Implantable Intracranial Pressure Sensor with Continuous Bluetooth Transmission via Mobile Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Key Design Requirements
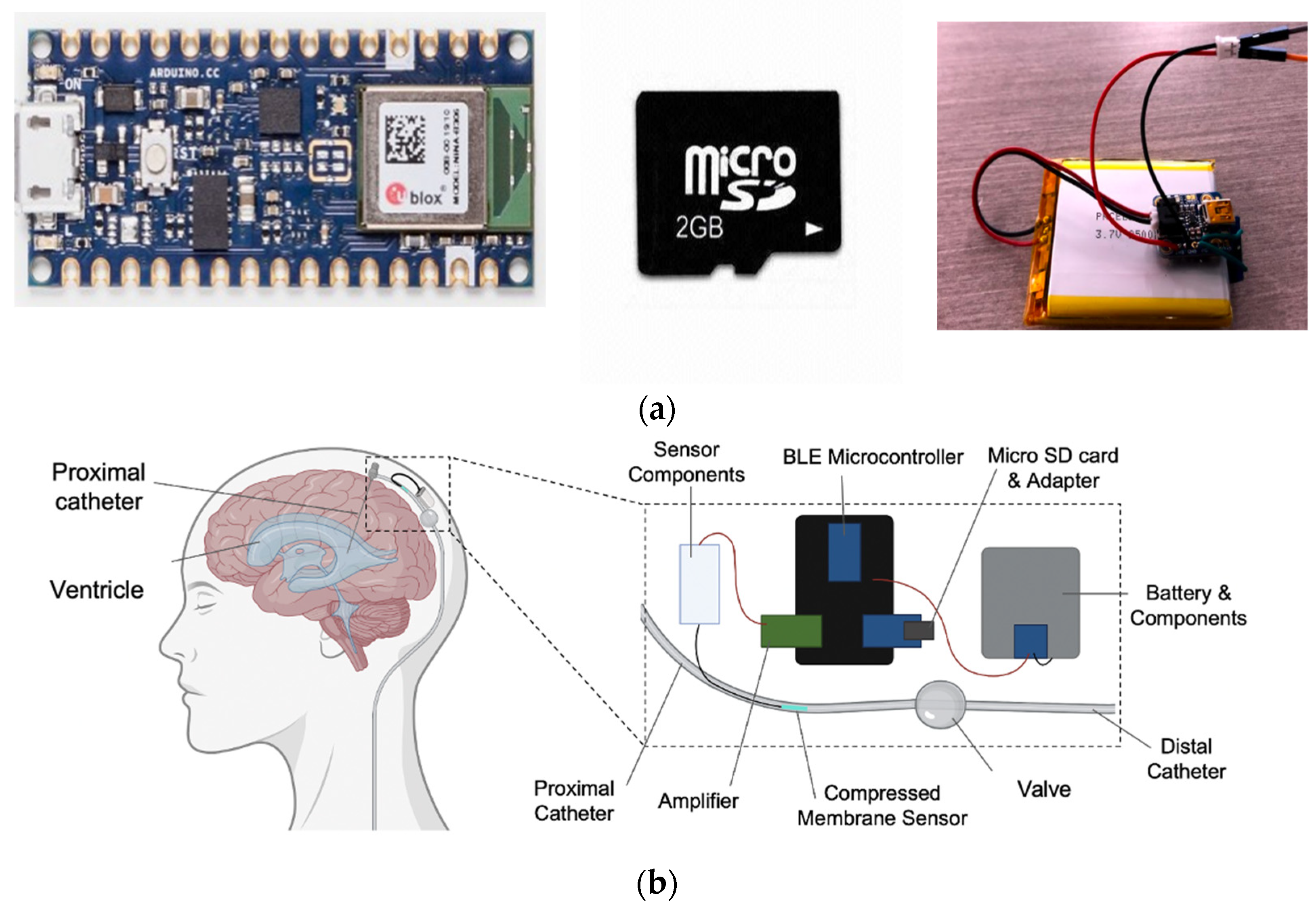
- Pressure Sensing: The system must measure ICP within a range of 0–100 mmHg (±2 mmHg from 0–20 mmHg and ±10% from 20–100 mmHg);
- Components: The system must wirelessly transmit data to a mobile device at a frequency of 2 Hz without compromising safety and store 54 mB of data locally;
- Safety: The system must be powered by a rechargeable battery that meets industry safety requirements, and information security must be addressed to ensure patient data safety;
- Mobile Application: The application must display continuous ICP readings and detect A and B ICP waveforms and acute elevations in ICP.
2.2. Trade Study and Design Solutions
3. Results
3.1. Prototype Development and Testing
3.1.1. Prototype Development
3.1.2. Prototype Testing
3.1.3. Prototype Calibration
3.1.4. Prototype Modeled ICP Detection
3.1.5. Prototype Battery and Data Safety
4. Discussion
5. Conclusions
6. Provisional Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supplementary Methods
Trade Study and Design Solutions
Appendix B. Supplementary Data
References
- Soler, G.J.; Bao, M.; Jaiswal, D.; Zaveri, H.P.; DiLuna, M.L.; Grant, R.A.; Hoshino, K. A Review of Cerebral Shunts, Current Technologies, and Future Endeavors. Yale J. Biol. Med. 2018, 91, 313–321. [Google Scholar] [PubMed]
- Miyake, H. Shunt Devices for the Treatment of Adult Hydrocephalus: Recent Progress and Characteristics. Neurol. Med.-Chir. 2016, 56, 274–283. [Google Scholar] [CrossRef]
- Zhang, X.; Medow, J.E.; Iskandar, B.J.; Wang, F.; Shokoueinejad, M.; Koueik, J.; Webster, J.G. Invasive and noninvasive means of measuring intracranial pressure: A review. Physiol. Meas. 2017, 38, R143–R182. [Google Scholar] [CrossRef] [PubMed]
- Kawoos, U.; McCarron, R.M.; Auker, C.R.; Chavko, M. Advances in Intracranial Pressure Monitoring and Its Significance in Managing Traumatic Brain Injury. Int. J. Mol. Sci. 2015, 16, 28979–28997. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.T. Raised intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2002, 73, i23–i27. [Google Scholar] [CrossRef]
- Spiegelberg, A.; Preuß, M.; Kurtcuoglu, V. B-waves revisited. Interdiscip. Neurosurg. 2016, 6, 13–17. [Google Scholar] [CrossRef]
- Czosnyka, M.; Pickard, J.D. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef]
- Krishnan, S.R.; Arafa, H.M.; Kwon, K.; Deng, Y.; Su, C.J.; Reeder, J.T.; Freudman, J.; Stankiewicz, I.; Chen, H.M.; Loza, R.; et al. Continuous, noninvasive wireless monitoring of flow of cerebrospinal fluid through shunts in patients with hydrocephalus. NPJ Digit. Med. 2020, 3, 29. [Google Scholar] [CrossRef]
- Swoboda, M.; Hochman, M.G.; Mattiucci, M.E.; Fritz, F.J. Real Time CSF Flow Measurement System & Method. U.S. Patent 20150045717A1, 17 June 2020. [Google Scholar]
- Hached, S.; Trigui, A.; El Khalloufi, I.; Sawan, M.; Loutochin, O.; Corcos, J. A Bluetooth-based Low-Energy Qi-compliant battery charger for implantable medical devices. In Proceedings of the 2014 IEEE International Symposium on Bioelectronics and Bioinformatics (IEEE ISBB 2014), Chung Li, Taiwan, 11–14 April 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Kiruthiga, G.; Sharmila, A.; Mahalakshmi, P.; Muruganandam, M. Power optimisation for wearable heart rate measurement device with wireless charging. J. Med. Eng. Technol. 2017, 41, 288–297. [Google Scholar] [CrossRef]
- De Silva, H.; Lakmal, S.; Chamikara, P.; Gunawardana, I.; Chandima, D. Qi Compliant Wireless Charging and Communication for Implantable Devices. In Proceedings of the 2018 2nd International Conference On Electrical Engineering (EECon), Colombo, Sri Lanka, 28 September 2018; pp. 177–183. [Google Scholar] [CrossRef]
- Qi Wireless Charging Standard: Electronics Notes. Available online: https://www.electronics-notes.com/articles/equipment-items-gadgets/wireless-battery-charging/qi-wireless-charging-standard.php (accessed on 17 June 2020).
- Miyake, H.; Ohta, T.; Kajimoto, Y.; Matsukawa, M. A New Ventriculoperitoneal Shunt with a Telemetric Intracranial Pressure Sensor: Clinical Experience in 94 Patients with Hydrocephalus. Neurosurgery 1997, 40, 931–935. [Google Scholar] [CrossRef]
- Antes, S.; Stadie, A.; Müller, S.; Linsler, S.; Breuskin, D.; Oertel, J. Intracranial Pressure–Guided Shunt Valve Adjustments with the Miethke Sensor Reservoir. World Neurosurg. 2018, 109, e642–e650. [Google Scholar] [CrossRef]
- Antes, S.; Tschan, C.A.; Heckelmann, M.; Breuskin, D.; Oertel, J. Telemetric Intracranial Pressure Monitoring with the Raumedic Neurovent P-tel. World Neurosurg. 2016, 91, 133–148. [Google Scholar] [CrossRef]
- Abay, T.Y.; Phillips, J.P.; Uff, C.; Roldan, M.; Kyriacou, P.A. In Vitro Evaluation of a Non-Invasive Photoplethysmography Based Intracranial Pressure Sensor. Appl. Sci. 2023, 13, 534. [Google Scholar] [CrossRef]
- Liu, H.; Pan, F.; Lei, X.; Hui, J.; Gong, R.; Feng, J.; Zheng, D. Effect of intracranial pressure on photoplethysmographic waveform in different cerebral perfusion territories: A computational study. Front. Physiol. 2023, 14, 1085871. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. Photoplethysmography for Quantitative Assessment of Sympathetic Nerve Activity (SNA) During Cold Stress. Front. Physiol. 2019, 9, 1863. [Google Scholar] [CrossRef] [PubMed]
- Lidington, D.; Wan, H.; Bolz, S.-S. Cerebral Autoregulation in Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 688362. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.G.; Ali, S.M.; Liu, H.; Qurashi, A.G.; Ali, U. Photoplethysmography temporal marker-based machine learning classifier for anesthesia drug detection. Med. Biol. Eng. Comput. 2022, 60, 3057–3068. [Google Scholar] [CrossRef]
- Evensen, K.B.; Paulat, K.; Prieur, F.; Holm, S.; Eide, P.K. Utility of the Tympanic Membrane Pressure Waveform for Non-invasive Estimation of The Intracranial Pressure Waveform. Sci. Rep. 2018, 8, 15776. [Google Scholar] [CrossRef]
- Shamim, M.; Khan, M.; Shallwani, H.; Khan, M. Noninvasive monitoring intracranial pressure—A review of available modalities. Surg. Neurol. Int. 2017, 8, 51. [Google Scholar] [CrossRef]
- Panescu, D. MEMS in medicine and biology. IEEE Eng. Med. Biol. Mag. 2006, 25, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Baheti, P.K.; Garudadri, H. An Ultra Low Power Pulse Oximeter Sensor Based on Compressed Sensing. In Proceedings of the Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 144–148. [Google Scholar] [CrossRef]
- Kockmann, N. Thermoelectric Flow Sensors. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Boston, MA, USA, 2008. [Google Scholar]
- EDN—The Evolution of Ultra Sonic Technology for Smarter Flow Measurement, EDN. 2019. Available online: https://www.edn.com/the-evolution-of-ultrasonic-technology-for-smarter-flow-measurement/ (accessed on 17 June 2020).
- Barlian, A.A.; Park, W.T.; Mallon, J.R.J.; Rastegar, A.J.; Pruitt, B.L. Review: Semiconductor Piezoresistance for Microsystems. Proc. IEEE Inst. Electr. Electron. Eng. 2009, 97, 513–552. [Google Scholar] [CrossRef]
- Demystifying Piezoresistive Pressure Sensors. Fierce Electronics. Available online: https://www.fierceelectronics.com/components/demystifying-piezoresistive-pressure-sensors (accessed on 17 June 2020).
- Gomez, C.; Oller, J.; Paradells, J. Overview and Evaluation of Bluetooth Low Energy: An Emerging Low-Power Wireless Technology. Sensors 2012, 12, 11734–11753. [Google Scholar] [CrossRef]
- Bluetooth vs. BLE—Difference between Bluetooth and BLE (Bluetooth Low Energy). RF Wireless World. Available online: https://www.rfwireless-world.com/Terminology/Bluetooth-vs-BLE.html (accessed on 17 June 2020).
- Darroudi, S.M.; Gomez, C. Bluetooth Low Energy Mesh Networks: A Survey. Sensors 2017, 17, 1467. [Google Scholar] [CrossRef] [PubMed]
- Learn About Bluetooth. Bluetooth. Available online: https://www.bluetooth.com/learn-about-bluetooth/bluetooth-technology/radio-versions/ (accessed on 17 June 2020).
- Get Connected Blog: The Difference between Classic Bluetooth and Bluetooth Low Energy. Nordic Semiconductor. 2019. Available online: https://blog.nordicsemi.com/getconnected/the-difference-between-classic-bluetooth-and-bluetooth-low-energy (accessed on 17 June 2020).
- World’s Smallest BLE Chip Claim, Electronics Weekly. 2019. Available online: https://www.electronicsweekly.com/news/business/714726-2019-11/ (accessed on 17 June 2020).
- Arduino—Making a Simple BlueTooth Data Logger—GeoThread. Available online: http://www.geothread.net/arduino-making-a-simple-bluetooth-data-logger (accessed on 17 June 2020).
- How Does Flash Memory Work? Types of Flash Memory and Uses. Available online: https://www.arrow.com/en/research-and-events/articles/how-flash-memory-works-advantages-and-disadvantages (accessed on 17 June 2020).
- New microSD Memory Card—World’s Smallest Memory Card. Available online: https://phys.org/news/2005-03-microsd-memory-card-world.html (accessed on 17 June 2020).
- Myths Surrounding USB Flash Drives, Machine Design. Available online: https://www.machinedesign.com/automation-iiot/article/21832250/5-myths-surrounding-usb-flash-drives (accessed on 17 June 2020).
- Memory—Microchip Technology. Available online: https://www.microchip.com/design-centers/memory (accessed on 17 June 2020).
- Best Qi Wireless Charger for iPhone and Android 2020. The New York Times, 9 October 2017. Available online: www.nytimes.com/wirecutter/reviews/best-qi-wireless-charger-for-iphone-and-android-phones/ (accessed on 17 June 2020).











| Pressure Measurement | Precision (25%) | Direct Measurement (10%) | Surgical Integration (20%) | Durability (5%) | Attainability (20%) | Power Req (20%) | Score | |||
| Compressed Membrane | 5 | 5 | 4 | 5 | 4 | 3 | 4.20 | |||
| Light Pulse | 3 | 1 | 2 | 4 | 3 | 4 | 2.85 | |||
| Thermal Flow | 4.5 | 2.5 | 4 | 4.5 | 4 | 2 | 3.60 | |||
| Non-thermal Flow | 4 | 2.5 | 4 | 4.5 | 4 | 2 | 3.48 | |||
| Wireless Transmission | Fully Internal (17.5%) | Data Transfer (7.5%) | Continuity (17.5%) | Speed (7.5%) | Transmission Range (12.5%) | Security (5%) | Power Req (17.5%) | Surgical Integration (10%) | Accessibility (5%) | Score |
| Bluetooth | 5 | 4.5 | 5 | 4 | 4 | 5 | 3 | 4.5 | 5 | 4.36 |
| Bluetooth Low Energy | 5 | 4 | 4.5 | 4.5 | 5 | 4 | 4.5 | 5 | 5 | 4.66 |
| Near Field Communication | 1 | 5 | 3 | 4 | 1 | 5 | 5 | 4.5 | 5 | 3.33 |
| Radio Frequency | 1 | 5 | 5 | 5 | 5 | 2 | 5 | 1 | 3 | 3.65 |
| Storage | System Integration (40%) | Storage Capacity (30%) | Security (20%) | Cost (10%) | Score | |||||
| Cloud | 1 | 5 | 2 | 4 | 2.70 | |||||
| Internal | 4 | 5 | 4 | 5 | 4.40 | |||||
| Battery Recharging | Power Delivery (20%) | Safety (15%) | All Internal (15%) | Durability (15%) | Size (15%) | Charging Distance (10%) | Accessibility (10%) | Score | ||
| Subcutaneous Solar Cells | 1 | 3 | 5 | 3 | 3 | 4 | 1 | 2.80 | ||
| Qi Charging | 5 | 5 | 1 | 5 | 3 | 4.5 | 3 | 3.85 | ||
| Radio Frequency | 2 | 1 | 1 | 5 | 5 | 5 | 2 | 2.90 | ||
| Thermoelectric | 1 | 5 | 5 | 3 | 4 | 5 | 2 | 3.45 | ||
| Piezoelectric | 1.5 | 3 | 5 | 3 | 4.5 | 5 | 1 | 3.23 |
| Pressure (mmHg) | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 40 | 60 | 80 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rep 1 (SD) | 0.09 (0.07) | 4.03 (0.13) | 10.77 (0.86) | 17.11 (0.28) | 22.66 (0.15) | 28.99 (0.14) | 34.83 (0.20) | 44.45 (0.55) | 68.12 (0.53) | 94.12 (0.99) | 1117.33 (0.20) |
| Rep 2 (SD) | −0.44 (0.11) | 7.73 (0.59) | 8.95 (0.22) | 12.13 (0.12) | 17.34 (0.41) | 22.23 (0.20) | 28.54 (0.38) | 38.33 (0.19) | 59.37 (0.17) | 84.55 (0.33) | 108.86 (0.29) |
| Rep 3 (SD) | −0.44 (0.04) | 5.6 (0.13) | 9.22 (0.22) | 15.43 (0.12) | 21.04 (0.10) | 27.38 (0.20) | 32.58 (0.17) | 44.13 (0.19) | 66.47 (0.18) | 91.39 (0.11) | 115.86 (0.16) |
| Rep 4 (SD) | 0.38 (0.25) | 5.45 (0.15) | 10.22 (0.28) | 16.04 (0.12) | 20.95 (0.20) | 26.76 (0.12) | 32.28 (0.23) | 44.31 (0.16) | 66.6 (0.18) | 90.18 (0.33) | 113.11 (0.15) |
| Rep 5 (SD) | −0.72 (0.12) | 5.09 (0.26) | 13.2 (1.48) | 17.55 (0.14) | 21.78 (0.12) | 29.11 (0.24) | 33.5 (0.17) | 44.68 (0.22) | 68.34 (0.20) | 91.52 (0.52) | 114.92 (0.48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsawaf, Y.; Jaklitsch, E.; Belyea, M.; Rodriguez, L.; Silverman, A.; Valley, H.; Koleilat, I.; Yaghi, N.K.; Jaeggli, M. Implantable Intracranial Pressure Sensor with Continuous Bluetooth Transmission via Mobile Application. J. Pers. Med. 2023, 13, 1318. https://doi.org/10.3390/jpm13091318
Elsawaf Y, Jaklitsch E, Belyea M, Rodriguez L, Silverman A, Valley H, Koleilat I, Yaghi NK, Jaeggli M. Implantable Intracranial Pressure Sensor with Continuous Bluetooth Transmission via Mobile Application. Journal of Personalized Medicine. 2023; 13(9):1318. https://doi.org/10.3390/jpm13091318
Chicago/Turabian StyleElsawaf, Yasmeen, Erik Jaklitsch, Madison Belyea, Levon Rodriguez, Alexandra Silverman, Halyn Valley, Issam Koleilat, Nasser K. Yaghi, and Michael Jaeggli. 2023. "Implantable Intracranial Pressure Sensor with Continuous Bluetooth Transmission via Mobile Application" Journal of Personalized Medicine 13, no. 9: 1318. https://doi.org/10.3390/jpm13091318






