Incidence and Patterns of Digestive Organ Cancer in Georgia: Insights from a Population-Based Registry Study in 2021
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Site Classification
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- National Statistics Office of Georgia. Demographic Situation in Georgia; National Statistics Office of Georgia: Tbilisi, Georgia, 2021. [Google Scholar]
- The World Bank. The World by Income and Region. 2021. Available online: https://datatopics.worldbank.org/worlddevelopment-indicators/the-world-by-income-and-region.html (accessed on 22 August 2022).
- The World Bank. GDP per Capita (Current US$). 2021. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD (accessed on 22 August 2022).
- Richardson, E.; Berdzuli, N. Georgia: Health system review. In Georgia Health System Review; World Health Organization: Geneva, Switzerland, 2017; Volume 19, Available online: https://apps.who.int/iris/handle/10665/330206 (accessed on 3 August 2022).
- National Centre for Disease Control and Public Health, Cancer in Georgia, 2015–2019, Tbilisi. 2020. Available online: https://test.ncdc.ge/Handlers/GetFile.ashx?ID=61a5547b-8e8b-482b-8425-12f8bdfbb7bb (accessed on 3 August 2022).
- Fritz, A.G.; World Health Organization. International Classification of Diseases for Oncology = ICD-O, 3rd ed.; World Health Organization: Geneva, Switzerland, 2000; p. 240. [Google Scholar]
- European Union. Revision of the European Standard Population—Report of Eurostat’s Task Force—2013 edition. 2013. Available online: https://ec.europa.eu/eurostat/web/products-manuals-and-guidelines/-/ks-ra-13-028 (accessed on 3 August 2022).
- World Health Organization. Age Standardization of Rates: A New WHO Standard. 2001. Available online: https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/gpe_discussion_paper_series_paper31_2001_age_standardization_rates.pdf (accessed on 3 August 2022).
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. Eclinicalmedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern. Med. 2008, 47, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–538. [Google Scholar]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; E Schoen, R.; Sung, J.J.Y.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Keum, N.; Nishihara, R.; Giovannucci, E.L. Cancers of the colon and rectum. In Cancer Epidemiology and Prevention, 4th ed.; Thun, M.J., Linet, M.S., Cerhan, J.R., Haiman, C.A., Schottenfeld, D., Eds.; Oxford University Press: New York, NY, USA, 2018; pp. 681–706. [Google Scholar]
- Subedi, R.; Budukh, A.; Chapagain, S.; Gyanwali, P.; Gyawali, B.; Khadka, K.; Thakur, C.; Dahal, U.; Dikshit, R.; Jha, A.K.; et al. Differences in cancer incidence and pattern between urban and rural Nepal: One-year experience from two population-based cancer registries. Ecancermedicalscience 2021, 15, 1229. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.R.; Blackburn, B.E.; Huntington, M.; Curtin, K.; Thorpe, R.J., Jr.; Rowe, K.; Snyder, J.; Deshmukh, V.; Newman, M.; Fraser, A.; et al. Rural-urban disparities in colorectal cancer survival and risk among men in Utah: A statewide population-based study. Cancer Causes Control 2020, 31, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, D.E.; Shah, M.A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013, 107, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Shimizu, S.; Shirotani, M.; Yorozu, K.; Kitamura, K.; Oehorumu, M.; Kawai, Y.; Fukuzawa, Y. Nutrition and Cancer Risk from the Viewpoint of the Intestinal Microbiome. Nutrients 2021, 13, 3326. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; von Karsa, L.; Herrero, R. Prevention strategies for gastric cancer: A global perspective. Clin. Endosc. 2014, 47, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Kamangar, F.; Nasrollahzadeh, D.; Aghcheli, K.; Sotoudeh, M.; Abedi-Ardekani, B.; Merat, S.; Nasseri-Moghaddam, S.; Semnani, S.; Sepehr, A.; et al. Socio-economic status and oesophageal cancer: Results from a population-based case-control study in a high-risk area. Int. J. Epidemiol. 2009, 38, 978–988. [Google Scholar] [CrossRef] [PubMed]
- London, W.T.; Petrick, J.L.; McGlynn, K.A. Liver cancer. In Cancer Epidemiology and Prevention, 4th ed.; Thun, M.J., Linet, M.S., Cerhan, J.R., Haiman, C.A., Schottenfeld, D., Eds.; Oxford University Press: New York, NY, USA, 2018; pp. 635–660. [Google Scholar]
- Centers for Diseases Control and Prevention; Division of Viral Hepatitis, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. Global Viral Hepatitis: Georgia’s Hepatitis C Elimination Program. 2021. Available online: https://www.cdc.gov/hepatitis/global/GeorgiaHepCProg.htm (accessed on 8 June 2023).
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
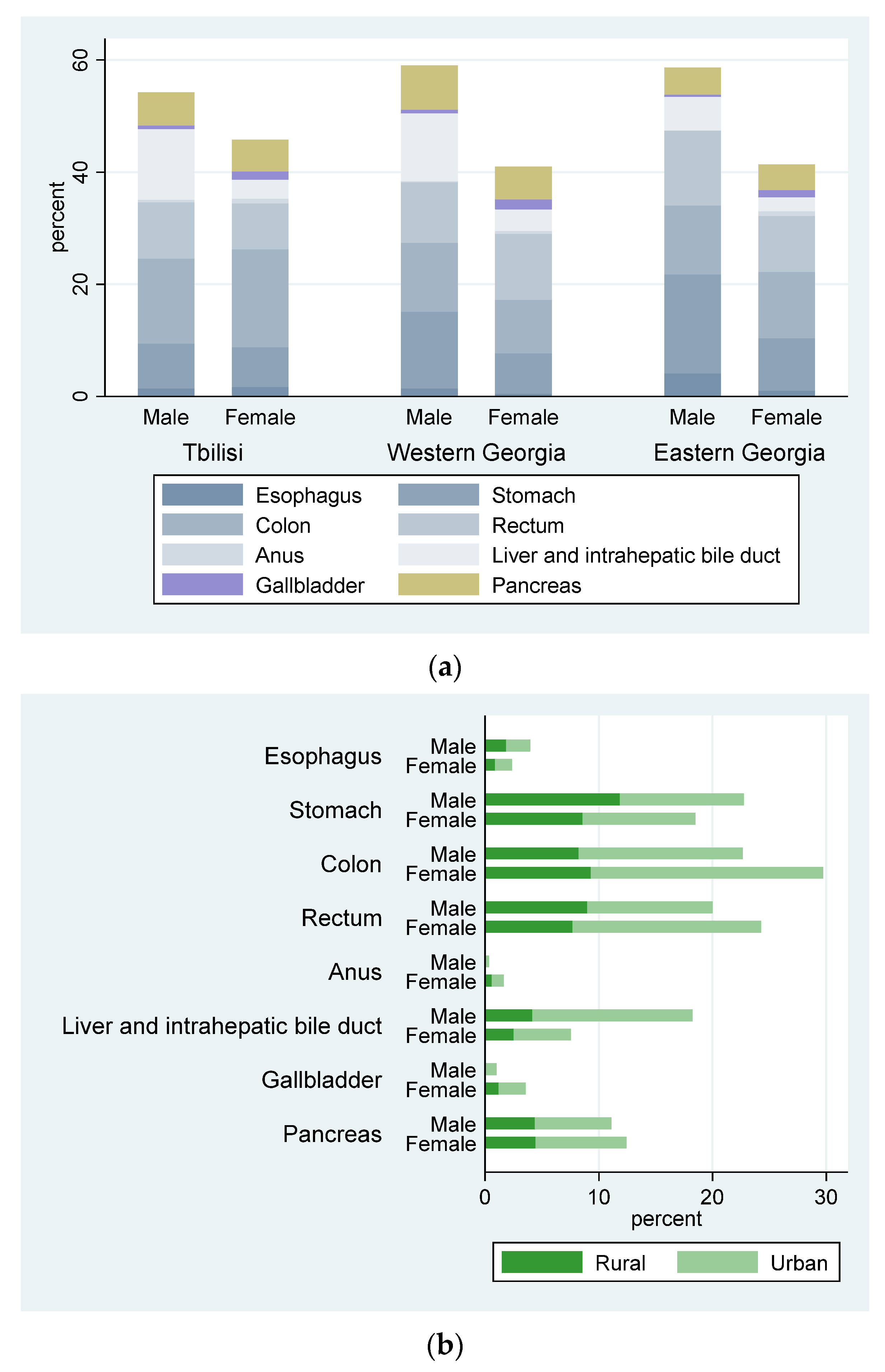
| Cancer Site (ICD-10) | CR 1 | ESR 2 | WSR 3 |
|---|---|---|---|
| Esophagus (C15) | 1.4 | 1.6 | 0.6 |
| Stomach (C16) | 9.0 | 10.4 | 4.2 |
| Colon (C18) | 11.4 | 13.2 | 5.3 |
| Rectum (C19–20) | 9.6 | 11.2 | 4.3 |
| Anus (C21) | 0.4 | 0.4 | 0.1 |
| Liver and intrahepatic bile ducts (C22) | 6.0 | 6.7 | 3.1 |
| Gallbladder (C23) | 0.9 | 1.0 | 0.3 |
| Pancreas (C25) | 5.1 | 5.7 | 2.5 |
| Cancer Site and Subsite | ICD-10 Code | Overall | ≤49 | 50–69 | ≥70 | Median (IQR) | Male | Female |
|---|---|---|---|---|---|---|---|---|
| Esophagus | C15 | 52 | 5 (3.62%) | 28 (3.11%) | 19 (3.19%) | 63 (60–66) | 36 (3.83%) | 16 (2.30%) |
| Stomach | C16 | 337 | 24 (17.39%) | 187 (20.75%) | 126 (21.14%) | 62 (58.5–66) | 211 (22.47%) | 126 (18.10%) |
| Colon | C18 | 423 | 39 (28.26%) | 226 (25.08%) | 158 (26.51%) | 62 (59–66) | 214 (22.79%) | 209 (30.03%) |
| Rectum | C19, C20 | 358 | 26 (18.84%) | 175 (19.42%) | 157 (26.34%) | 62 (59–66) | 186 (19.81%) | 172 (24.71%) |
| Anus | C21 | 15 | 1 (0.72%) | 10 (1.11%) | 4 (0.67%) | 63 (60–68) | 3 (0.32%) | 12 (1.72%) |
| Liver and intrahepatic bile ducts | C22 | 226 | 18 (13.04%) | 151 (16.76%) | 57 (9.56%) | 60 (57–64) | 174 (18.53%) | 52 (7.47%) |
| Gallbladder | C23 | 34 | 1 (0.72%) | 20 (2.22%) | 13 (2.18%) | 63 (59–67) | 10 (1.06%) | 24 (3.45%) |
| Pancreas | C25 | 190 | 24 (17.39%) | 104 (11.54%) | 62 (10.40%) | 61 (58–67) | 105 (11.18%) | 85 (12.21%) |
| Histological Groups | Esophagus (C15) n (%) | Stomach (C16) n (%) | Colon (C18) n (%) | Rectum (C19–C20) n (%) | Anus (C21) n (%) | Liver and Intrahepatic Bile Ducts (C22) n (%) | Gallbladder (C23) n (%) | Pancreas (C25) n (%) |
|---|---|---|---|---|---|---|---|---|
| Carcinoma | 43 (82.7%) | 271 (80.4%) | 377 (89.9%) | 316 (88.2%) | 14 (93.3%) | 83 (36.7%) | 24 (70.6%) | 88 (46.5%) |
| Squamous cell carcinoma | 35 (67.3%) | 3 (0.9%) | 3 (0.7%) | 13 (3.6%) | 9 (60.0%) | 23 (10.2%) | 18 (52.9%) | 12 (6.3%) |
| Adenocarcinoma | 6 (11.5%) | 252 (74.7%) | 372 (88.8%) | 297 (82.9%) | 5 (33.3%) | 60 (26.5%) | 6 (17.6%) | 76 (40.2%) |
| Other specified carcinoma | - | - | - | - | - | - | - | - |
| Unspecified carcinoma | 2 (3.8%) | 15 (4.4%) | 2 (0.4%) | 6 (1.6%) | - | - | - | - |
| Hepatoblastoma | - | - | - | - | - | 2 (0.9%) | - | - |
| Sarcoma | 2 (3.8%) | 10 (2.9%) | 6 (1.4%) | 2 (0.5%) | - | 3 (1.3%) | - | - |
| Other specified malignant neoplasm | - | - | - | - | - | - | - | - |
| Unspecified malignant neoplasm | 7 (13.5%) | 50 (14.8%) | 36 (8.6%) | 38 (10.6%) | 1 (6.6%) | 138 (61.1%) | 10 (29.4%) | 101 (53.4%) |
| Variable | Esophagus (C15) n (%) | Stomach (C16) n (%) | Colon (C18) n (%) | Rectum (C19–C20) n (%) | Anus (C21) n (%) | Liver and Intrahepatic Bile Ducts (C22) n (%) | Gallbladder (C23) n (%) | Pancreas (C25) n (%) | p Value * |
|---|---|---|---|---|---|---|---|---|---|
| Sex | <0.000 | ||||||||
| Male | 36 (69.2) | 211 (62.6) | 214 (50.5) | 186 (51.9) | <5 | 174 (76.9) | 10 (29.4) | 105 (55.2) | |
| Female | 16 (30.7) | 126 (37.3) | 209 (49.4) | 172 (48.0) | 12 (80.0) | 52 (23.0) | 24 (70.5) | 85 (44.7) | |
| Age | <0.007 | ||||||||
| Birth to 49 | 5 (9.6) | 24 (7.1) | 39 (9.2) | 26 (7.2) | <5 | 18 (7.9) | <5 | 24 (12.6) | |
| 50–69 | 28 (53.8) | 187 (55.4) | 226 (53.4) | 175 (48.8) | 10 (66.6) | 151 (66.8) | 20 (58.8) | 104 (54.7) | |
| ≥70 | 19 (36.5) | 126 (37.3) | 158 (37.3) | 157 (43.8) | <5 | 57 (25.2) | 13 (38.2) | 62 (32.6) | |
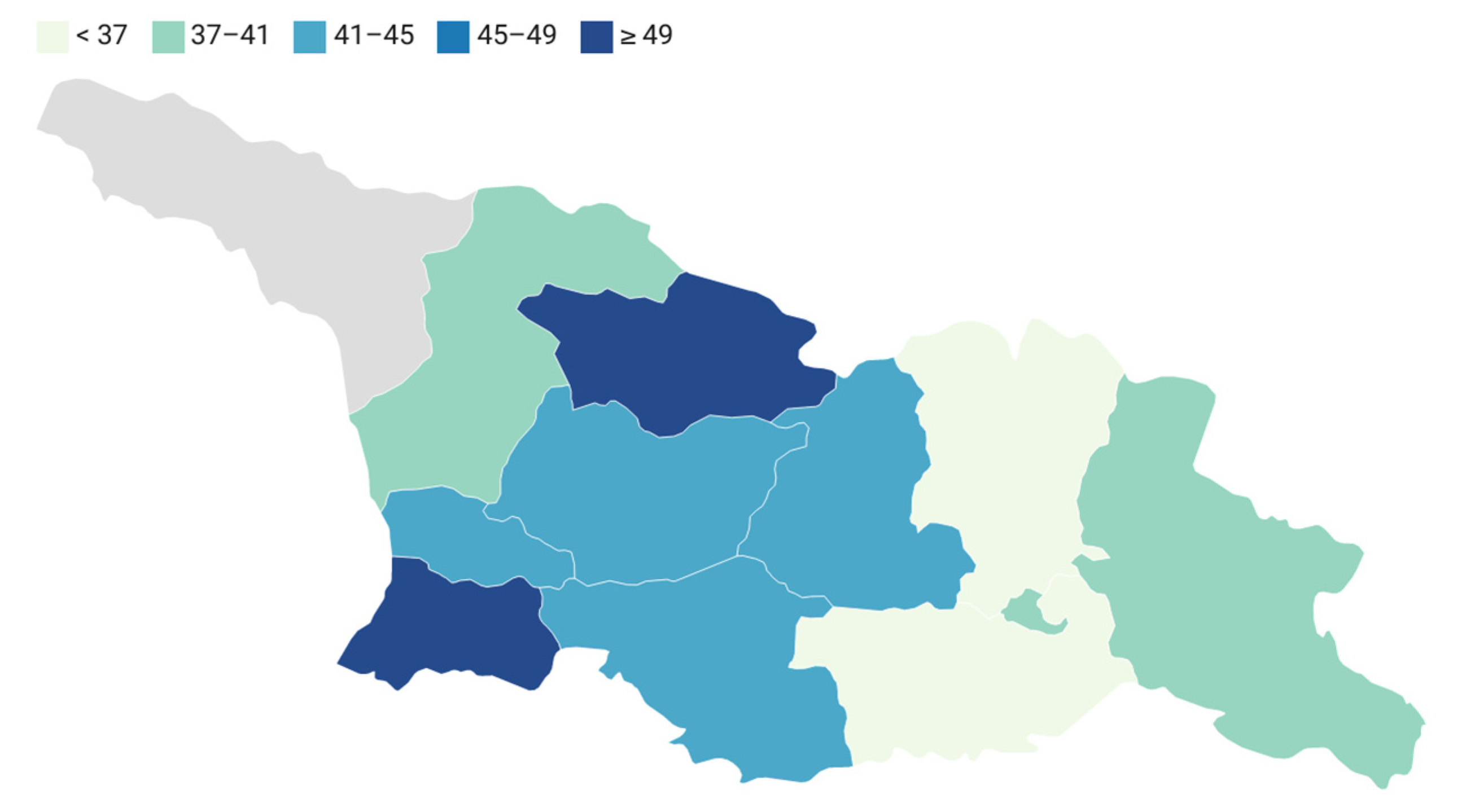
| Region | <0.000 | ||||||||
| Tbilisi (Capital) | 15 (28.8) | 72 (21.6) | 155 (38.1) | 87 (25.6) | 6 (42.8) | 76 (35.1) | 10 (30.3) | 55 (29.7) | |
| Western Georgia | 12 (23.0) | 130 (39.1) | 135 (33.2) | 140 (41.3) | <5 | 99 (45.8) | 15 (45.4) | 85 (45.9) | |
| Eastern Georgia | 25 (48.0) | 130 (39.1) | 116 (28.5) | 112 (33.0) | <5 | 41 (18.9) | 8 (24.2) | 45 (24.3) | |
| Settlement Type | <0.000 | ||||||||
| Rural | 23 (44.2) | 166 (50.0) | 138 (33.9) | 134 (38.7) | <5 | 55 (25.3) | 9 (27.2) | 70 (37.8) | |
| Urban | 29 (55.7) | 166 (50.0) | 269 (66.0) | 212 (61.2) | 10 (71.4) | 162 (74.6) | 24 (72.7) | 155 (62.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonikashvili, M.; Kereselidze, M.; Toidze, O.; Beruchashvili, T. Incidence and Patterns of Digestive Organ Cancer in Georgia: Insights from a Population-Based Registry Study in 2021. J. Pers. Med. 2023, 13, 1121. https://doi.org/10.3390/jpm13071121
Nonikashvili M, Kereselidze M, Toidze O, Beruchashvili T. Incidence and Patterns of Digestive Organ Cancer in Georgia: Insights from a Population-Based Registry Study in 2021. Journal of Personalized Medicine. 2023; 13(7):1121. https://doi.org/10.3390/jpm13071121
Chicago/Turabian StyleNonikashvili, Miranda, Maia Kereselidze, Otar Toidze, and Tina Beruchashvili. 2023. "Incidence and Patterns of Digestive Organ Cancer in Georgia: Insights from a Population-Based Registry Study in 2021" Journal of Personalized Medicine 13, no. 7: 1121. https://doi.org/10.3390/jpm13071121
APA StyleNonikashvili, M., Kereselidze, M., Toidze, O., & Beruchashvili, T. (2023). Incidence and Patterns of Digestive Organ Cancer in Georgia: Insights from a Population-Based Registry Study in 2021. Journal of Personalized Medicine, 13(7), 1121. https://doi.org/10.3390/jpm13071121






