Cardiac Troponin I but Not N-Terminal Pro-B-Type Natriuretic Peptide Predicts Outcomes in Cardiogenic Shock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients, Design and Data Collection
2.2. Inclusion and Exclusion Criteria, Study Endpoints
2.3. Measurement of cTNI and NT-proBNP
2.4. Statistical Methods
3. Results
3.1. Study Population
3.2. Association of cTNI and NT-proBNP with Clinical and Laboratory Data
3.3. Prognostic Performance of cTNI and NT-proBNP Levels
3.4. Multivariable Risk Prediction Models
4. Discussion
- -
- cTNI levels were consistently higher among 30-day non-survivors as compared to survivors in consecutive CS patients.
- -
- cTNI, but not NT-proBNP levels, were able to discriminate 30-day non-survivors, alongside increased risk of 30-day all-cause mortality in patients with higher cTNI levels.
- -
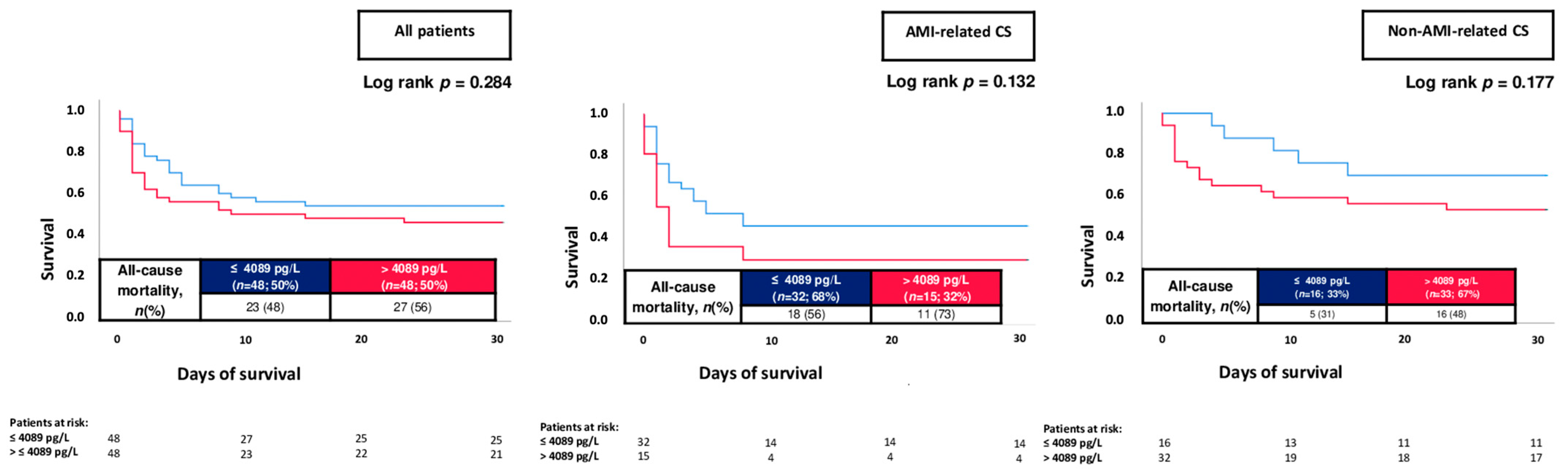
- The negative prognostic impact of increased cTNI levels was demonstrated irrespective of AMI- or non-AMI-related CS and confirmed even after multivariable adjustment.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.M.; Collins, S.P.M.; Ambrosy, A.P.; Pang, P.S.M.; Radu, R.I.; Antohi, E.-L.; Masip, J.; Butler, J.M.; Iliescu, V.A.M. Therapeutic Advances in the Management of Cardiogenic Shock. Am. J. Ther. 2019, 26, e234–e247. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.; Jäättelä, A.; Lahdensuu, M.; Rokkanen, P.; Avikainen, V.; Karaharju, E.; Tervo, T.; Lepistö, P. Catecholamines in shock. Ann. Clin. Res. 1977, 9, 157–163. [Google Scholar] [PubMed]
- Bruoha, S.; Yosefy, C.; Taha, L.; Dvir, D.; Shuvy, M.; Jubeh, R.; Carasso, S.; Glikson, M.; Asher, E. Mechanical Circulatory Support Devices for the Treatment of Cardiogenic Shock Complicating Acute Myocardial Infarction—A Review. J. Clin. Med. 2022, 11, 5241. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.; Dixon, S.; Alaswad, K.; Patel, K.; Almany, S.; Khandelwal, A.; Hanson, I.; George, A.; Ashbrook, M.; et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter. Cardiovasc. Interv. 2018, 91, 454–461. [Google Scholar] [CrossRef]
- Choi, K.H.; Yang, J.H.; Hong, D.; Park, T.K.; Lee, J.M.; Song, Y.B.; Hahn, J.Y.; Choi, S.H.; Choi, J.H.; Chung, S.R.; et al. Optimal Timing of Venoarterial-Extracorporeal Membrane Oxygenation in Acute Myocardial Infarction Patients Suffering from Refractory Cardiogenic Shock. Circ. J. 2020, 84, 1502–1510. [Google Scholar] [CrossRef]
- Thiele, H.; de Waha-Thiele, S.; Freund, A.; Zeymar, U.; Desch, S.; Fitzgerald, S. Management of cardiogenic shock. EuroIntervention 2021, 17, 451–465. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; de Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Defilippis, E.M.; Biery, D.W.; Singh, A.; Wu, W.Y.; Divakaran, S.; Berman, A.N.; Rizk, T.; Januzzi, J.L.; Bohula, E.; et al. Mortality and Heart Failure Hospitalization among Young Adults with and without Cardiogenic Shock after Acute Myocardial Infarction. J. Card. Fail. 2022, 29, 18–29. [Google Scholar] [CrossRef]
- Ting, H.H.; Rihal, C.S.; Gersh, B.J.; Haro, L.H.; Bjerke, C.M.; Lennon, R.J.; Lim, C.C.; Bresnahan, J.F.; Jaffe, A.S.; Holmes, D.R.; et al. Regional systems of care to optimize timeliness of reperfusion therapy for ST-elevation myocardial infarction: The Mayo Clinic STEMI Protocol. Circulation 2007, 116, 729–736. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Dzavik, V.; Buller, C.E.; Aylward, P.; Col, J.; White, H.D. Early Revascularization and Long-term Survival in Cardiogenic Shock Complicating Acute Myocardial Infarction. JAMA 2006, 295, 2511–2515. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Ohman, E.M.; Desch, S.; Eitel, I.; de Waha, S. Management of cardiogenic shock. Eur. Heart J. 2015, 36, 1223–1230. [Google Scholar] [CrossRef]
- Agewall, S.; Giannitsis, E.; Jernberg, T.; Katus, H. Troponin elevation in coronary vs. non-coronary disease. Eur. Heart J. 2011, 32, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Babuin, L.; Jaffe, A.S. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ 2005, 173, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Moorthy, M.; Tikkanen, J.T.; Cook, N.R.; Albert, C.M. Markers of Myocardial Stress, Myocardial Injury, and Subclinical Inflammation and the Risk of Sudden Death. Circulation 2020, 142, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Iborra-Egea, O.; Montero, S.; Bayes-Genis, A. An outlook on biomarkers in cardiogenic shock. Curr. Opin. Crit. Care 2020, 26, 392–397. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Lee, J.-S.; Kim, Y.-H.; Kim, B.J.; Kim, Y.-J.; Kang, D.-W.; Kim, J.S.; Kwon, S.U. Prognostic Significance of Troponin Elevation for Long-Term Mortality after Ischemic Stroke. J. Stroke 2017, 19, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Ghersin, I.; Zahran, M.; Azzam, Z.S.; Suleiman, M.; Bahouth, F. Prognostic value of cardiac troponin levels in patients presenting with supraventricular tachycardias. J. Electrocardiol. 2020, 62, 200–203. [Google Scholar] [CrossRef]
- Forner, J.; Schupp, T.; Weidner, K.; Rusnak, J.; Jawhar, S.; Dulatahu, F.; Brück, L.M.; Behnes, M.; Hoffmann, U.; Bertsch, T.; et al. Cardiac Troponin I Reveals Diagnostic and Prognostic Superiority to Aminoterminal Pro-B-Type Natriuretic Peptide in Sepsis and Septic Shock. J. Clin. Med. 2022, 11, 6592. [Google Scholar] [CrossRef]
- Akin, I.; Behnes, M.; Müller, J.; Forner, J.; Abumayyaleh, M.; Mashayekhi, K.; Akin, M.; Bertsch, T.; Weidner, K.; Rusnak, J.; et al. Prognostic Value of Cardiac Troponin I in Patients with Ventricular Tachyarrhythmias. J. Clin. Med. 2022, 11, 2987. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.; Morss, A.; Tung, R.; Pino, R.; Fifer, M.; Thompson, B.T.; Lee-Lewandrowski, E. Natriuretic peptide testing for the evaluation of critically ill patients with shock in the intensive care unit: A prospective cohort study. Crit. Care 2006, 10, R37. [Google Scholar] [CrossRef]
- Hoffmann, U.; Borggrefe, M.; Brueckmann, M. New horizons: NT-proBNP for risk stratification of patients with shock in the intensive care unit. Crit. Care 2006, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Tolppanen, H.; Rivas-Lasarte, M.; Lassus, J.; Sadoune, M.; Gayat, E.; Pulkki, K.; Arrigo, M.; Krastinova, E.; Sionis, A.; Parissis, J.; et al. Combined Measurement of Soluble ST2 and Amino-Terminal Pro-B-Type Natriuretic Peptide Provides Early Assessment of Severity in Cardiogenic Shock Complicating Acute Coronary Syndrome. Crit. Care Med. 2017, 45, e666–e673. [Google Scholar] [CrossRef] [PubMed]
- Raynor, A.; Vallée, C.; Belkarfa, A.-L.; Lunte, K.; Laney, M.; Belhadjer, Z.; Vicca, S.; Boutten, A.; Bonnet, D.; Nivet-Antoine, V. Multisystem inflammatory syndrome in children: Inputs of BNP, NT-proBNP and Galectin-3. Clin. Chim. Acta 2022, 529, 109–113. [Google Scholar] [CrossRef]
- Sharma, Y.P.; Kanabar, K.; Santosh, K.; Kasinadhuni, G.; Krishnappa, D. Role of N-terminal pro-B-type natriuretic peptide in the prediction of outcomes in ST-elevation myocardial infarction complicated by cardiogenic shock. Indian Heart J. 2020, 72, 302–305. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Mei, Y.; Lyu, J.; Hu, D.; Sun, F.; Zhang, G.; Zhang, H.; Zhang, J. Value of cardiac troponin T in predicting the prognosis of patients with cardiogenic shock receiving veno-arterial extracorporeal membrane oxygenation treatment: A consecutive 5-year retrospective study. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 1091–1095. [Google Scholar]
- Schupp, T.; Rusnak, J.; Egner-Walter, S.; Ruka, M.; Dudda, J.; Bertsch, T.; Müller, J.; Mashayekhi, K.; Tajti, P.; Ayoub, M.; et al. Prognosis of cardiogenic shock with and without acute myocardial infarction: Results from a prospective, monocentric registry. Clin. Res. Cardiol. 2023. [Google Scholar] [CrossRef]
- Rusnak, J.; Schupp, T.; Weidner, K.; Ruka, M.; Egner-Walter, S.; Forner, J.; Bertsch, T.; Kittel, M.; Mashayekhi, K.; Tajti, P.; et al. Impact of Lactate on 30-Day All-Cause Mortality in Patients with and without Out-of-Hospital Cardiac Arrest Due to Cardiogenic Shock. J. Clin. Med. 2022, 11, 7295. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef]
- Zeymer, U.; Bueno, H.; Granger, C.B.; Hochman, J.; Huber, K.; Lettino, M.; Price, S.; Schiele, F.; Tubaro, M.; Vranckx, P.; et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: A document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 183–197. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar]
- Apple, F.S.; Collinson, P.O. Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays. Clin. Chem. 2012, 58, 54–61. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef]
- Verschuren, F.; Bonnet, M.; Benoit, M.-O.; Gruson, D.; Zech, F.; Couturaud, F.; Meneveau, N.; Roy, P.-M.; Righini, M.; Meyer, G.; et al. The prognostic value of pro-B-Type natriuretic peptide in acute pulmonary embolism. Thromb. Res. 2013, 131, e235–e239. [Google Scholar] [CrossRef]
- Potapov, E.V.; Hennig, F.; Wagner, F.D.; Volk, H.-D.; Sodian, R.; Hausmann, H.; Lehmkuhl, H.B.; Hetzer, R. Natriuretic peptides and E-selectin as predictors of acute deterioration in patients with inotrope-dependent heart failure. Eur. J. Cardiothorac. Surg. 2005, 27, 899–905. [Google Scholar] [CrossRef]
- Stiermaier, T.; Santoro, F.; Graf, T.; Guastafierro, F.; Tarantino, N.; De Gennaro, L.; Caldarola, P.; Di Biase, M.; Thiele, H.; Brunetti, N.D.; et al. Prognostic value of N-Terminal Pro-B-Type Natriuretic Peptide in Takotsubo syndrome. Clin. Res. Cardiol. 2018, 107, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Nakashima, H.; Takagi, C.; Honda, Y.; Suzuki, S.; Yano, K. Predictors of Mortality in Patients with Acute Myocardial Infarction and Cardiogenic Shock. Circ. J. 2005, 69, 83–88. [Google Scholar] [CrossRef]
- Jarai, R.; Fellner, B.; Haoula, D.; Jordanova, N.; Heinz, G.; Karth, G.D.; Huber, K.; Geppert, A. Early assessment of outcome in cardiogenic shock: Relevance of plasma N-terminal pro-B-type natriuretic peptide and interleukin-6 levels. Crit. Care Med. 2009, 37, 1837–1844. [Google Scholar] [CrossRef]
- Brueckmann, M.; Huhle, G.; Lang, S.; Haase, K.K.; Bertsch, T.; Weiß, C.; Kaden, J.J.; Putensen, C.; Borggrefe, M.; Hoffmann, U.; et al. Prognostic Value of Plasma N-Terminal Pro-Brain Natriuretic Peptide in Patients with Severe Sepsis. Circulation 2005, 112, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Pöss, J.; Mahfoud, F.; Seiler, S.; Heine, G.H.; Fliser, D.; Böhm, M.; Link, A. FGF-23 is associated with increased disease severity and early mortality in cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 211–218. [Google Scholar] [CrossRef]
- Ceglarek, U.; Schellong, P.; Rosolowski, M.; Scholz, M.; Willenberg, A.; Kratzsch, J.; Zeymer, U.; Fuernau, G.; de Waha-Thiele, S.; Büttner, P.; et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur. Heart J. 2021, 42, 2344–2352. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, F.; Zhang, C.; Zheng, L.-R.; Yang, J. The Biomarkers for Acute Myocardial Infarction and Heart Failure. BioMed. Res. Int. 2020, 2020, 2018035. [Google Scholar] [CrossRef]
- Wereski, R.; Kimenai, D.M.; Taggart, C.; Doudesis, D.; Lee, K.K.; Lowry, M.T.H.; Bularga, A.; Lowe, D.J.; Fujisawa, T.; Apple, F.S.; et al. Cardiac Troponin Thresholds and Kinetics to Differentiate Myocardial Injury and Myocardial Infarction. Circulation 2021, 144, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.S.; Shenkman, H.; Brieger, D.; Fox, K.A.; Yan, A.T.; Eagle, K.A.; Steg, P.G.; Lim, K.-D.; Quill, A.; Goodman, S.G.; et al. Quantitative troponin and death, cardiogenic shock, cardiac arrest and new heart failure in patients with non-ST-segment elevation acute coronary syndromes (NSTE ACS): Insights from the Global Registry of Acute Coronary Events. Heart 2011, 97, 197–202. [Google Scholar] [CrossRef]
- Wanamaker, B.L.; Seth, M.M.; Sukul, D.; Dixon, S.R.; Bhatt, D.L.; Madder, R.D.; Rumsfeld, J.S.; Gurm, H.S. Relationship Between Troponin on Presentation and In-Hospital Mortality in Patients with ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e013551. [Google Scholar] [CrossRef]
- Samman Tahhan, A.; Sandesara, P.; Hayek, S.S.; Hammadah, M.; Alkhoder, A.; Kelli, H.M.; Topel, M.; O’Neal, W.T.; Ghasemzadeh, N.; Ko, Y.A.; et al. High-Sensitivity Troponin I Levels and Coronary Artery Disease Severity, Progression, and Long-Term Outcomes. J. Am. Heart Assoc. 2018, 7, e007914. [Google Scholar] [CrossRef]
- González-Pacheco, H.; Manzur-Sandoval, D.; Gopar-Nieto, R.; Álvarez-Sangabriel, A.; Martínez-Sánchez, C.; Eid-Lidt, G.; Altamirano-Castillo, A.; Mendoza-García, S.; Briseño-Cruz, J.L.; Azar-Manzur, F.; et al. Cardiogenic Shock Among Patients with and without Acute Myocardial Infarction in a Latin American Country: A Single-Institution Study. Glob. Heart 2021, 16, 78. [Google Scholar] [CrossRef]
- Kapur, N.K.; Thayer, K.L.; Zweck, E. Cardiogenic Shock in the Setting of Acute Myocardial Infarction. Methodist DeBakey Cardiovasc. J. 2020, 16, 16–21. [Google Scholar] [CrossRef]
- Lim, W.; Qushmaq, I.; Cook, D.J.; Crowther, M.A.; Heels-Ansdell, D.; Devereaux, P.J. Elevated troponin and myocardial infarction in the intensive care unit: A prospective study. Crit. Care 2005, 9, R636–R644. [Google Scholar] [CrossRef]



| Survivor (n = 95) | Non-Survivor (n = 122) | p Value | |||
|---|---|---|---|---|---|
| Age, median; (IQR) | 71 | (61–79) | 73 | (63–81) | 0.409 |
| Male sex, n (%) | 63 | (66.3) | 75 | (61.5) | 0.462 |
| Body mass index, kg/m2 (median, (IQR)) | 26.2 | (24.2–29.4) | 26.8 | (24.5–30.5) | 0.380 |
| Vital signs on admission, (median, (IQR)) | |||||
| Body temperature (°C) | 36.0 | (35.3–36.6) | 35.7 | (34.4–36.5) | 0.034 |
| Heart rate (bpm) | 84 | (69–104) | 95 | (72–113) | 0.039 |
| Systolic blood pressure (mmHg) | 110 | (94–131) | 111 | (93–131) | 0.432 |
| Respiratory rate (breaths/min) | 19 | (16–22) | 20 | (17–24) | 0.161 |
| Cardiovascular risk factors, n (%) | |||||
| Arterial hypertension | 71 | (74.7) | 83 | (68.0) | 0.280 |
| Diabetes mellitus | 33 | (34.7) | 51 | (41.8) | 0.289 |
| Hyperlipidemia | 53 | (55.8) | 63 | (51.6) | 0.543 |
| Smoking | 34 | (35.8) | 48 | (39.3) | 0.592 |
| Prior medical history, n (%) | |||||
| Coronary artery disease: | 38 | (40.0) | 43 | (35.2) | 0.696 |
| Congestive heart failure | 31 | (32.6) | 37 | (30.3) | 0.717 |
| Atrial fibrillation | 27 | (28.4) | 35 | (28.7) | 0.965 |
| Chronic kidney disease | 32 | (33.7) | 39 | (32.0) | 0.789 |
| Stroke | 11 | (11.6) | 11 | (9.0) | 0.535 |
| COPD | 16 | (16.8) | 29 | (23.8) | 0.212 |
| Liver cirrhosis | 4 | (4.2) | 3 | (2.5) | 0.469 |
| Prior medical history, n (%) | |||||
| ACE-inhibitor | 34 | (35.8) | 37 | (30.3) | 0.395 |
| ARB | 18 | (18.9) | 19 | (15.6) | 0.512 |
| Beta-blocker | 48 | (50.5) | 54 | (44.3) | 0.359 |
| ARNI | 4 | (4.2) | 1 | (0.8) | 0.099 |
| Aldosterone antagonist | 14 | (14.7) | 16 | (13.1) | 0.731 |
| Diuretics | 35 | (36.8) | 50 | (41.0) | 0.535 |
| ASA | 29 | (30.5) | 34 | (27.9) | 0.669 |
| P2Y12-inhibitor | 9 | (9.5) | 11 | (9.0) | 0.908 |
| Statin | 47 | (49.5) | 46 | (37.7) | 0.082 |
| Survivor (n = 95) | Non-Survivor (n = 122) | p Value | |||
|---|---|---|---|---|---|
| Cause of CS, n (%) | |||||
| Acute myocardial infarction (AMI) | 41 | (43.2) | 72 | (59.0) | 0.020 |
| Arrhythmic | 20 | (21.1) | 7 | (5.7) | 0.001 |
| ADHF | 20 | (21.1) | 29 | (23.8) | 0.635 |
| Pulmonary embolism | 4 | (4.2) | 9 | (7.4) | 0.329 |
| Valvular | 3 | (3.2) | 2 | (1.6) | 0.656 |
| Cardiomyopathy | 4 | (4.2) | 3 | (2.5) | 0.702 |
| Pericardial tamponade | 3 | (3.2) | 0 | (0.0) | 0.082 |
| Onset of cardiogenic shock | |||||
| Primary CS, n (%) | 70 | (73.7) | 83 | (68.0) | 0.365 |
| AMI-onset-balloon-time, min (median, (IQR)) | 164 | (123–256) | 157 | (119–208) | 0.474 |
| CS-onset-balloon-time, min (median, (IQR)) | 146 | (93–193) | 157 | (118–201) | 0.308 |
| Secondary CS, n (%) | 25 | (26.3) | 39 | (32.0) | 0.365 |
| Coronary angiography, n (%) | 75 | (79.8) | 87 | (71.3) | 0.154 |
| No evidence of CAD | 12 | (16.0) | 8 | (9.2) | 0.189 |
| 1-vessel disease | 16 | (21.3) | 14 | (16.1) | 0.392 |
| 2-vessel disease | 13 | (17.3) | 15 | (17.2) | 0.988 |
| 3-vessel disease | 34 | (45.3) | 50 | (57.5) | 0.123 |
| Left main trunk | 9 | (12.0) | 8 | (9.2) | 0.561 |
| Left anterior descending | 36 | (48.0) | 57 | (65.5) | 0.025 |
| Right coronary artery | 36 | (48.0) | 45 | (51.7) | 0.636 |
| Left circumflex | 26 | (34.7) | 50 | (57.5) | 0.004 |
| PCI, n (%) | 41 | (43.2) | 67 | (54.9) | 0.086 |
| Number of stents, (median; (IQR)) | 75 | (79.8) | 87 | (71.3) | 0.154 |
| CABG, n (%) | 12 | (16.0) | 8 | (9.2) | 0.189 |
| Chronic total occlusion, n (%) | 16 | (21.3) | 14 | (16.1) | 0.392 |
| Infection, n (%) | 13 | (17.3) | 15 | (17.2) | 0.988 |
| Classification of CS, n (%) | |||||
| Stage A | 0 | (0.0) | 0 | (0.0) | 0.001 |
| Stage B | 5 | (5.3) | 0 | (0.0) | |
| Stage C | 40 | (42.1) | 31 | (25.4) | |
| Stage D | 8 | (8.4) | 8 | (6.6) | |
| Stage E | 42 | (44.2) | 83 | (68.0) | |
| Transthoracic echocardiography | |||||
| LVEF >55%, (n, %) | 12 | (13.2) | 11 | (9.7) | 0.438 |
| LVEF 54–41%, (n, %) | 17 | (18.7) | 9 | (8.0) | 0.023 |
| LVEF 40–30%, (n, %) | 28 | (30.8) | 24 | (21.2) | 0.121 |
| LVEF <30%, (n, %) | 34 | (37.4) | 69 | (61.1) | 0.001 |
| LVEF not documented, (n, %) | 4 | - | 9 | - | - |
| VCI, cm (median, (IQR)) | 1.8 | (1.4–2.2) | 1.9 | (1.6–2.2) | 0.334 |
| TAPSE, mm (median, (IQR)) | 17 | (12–20) | 14 | (11–17) | 0.133 |
| Cardiopulmonary resuscitation | |||||
| OHCA, n (%) | 35 | (36.8) | 60 | (49.2) | 0.069 |
| IHCA, n (%) | 7 | (7.4) | 23 | (18.9) | 0.015 |
| Shockable rhythm, n (%) | 32 | (33.7) | 37 | (30.3) | 0.598 |
| Non-shockable rhythm, n (%) | 63 | (66.3) | 85 | (69.7) | 0.598 |
| ROSC, min (median, IQR) | 11 | (5–19) | 19 | (12–31) | 0.001 |
| Respiratory status | |||||
| Mechanical ventilation, n (%) | 50 | (52.6) | 85 | (69.7) | 0.010 |
| Duration of mechanical ventilation, days, (mean, (IQR)) | 2 | (0–8) | 2 | (1–6) | 0.196 |
| PaO2/FiO2 ratio, (median, (IQR)) | 230 | (147–367) | 214 | (122–331) | 0.403 |
| PaO2, mmHg (median, (IQR)) | 103 | (80–162) | 103 | (77–168) | 0.919 |
| Multiple-organ support during ICU | |||||
| Norepinephrine on admission, µg/kg/min (median, (IQR)) | 0.1 | (0.0–0.1) | 0.2 | (0.1–0.6) | 0.001 |
| Dobutamine, n (%) | 19 | (20.0) | 48 | (39.3) | 0.002 |
| Levosimendan, n (%) | 16 | (16.8) | 42 | (34.4) | 0.004 |
| Mechanical circulatory assist device, n (%) | 3 | (3.2) | 19 | (15.6) | 0.003 |
| Baseline laboratory values, (median, (IQR)) | |||||
| pH | 7.30 | (7.23–7.36) | 7.27 | (7.17–7.36) | 0.056 |
| Lactate (mmol/L) | 2.7 | (1.6–4.2) | 4.5 | (2.2–9.7) | 0.001 |
| Sodium (mmol/L) | 139 | (136–141) | 138 | (136–141) | 0.600 |
| Potassium (mmol/L) | 4.2 | (3.7–4.9) | 4.4 | (3.8–5.0) | 0.574 |
| Creatinine (mg/dL) | 1.32 | (1.10–1.75) | 1.58 | (1.21–2.27) | 0.021 |
| Hemoglobin (g/dL) | 12.7 | (10.6–14.6) | 12.5 | (10.8–13.9) | 0.428 |
| WBC (106/mL) | 13.67 | (10.00–17.72) | 15.49 | (12.16–19.86) | 0.026 |
| Platelets (106/mL) | 225 | (171–287) | 224 | (175–265) | 0.587 |
| INR | 1.13 | (1.04–1.29) | 1.19 | (1.10–1.41) | 0.001 |
| D-dimer (mg/L) | 6.23 | (2.36–15.08) | 16.95 | (3.64–32.00) | 0.006 |
| AST (U/L) | 116 | (37–228) | 171 | (64–488) | 0.038 |
| ALT (U/L) | 60 | (30–149) | 96 | (39–312) | 0.048 |
| Bilirubin (mg/dL) | 0.58 | (0.40–1.02) | 0.62 | (0.43–0.94) | 0.456 |
| Troponin I (µg/L) | 0.332 | (0.087–2.494) | 1.850 | (0.344–12.431) | 0.001 |
| NT-proBNP (pg/mL) | 3386 | (407–10,824) | 4387 | (1090–15,627) | 0.152 |
| Procalcitonin (ng/mL) | 0.25 | (0.06–0.60) | 0.28 | (0.17–1.52) | 0.314 |
| CRP (mg/L) | 6 | (4–28) | 13 | (4–35) | 0.335 |
| Follow up data, n (%) | |||||
| ICU time, days (median, (IQR)) | 4 | (3–11) | 3 | (2–6) | 0.001 |
| cTNI | NT-proBNP | |||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Age | −0.017 | 0.805 | 0.369 | 0.001 |
| Body mass index (kg/m2) | −0.006 | 0.935 | 0.054 | 0.602 |
| Heart rate (bpm) | 0.115 | 0.095 | −0.077 | 0.454 |
| Systolic blood pressure (mmHg) | 0.064 | 0.356 | −0.200 | 0.051 |
| Norepinephrine (µg/kg/min) | 0.054 | 0.437 | 0.045 | 0.666 |
| Lactate (mmo/L) | 0.101 | 0.140 | 0.093 | 0.369 |
| Creatinine (mg/dL) | −0.050 | 0.467 | 0.458 | 0.001 |
| Hemoglobin (g/dL) | 0.113 | 0.097 | −0.263 | 0.010 |
| WBC count (106/mL) | 0.303 | 0.001 | −0.136 | 0.187 |
| Platelet count (106/mL) | 0.041 | 0.548 | −0.124 | 0.228 |
| INR | 0.074 | 0.287 | 0.403 | 0.001 |
| cTNI (µg/L) | - | - | −0.028 | 0.787 |
| NT-proBNP (pg/mL) | −0.028 | 0.787 | - | - |
| Procalcitonin (ng/mL) | 0.122 | 0.337 | 0.355 | 0.010 |
| CRP (mg/L) | 0.001 | 0.986 | 0.555 | 0.001 |
| LVEF (%) | 0.305 | 0.001 | 0.281 | 0.006 |
| TAPSE (mm) | −0. 018 | 0. 889 | −0. 425 | 0. 007 |
| AMI-onset-balloon-time (min) | −0. 173 | 0. 151 | 0. 408 | 0. 053 |
| CS-onset-balloon-time (min) | −0.332 | 0.005 | 0.468 | 0.024 |
| cTNI | NT-pro BNP | p Value | |
|---|---|---|---|
| Day 1 | 0.669 (0.597–0.741) p = 0.001 | 0.585 (0.470–0.700) p = 0.152 | 0.220 |
| Day 2 | 0.666 (0.576–0.756) p = 0.001 | 0.435 (0.286–0.584) p = 0.394 | 0.009 |
| Day 3 | 0.575 (0.454–0.695) p = 0.224 | 0.405 (0.210–0.599) p = 0.333 | 0.131 |
| Day 4 | 0.685 (0.527–0.843) p = 0.031 | 0.580 (0.320–0.840) p = 0.539 | 0.492 |
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| Model 1 | |||
| Age (years) | 1.016 | 1.001–1.031 | 0.038 |
| Male sex | 1.222 | 0.824–1.810 | 0.319 |
| Heart rate (bpm) | 1.006 | 0.999–1.013 | 0.083 |
| Norepinephrine (µg/kg/min) | 1.111 | 0.911–1.356 | 0.297 |
| Lactate (mmol/L) | 1.084 | 1.036–1.134 | 0.001 |
| Creatinine (mg/dL) | 1.138 | 1.013–1.279 | 0.030 |
| Cardiopulmonary resuscitation | 1.595 | 1.211–2.101 | 0.001 |
| cTNI > 0.763 µg/L | 1.915 | 1.298–2.824 | 0.001 |
| Model 2 * | |||
| cTNI > 0.763 µg/L | 2.822 | 1.487–5.356 | 0.002 |
| NT-proBNP > 4089 pg/mL | 1.295 | 0.669–2.507 | 0.443 |
| Variables | Non-AMI-Related CS | AMI-Related CS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age (years) | 1.014 | 0.989–1.041 | 0.278 | 1.011 | 0.989–1.033 | 0.327 |
| Male sex | 1.293 | 0.708–2.364 | 0.403 | 1.228 | 0.672–2.244 | 0.505 |
| Heart rate (bpm) | 1.001 | 0.991–1.011 | 0.889 | 1.010 | 0.999–1.022 | 0.065 |
| Norepinephrine (µg/kg/min) | 0.843 | 0.566–1.255 | 0.400 | 1.248 | 1.009–1.543 | 0.041 |
| Lactate (mmol/L) | 1.123 | 1.047–1.204 | 0.001 | 1.080 | 1.019–1.144 | 0.009 |
| Creatinine (mg/dL) | 1.187 | 0.991–1.421 | 0.063 | 1.101 | 0.933–1.299 | 0.257 |
| Cardiopulmonary resuscitation | 2.006 | 1.290–3.118 | 0.002 | 1.423 | 0.976–2.077 | 0.067 |
| cTNI > 0.763 µg/L | 1.953 | 1.021–3.735 | 0.043 | 1.807 | 1.019–3.202 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schupp, T.; Rusnak, J.; Forner, J.; Weidner, K.; Ruka, M.; Egner-Walter, S.; Dudda, J.; Bertsch, T.; Kittel, M.; Behnes, M.; et al. Cardiac Troponin I but Not N-Terminal Pro-B-Type Natriuretic Peptide Predicts Outcomes in Cardiogenic Shock. J. Pers. Med. 2023, 13, 1348. https://doi.org/10.3390/jpm13091348
Schupp T, Rusnak J, Forner J, Weidner K, Ruka M, Egner-Walter S, Dudda J, Bertsch T, Kittel M, Behnes M, et al. Cardiac Troponin I but Not N-Terminal Pro-B-Type Natriuretic Peptide Predicts Outcomes in Cardiogenic Shock. Journal of Personalized Medicine. 2023; 13(9):1348. https://doi.org/10.3390/jpm13091348
Chicago/Turabian StyleSchupp, Tobias, Jonas Rusnak, Jan Forner, Kathrin Weidner, Marinela Ruka, Sascha Egner-Walter, Jonas Dudda, Thomas Bertsch, Maximilian Kittel, Michael Behnes, and et al. 2023. "Cardiac Troponin I but Not N-Terminal Pro-B-Type Natriuretic Peptide Predicts Outcomes in Cardiogenic Shock" Journal of Personalized Medicine 13, no. 9: 1348. https://doi.org/10.3390/jpm13091348





