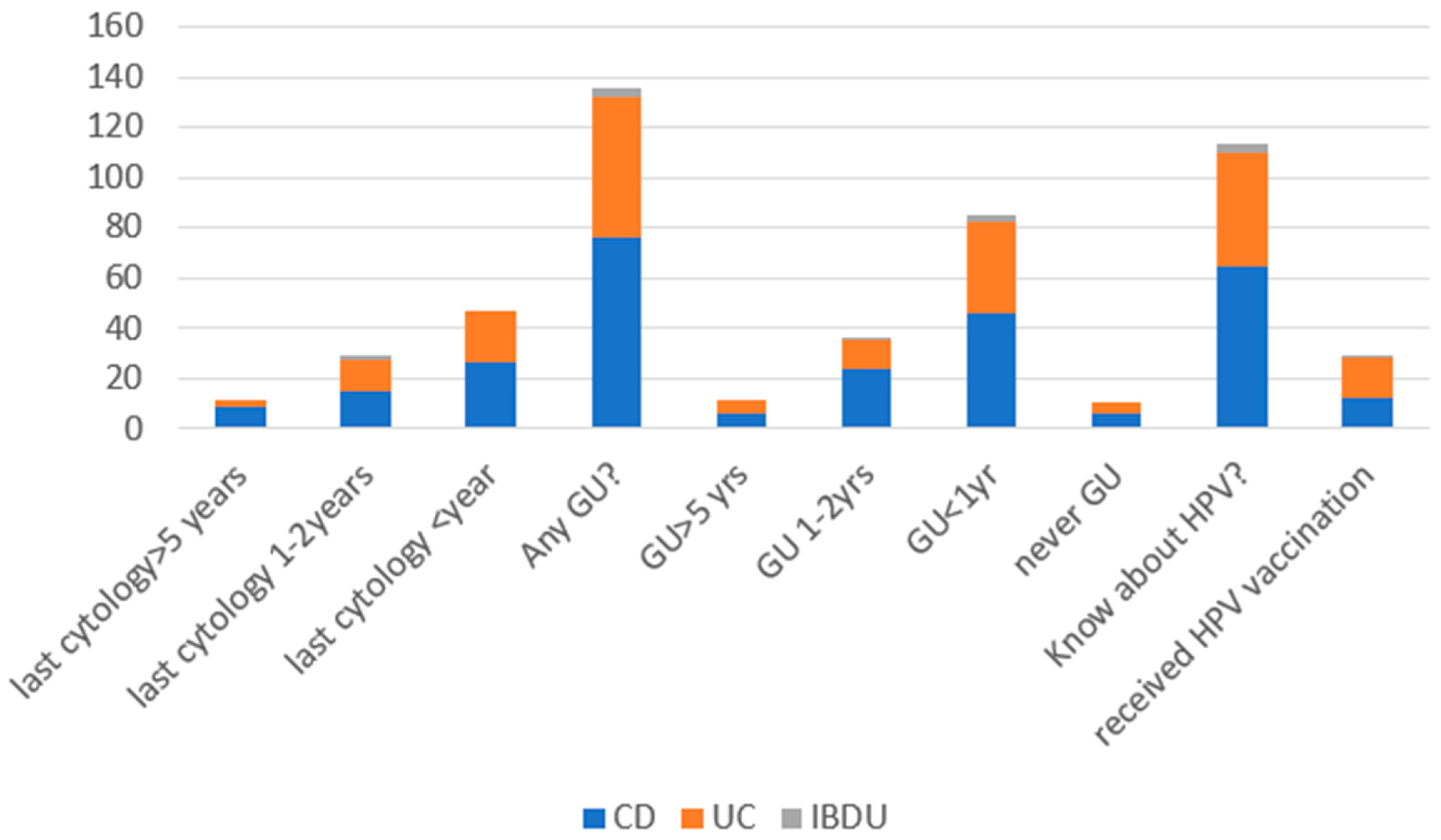
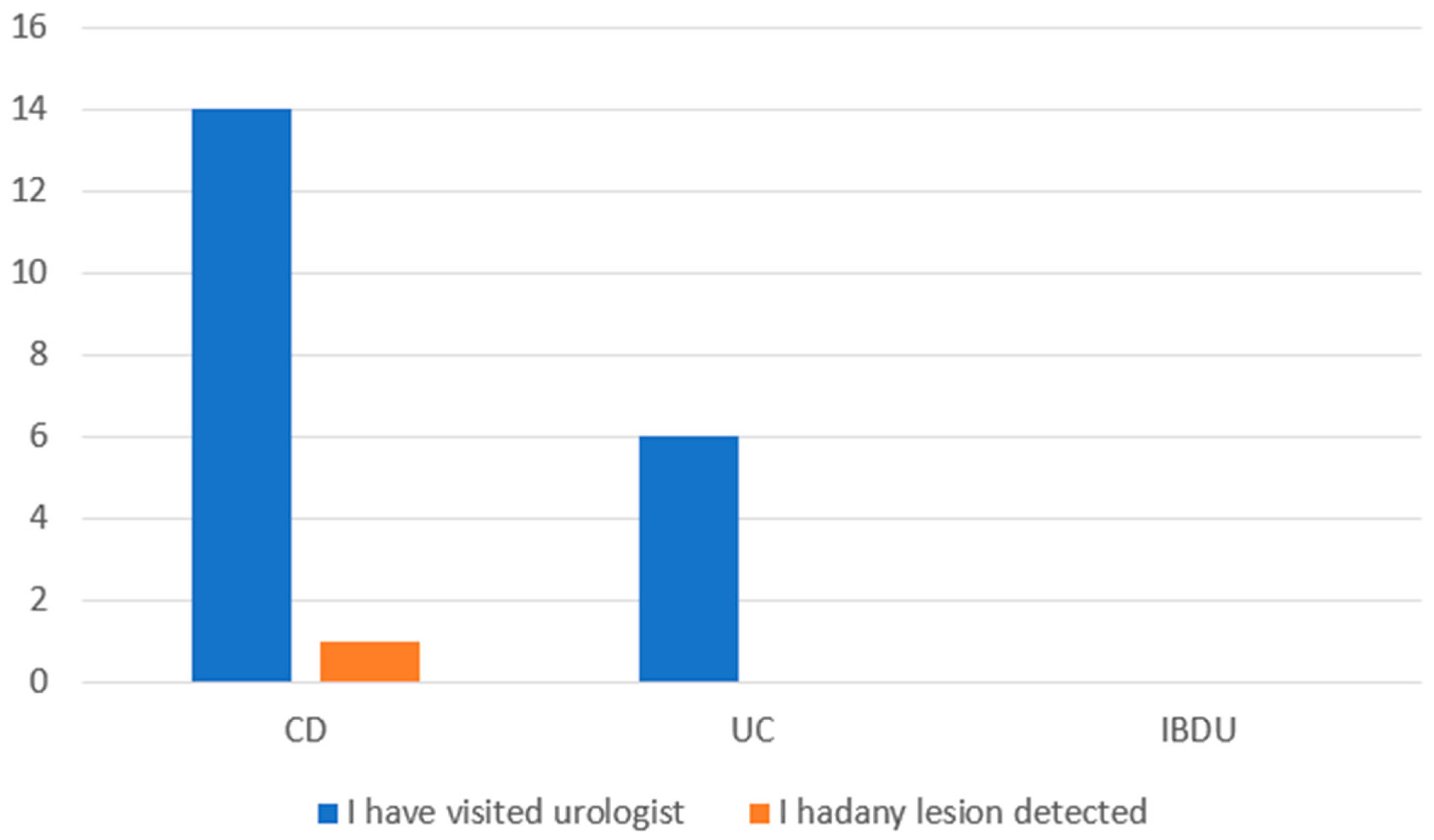
3.2. Breast Cancer, HPV-Related Dysplasia, and Cancer of the Uterine Cervix Prevention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spratt, J.S. The primary and secondary prevention of cancer. J. Surg. Oncol. 1981, 18, 219–230. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778, Erratum in Lancet 2020, 396, e56. [Google Scholar] [CrossRef]
- El-Tawil, A.M. Epidemiology and inflammatory bowel diseases. World J. Gastroenterol. 2013, 19, 1505–1507. [Google Scholar] [CrossRef]
- LoConte, N.K.; Gershenwald, J.E.; Thomson, C.A.; Crane, T.E.; Harmon, G.E.; Rechis, R. Lifestyle Modifications and Policy Implications for Primary and Secondary Cancer Prevention: Diet, Exercise, Sun Safety, and Alcohol Reduction. Am. Soc. Clin. Oncol. Educ. Book. 2018, 38, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Eder, P. Choroba nowotworowa u pacjenta z nieswoistą chorobą zapalną jelit—Zasady postępowania. Gastroenterol. Klin. 2016, 8, 119–130. [Google Scholar]
- Lech, G.; Korcz, W.; Kowalczyk, E.; Chaber, A.; Słodkowski, M. The risk of small bowel adenocarcinoma in patients with Crohn’s disease. Gastroenterol. Rev. Przegląd Gastroenterol. 2020, 15, 309–313. [Google Scholar] [CrossRef]
- Eder, P.; Łodyga, M.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of ulcerative colitis. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Gastroenterol. Rev. Przegląd Gastroenterol. 2023, 18, 1–42. [Google Scholar] [CrossRef]
- Łodyga, M.; Eder, P.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of patients with Crohn’s disease. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Prz. Gastroenterol. 2021, 16, 257–296. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Sulais, E.A.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohn's Colitis. 2022, jjac187. [Google Scholar] [CrossRef]
- Annese, V.; Beaugerie, L.; Egan, L.; Biancone, L.; Bolling, C.; Brandts, C.; Dierickx, D.; Dummer, R.; Fiorino, G.; Gornet, J.M.; et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J. Crohn's Colitis 2015, 9, 945–965. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef]
- Islam, M.R.; Arthur, S.; Haynes, J.; Butts, M.R.; Nepal, N.; Sundaram, U. The Role of Gut Microbiota and Metabolites in Obesity-Associated Chronic Gastrointestinal Disorders. Nutrients 2022, 14, 624. [Google Scholar] [CrossRef] [PubMed]
- Rozich, J.J.; Holmer, A.; Singh, S. Effect of Lifestyle Factors on Outcomes in Patients With Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2020, 115, 832–840. [Google Scholar] [CrossRef]
- Kadenczki, O.; Dezsofi, A.; Cseh, A.; Szucs, D.; Vass, N.; Nemes, E.; Tarnok, A.; Szakos, E.; Guthy, I.; Kovacs, M.; et al. Disease Activity Is Associated with Obesity in Newly Diagnosed Pediatric Patients with Ulcerative Colitis. Int. J. Environ. Res. Public Health 2022, 19, 16091. [Google Scholar] [CrossRef] [PubMed]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.; Etchevers, M.J.; Domènech, E.; García-Sánchez, V.; Ber, Y.; Peñalva, M.; Merino, O.; Nos, P.; Garcia-Planella, E.; Casbas, A.G.; et al. Smoking does influence disease behaviour and impacts the need for therapy in Crohn's disease in the biologic era. Aliment. Pharmacol. Ther. 2013, 38, 752–760. [Google Scholar] [CrossRef]
- Goetgebuer, R.L.; Kreijne, J.E.; Aitken, C.A.; Dijkstra, G.; Hoentjen, F.; de Boer, N.K.; Oldenburg, B.; van der Meulen, A.E.; Ponsioen, C.I.J.; Pierik, M.J.; et al. Increased Risk of High-grade Cervical Neoplasia in Women with Inflammatory Bowel Disease: A Case-controlled Cohort Study. J. Crohn’s Colitis 2021, 15, 1464–1473. [Google Scholar] [CrossRef]
- Piovezani Ramos, G.; Kane, S. Alcohol Use in Patients With Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2021, 17, 211–225. [Google Scholar]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef]
- Beaugerie, L.; Carrat, F.; Colombel, J.-F.; Bouvier, A.-M.; Sokol, H.; Babouri, A.; Carbonnel, F.; Laharie, D.; Faucheron, J.-L.; Simon, T.; et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut 2014, 63, 1416–1423. [Google Scholar] [CrossRef]
- Scharl, S.; Barthel, C.; Rossel, J.B.; Biedermann, L.; Misselwitz, B.; Schoepfer, A.M.; Straumann, A.; Vavricka, S.R.; Rogler, G.; Scharl, M.; et al. Malignancies in Inflammatory Bowel Disease: Frequency, Incidence and Risk Factors-Results from the Swiss IBD Cohort Study. Am. J. Gastroenterol. 2019, 114, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Madanchi, M.; Zeitz, J.; Barthel, C.; Samaras, P.; Scharl, S.; Sulz, M.C.; Biedermann, L.; Frei, P.; Vavricka, S.R.; Rogler, G.; et al. Malignancies in Patients with Inflammatory Bowel Disease: A Single-Centre Experience. Digestion 2016, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheddani, H.; Dauchet, L.; Fumery, M.; Charpentier, C.; Bouvier, A.M.; Dupas, J.-L.; Pariente, B.; Peyrin-Biroulet, L.; Savoye, G.; Gower-Rousseau, C. Cancer in Elderly Onset Inflammatory Bowel Disease: A Population-Based Study. Am. J. Gastroenterol. 2016, 111, 1428–1436. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef] [PubMed]
- Ballester, M.P.; Mesonero, F.; Flórez-Diez, P.; Gómez, C.; Fuentes-Valenzuela, E.; Martín, N.; Senosiain, C.; Vela, M.; Fernández-Clotet, A.; Pérez, P.; et al. Adherence to endoscopic surveillance for advanced lesions and colorectal cancer in inflammatory bowel disease: An AEG and GETECCU collaborative cohort study. Aliment. Pharmacol. Ther. 2022, 55, 1402–1413. [Google Scholar] [CrossRef]
- Friedman, S.; Cheifetz, A.S.; Farraye, F.A.; Banks, P.A.; Makrauer, F.L.; Burakoff, R.; Farmer, B.; Torgersen, L.N.; Wahl, K.E. Factors that affect adherence to surveillance colonoscopy in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 534–539. [Google Scholar] [CrossRef]
- Jackson, B.D.; Con, D.; Liew, D.; De Cruz, P. Clinicians' adherence to international guidelines in the clinical care of adults with inflammatory bowel disease. Scand J. Gastroenterol. 2017, 52, 536–542. [Google Scholar] [CrossRef]
- Hazenberg, H.M.; de Boer, N.K.; Mulder, C.J.; Mom, S.H.; van Bodegraven, A.A.; Tack, G.J. Neoplasia and Precursor Lesions of the Female Genital Tract in IBD: Epidemiology, Role of Immunosuppressants, and Clinical Implications. Inflamm. Bowel Dis. 2018, 24, 510–531. [Google Scholar] [CrossRef]
- Beaugerie, L.; Rahier, J.F.; Kirchgesner, J. Predicting, Preventing, and Managing Treatment-Related Complications in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1324–1335.e2. [Google Scholar] [CrossRef]
- Haddad, A.; Al-Sabbagh, M.Q.; Al-Ani, H.; Siyam, A.; Aborajooh, E.; Iwata, T.; Kimura, S.; Shariat, S.F.; Abufaraj, M. Inflammatory bowel disease and prostate cancer risk: A systematic review. Arab. J. Urol. 2020, 18, 207–212. [Google Scholar] [CrossRef]

| CD n = 182 | UC n = 120 | IBDU n = 11 | |
|---|---|---|---|
| Female, n (%) Male, n (%) | 84 (46.2%) 98 (53.8%) | 57 (47.5%) 63 (52.5%) | 4 (36.4%) 7 (63.6%) |
| Age (years) (%) | |||
| <20 | 10 (5.5%) | 2 (1.7%) | 0 (0%) |
| 21–30 | 63 (34.1%) | 35 (29.2%) | 1 (9.1%) |
| 31–40 | 55 (30.2%) | 36 (30.0%) | 3 (27.3%) |
| 41–50 | 39 (21.4%) | 21 (17.5%) | 3 (27.3%) |
| 51–60 | 11 (6.0%) | 17 (14.2%) | 3 (27.3%) |
| 61–65 | 2 (1.1%) | 0 (0%) | 0 (0%) |
| >65 | 3 (1.6%) | 9 (7.5%) | 1 (9.1%) |
| Duration of disease (years) | |||
| <1 year | 9 (4.9%) | 5 (4.2%) | 2 (18.2%) |
| 1–5 | 19 (10.4%) | 35 (29.2%) | 3 (27.3%) |
| 5–8 | 39 (21.4%) | 27 (22.5%) | 2 (18.2%) |
| >8 | 115 (63.2%) | 53 (44.2%) | 4 (36.4%) |
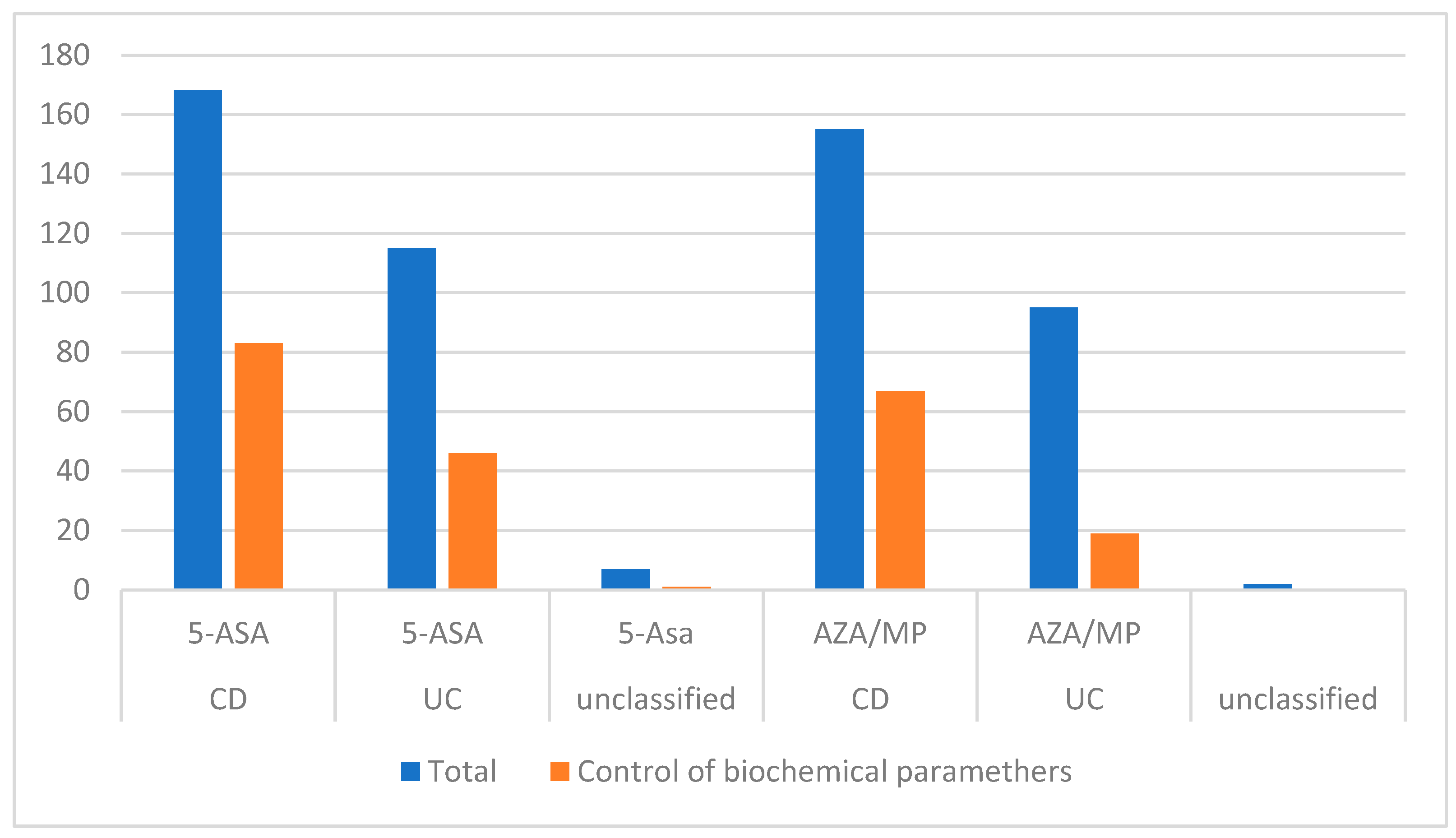
| Current use of medications | |||
| 5-ASA (Mesalazine or sulfasalazine) | 168 (92.3%) | 115 (95.8%) | 7(63.6%) |
| Corticosteroids | |||
| Prednisone or methylprednisolone | 102 (56.0%) | 72 (60.0%) | 1 (9.1%) |
| Budesonide | 86 (47.3%) | 67 (55.8%) | 2 (18.2%) |
| Immunosuppressants | |||
| Azathioprine/6-mercaptopurine | 155 (85.2%) | 95 (79.2%) | 2 (18.2%) |
| Methotrexate | 34 (18.7%) | 3 (2.5%) | 1 (9.1%) |
| Biologic agent | 149 (81.9%) | 71 (59.2%) | 2 (18.2%) |
| Contraceptive/hormone replacement therapy | 28 (15.4%) | 12 (10.0%) | 0 (0.0%) |
| Other medication | 2 (1.1%) | 5 (4.2%) | 0 (0.0%) |
| Characteristic | Overall, n = 313 1 | CD, n = 182 1 | UC, n = 120 1 | IBDU, n = 11 1 |
|---|---|---|---|---|
| Mesalazine, sulfasalazine | 290 (92.7%) | 168 (92.3%) | 115 (95.8%) | 7 (63.6%) |
| Azathioprine, 6-MP | 252 (80.5%) | 155 (85.2%) | 95 (79.2%) | 2 (18.2%) |
| MTX | 38 (12.1%) | 34 (18.7%) | 3 (2.5%) | 1 (9.1%) |
| Budesonide | 205 (65.5%) | 104 (57.1%) | 95 (79.2%) | 5 (4.5%) |
| Prednisone | 175 (55.9%) | 102 (56.0%) | 72 (60.0%) | 1 (9.1%) |
| Biological treatment | 222 (70.9%) | 149 (81.9%) | 71 (59.2%) | 2 (18.2%) |
| Contraceptive/ hormone replacement therapy | 40 (12.8%) | 28 (15.4%) | 12 (10.0%) | 0 (0.0%) |
| Other medication | 7 (2.2%) | 2 (1.1%) | 5 (4.2%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulewicz-Marti, E.; Stępień-Wrochna, B.; Maciejewska, K.; Łodyga, M.; Karłowicz, K.; Lewandowski, K.; Rydzewska, G. Awareness and Compliance with the Recommendations of Primary and Secondary Prevention of Cancer in Patients with Inflammatory Bowel Disease. J. Pers. Med. 2023, 13, 913. https://doi.org/10.3390/jpm13060913
Tulewicz-Marti E, Stępień-Wrochna B, Maciejewska K, Łodyga M, Karłowicz K, Lewandowski K, Rydzewska G. Awareness and Compliance with the Recommendations of Primary and Secondary Prevention of Cancer in Patients with Inflammatory Bowel Disease. Journal of Personalized Medicine. 2023; 13(6):913. https://doi.org/10.3390/jpm13060913
Chicago/Turabian StyleTulewicz-Marti, Edyta, Beata Stępień-Wrochna, Katarzyna Maciejewska, Michał Łodyga, Katarzyna Karłowicz, Konrad Lewandowski, and Grazyna Rydzewska. 2023. "Awareness and Compliance with the Recommendations of Primary and Secondary Prevention of Cancer in Patients with Inflammatory Bowel Disease" Journal of Personalized Medicine 13, no. 6: 913. https://doi.org/10.3390/jpm13060913
APA StyleTulewicz-Marti, E., Stępień-Wrochna, B., Maciejewska, K., Łodyga, M., Karłowicz, K., Lewandowski, K., & Rydzewska, G. (2023). Awareness and Compliance with the Recommendations of Primary and Secondary Prevention of Cancer in Patients with Inflammatory Bowel Disease. Journal of Personalized Medicine, 13(6), 913. https://doi.org/10.3390/jpm13060913







