Clinical Efficacy of Preoperative and Intraoperative Intravitreal Ranibizumab as Adjuvant Therapy of Ahmed Glaucoma Valve Implantation Combined with Vitrectomy in the Management of Neovascular Glaucoma with Diabetic Vitreous Hemorrhage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Procedures
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayreh, S.S. Neovascular glaucoma. Prog. Retin. Eye Res. 2007, 26, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Simha, A.; Aziz, K.; Braganza, A.; Abraham, L.; Samuel, P.; Lindsley, K.B. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst. Rev. 2020, 2, CD007920. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shi, Y.; Fan, Z. The mechanism and therapeutic strategies for neovascular glaucoma secondary to diabetic retinopathy. Front. Endocrinol. 2023, 14, 1102361. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, D.; Moussa, G.; Sung, V.C.; Pappa, C.; Kalogeropoulos, C. Neovascular Glaucoma: An Update. Klin. Monbl Augenheilkd. 2023, 240, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Senthil, S.; Dada, T.; Das, T.; Kaushik, S.; Puthuran, G.V.; Philip, R.; Rani, P.; Rao, H.; Singla, S.; Vijaya, L. Neovascular glaucoma—A review. Indian. J. Ophthalmol. 2021, 69, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Shazly, T.A.; Latina, M.A. Neovascular glaucoma: Etiology, diagnosis and prognosis. Semin. Ophthalmol. 2009, 24, 113–121. [Google Scholar] [CrossRef]
- European Glaucoma Society. European Glaucoma society terminology and guidelines for glaucoma, 4th edition—Chapter 2: Classification and terminologySupported by the EGS foundation: Part 1: Foreword; introduction; glossary; chapter 2 classification and terminology. Br. J. Ophthalmol. 2017, 101, 73–127. [Google Scholar] [CrossRef]
- Sánchez-Tabernero, S.; Juberías, J.R.; Artells, N.; Crespo-Millas, S.; Meneses, C.; Muñoz-Moreno, M.F.; Manzanas, L.; López Gálvez, M.I. Management and Systemic Implications of Diabetic Neovascular Glaucoma. Ophthalmic Res. 2019, 62, 111–115. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.H.; Shen, X.; Zhong, Y.S. Ahmed valve implantation for neovascular glaucoma after 23-gauge vitrectomy in eyes with proliferative diabetic retinopathy. Int. J. Ophthalmol. 2013, 6, 316–320. [Google Scholar]
- Lin, Z.J.; Chen, Z.H.; Huang, S.Y.; Sun, J.; Shen, X.; Zhong, Y.S. Clinical efficacy of Ahmed glaucoma valve implantation combined with 23-gauge vitrectomy for medically uncontrolled neovascular glaucoma with proliferative diabetic retinopathy. Int. J. Ophthalmol. 2020, 13, 832–836. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, Y.; Zhou, P.; Wu, H.; Hou, X.; Ren, Z.; Li, X.; Zhao, M. Anti-VEGF treatment is the key strategy for neovascular glaucoma management in the short term. BMC Ophthalmol. 2016, 16, 150. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Roberti, G.; Michelessi, M. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst. Rev. 2023, 4, CD007920. [Google Scholar]
- Liang, X.; Zhang, Y.; Li, Y.P.; Huang, W.R.; Wang, J.X.; Li, X. Frequency and Risk Factors for Neovascular Glaucoma After Vitrectomy in Eyes with Diabetic Retinopathy: An Observational Study. Diabetes Ther. 2019, 10, 1801–1809. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Du, Y.; Bao, H.; Zhu, J.; Liu, X. Influencing factors of low vision 2 years after vitrectomy for proliferative diabetic retinopathy: An observational study. BMC Ophthalmol. 2023, 23, 309. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Jiang, F.; You, C.; Mao, C.; Yu, J.; Han, J.; Zhang, Z.; Yan, H. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS ONE 2014, 9, e110531. [Google Scholar] [CrossRef]
- Shchomak, Z.; Cordeiro Sousa, D.; Leal, I.; Abegão Pinto, L. Surgical treatment of neovascular glaucoma: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1079–1089. [Google Scholar] [CrossRef]
- Ramji, S.; Nagi, G.; Ansari, A.S.; Kailani, O. A systematic review and meta-analysis of randomised controlled trials in the management of neovascular glaucoma: Absence of consensus and variability in practice. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 477–501. [Google Scholar] [CrossRef]
- Bernal-Morales, C.; Dotti-Boada, M.; Olate-Perez, A.; Navarro-Angulo, M.J.; Pelegrín, L.; Figueras-Roca, M. Simultaneous pars plana vitrectomy, panretinal photocoagulation, cryotherapy, and Ahmed valve implantation for neovascular glaucoma. Int. J. Ophthalmol. 2021, 14, 1396–1401. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, X.; Zhang, X.; Sun, X. Clinical outcomes of Ahmed glaucoma valve implantation with or without intravitreal bevacizumab pretreatment for neovascular glaucoma: A systematic review and meta-analysis. J. Glaucoma 2016, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Zhao, Q.; He, J.; Fan, W.; He, W.; Lai, M. Comparisons of the short-term effectiveness and safety of surgical treatment for neovascular glaucoma: A systematic review and network meta-analysis. BMJ Open 2022, 12, e051794. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Zhao, X.; Wan, G. A systematic review and meta-analysis of clinical outcomes of small gauge vitrectomy with or without intravitreal antivascular endothelial growth factor agents pretreatment for proliferative diabetic retinopathy. Ophthalmic Res. 2023, 66, 781–794. [Google Scholar]
- Wang, M.H.; Li, Q.M.; Dong, H.T.; Dong, S.Q.; Li, Y.; Zheng, C.Y. Ahmed valves vs trabeculectomy combined with pans plana vitrectomy for neovascular glaucoma with vitreous hemorrhage. Eur. J. Ophthalmol. 2017, 27, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lin, Z.; Chen, Y.; Xu, J.; Zhang, Q.; Chen, J.; Shen, X. Intravitreal conbercept injection as an adjuvant in vitrectomy with silicone oil infusion for severe proliferative diabetic retinopathy. J. Ocul. Pharmacol. Ther. 2020, 36, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Fu, Y.; Wang, Y.; Zheng, Z.; Fan, Y.; Sun, X.; Xu, X. Efficacy of intravitreal ranibizumab combined with Ahmed glaucoma valve implantation for the treatment of neovascular glaucoma. BMC Ophthalmol. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Woo, S.J.; Chung, H.; Park, K.H. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology 2011, 118, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- Edington, M.; Connolly, J.; Chong, N.V. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 295–307. [Google Scholar] [CrossRef]
- Xu, M.; Fan, R.; Fan, X.; Shao, Y.; Li, X. Progress and Challenges of Anti-VEGF Agents and Their Sustained-Release Strategies for Retinal Angiogenesis. Drug Des. Devel Ther. 2022, 16, 3241–3262. [Google Scholar] [CrossRef]
- Tanetsugu, Y.; Tagami, T.; Terukina, T.; Ogawa, T.; Ohta, M.; Ozeki, T. Development of a Sustainable Release System for a Ranibizumab Biosimilar Using Poly(lactic-co-glycolic acid) Biodegradable Polymer-Based Microparticles as a Platform. Biol. Pharm. Bull. 2017, 40, 145–150. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, X.; Wang, Q.; He, M.; Chau, Y. Long-term therapeutic effect in nonhuman primate eye from a single injection of anti-VEGF controlled release hydrogel. Bioeng. Transl. Med. 2019, 4, e10128. [Google Scholar] [CrossRef]
- Abrishami, M.; Zarei-Ghanavati, S.; Soroush, D.; Rouhbakhsh, M.; Jaafari, M.R.; Malaekeh-Nikouei, B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina 2009, 29, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Huu, V.A.; Luo, J.; Zhu, J.; Zhu, J.; Patel, S.; Boone, A.; Mahmoud, E.; McFearin, C.; Olejniczak, J.; Lux, C.d.G.; et al. Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. J. Control Release 2015, 200, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ohlhausen, M.; Payne, C.; Greenlee, T.; Chen, A.X.; Conti, T.; Singh, R.P. Impact and Characterization of Delayed Pan-Retinal Photocoagulation in Proliferative Diabetic Retinopathy. Am. J. Ophthalmol. 2021, 223, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lin, Z.; Shen, X. Anti-Vascular Endothelial Growth Factor Therapy as an Alternative or Adjunct to Pan-Retinal Photocoagulation in Treating Proliferative Diabetic Retinopathy: Meta-Analysis of Randomized Trials. Front. Pharmacol. 2020, 11, 849. [Google Scholar] [CrossRef]
| Group 1 | Group 2 | p Value | |
|---|---|---|---|
| Number of eyes | 22 | 17 | |
| Age (years) | 48.0 ± 14.6 | 45.5 ± 12.3 | 0.570 |
| Gender (male/female) | 10/12 | 7/10 | 0.789 |
| Systemic profile | |||
| Hypertension | 15 (68.2%) | 11 (64.7%) | 0.819 |
| HbA1c at time of surgery | 8.6 ± 2.2 | 9.1 ± 2.2 | 0.521 |
| Duration of diabetes (years) | 14.0 ± 4.8 | 12.4 ± 5.1 | 0.325 |
| Previous PRP | 9 (40.9%) | 6 (35.3%) | 0.721 |
| Lens status | 0.966 | ||
| Phakic | 18 (81.82%) | 14 (82.35%) | |
| Pseudophakic | 4 (18.18%) | 3 (17.65%) | |
| Follow-up (months) | 11.9 ± 2.3 | 12.4 ± 2.5 | 0.569 |
| Baseline BCVA | 1.98 ± 0.42 | 1.96 ± 0.36 | 0.894 |
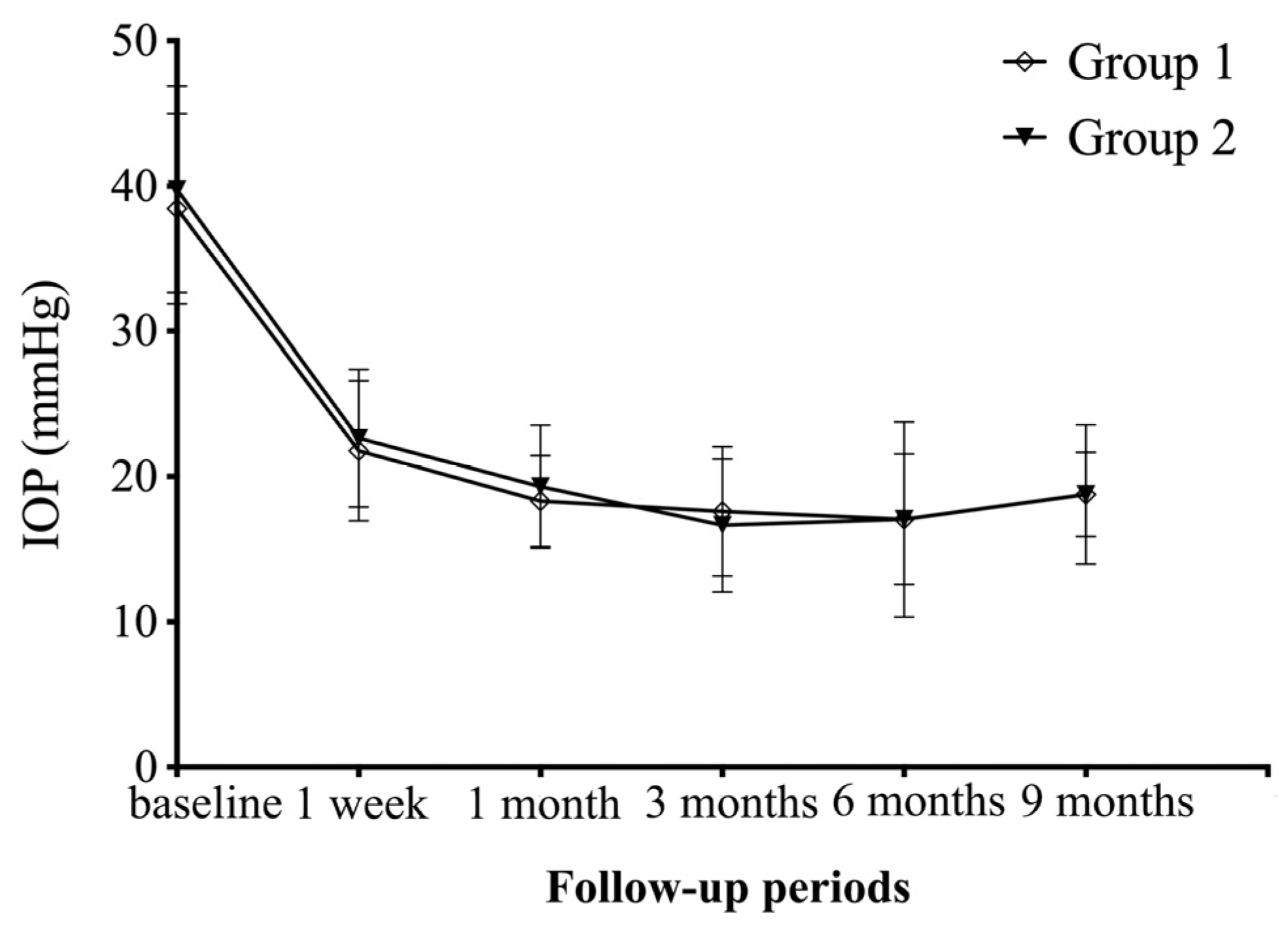
| Follow-Up Periods | IOP | BCVA | Number of Anti-Glaucoma Medications | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | p Value | Group 1 | Group 2 | p Value | Group 1 | Group 2 | p Value | |
| 1 week | 21.8 ± 4.8 | 22.6 ± 4.7 | 0.576 | 2.03 ± 0.40 | 1.96 ± 0.30 | 0.567 | 1.4 ± 1.1 | 1.1 ± 1.0 | 0.370 |
| 1 month | 18.3 ± 3.2 | 19.3 ± 4.3 | 0.416 | 1.83 ± 0.44 | 1.67 ± 0.52 | 0.330 | 1.1 ± 0.9 | 0.8 ± 1.0 | 0.381 |
| 3 months | 17.6 ± 4.5 | 16.6 ± 4.6 | 0.521 | 1.75 ± 0.51 | 1.62 ± 0.50 | 0.410 | 0.9 ± 1.0 | 1.0 ± 0.9 | 0.763 |
| 6 months | 17.0 ± 6.7 | 17.1 ± 4.5 | 0.994 | 1.68 ± 0.61 | 1.58 ± 0.54 | 0.569 | 1.0 ± 1.0 | 0.8 ± 0.8 | 0.432 |
| 9 months | 18.8 ± 4.8 | 18.8 ± 2.9 | 0.995 | 1.48 ± 0.67 | 1.29 ± 0.56 | 0.363 | 1.0 ± 1.0 | 0.9 ± 0.8 | 0.687 |
| Follow-Up Periods | Complete Success | Qualified Success | ||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | p Value | Group 1 | Group 2 | p Value | |
| 1 week | 7 (31.82%) | 6 (35.29%) | >0.999 | 10 (45.45%) | 6 (35.29%) | 0.744 |
| 1 months | 7 (31.82%) | 8 (47.06%) | 0.508 | 15 (68.18%) | 12 (70.59%) | >0.999 |
| 3 months | 10 (45.45%) | 5 (29.41%) | 0.343 | 15 (68.18%) | 14 (82.35%) | 0.464 |
| 6 months | 9 (40.91%) | 8 (47.06%) | 0.754 | 17 (77.27%) | 14 (82.35%) | >0.999 |
| 9 months | 9 (40.91%) | 6 (35.29%) | 0.753 | 14 (63.64%) | 12 (70.59%) | 0.740 |
| Group 1 | Group 2 | p Value | |
|---|---|---|---|
| Intraoperative tamponade | 0.709 | ||
| BSS | 7 (31.8%) | 4 (23.5%) | |
| Air | 5 (22.7%) | 3 (17.6%) | |
| Silicone oil | 10 (45.5%) | 10 (58.8%) | |
| Postoperative anti-VEGF | 10 (45.5%) | 8 (47.1%) | 0.921 |
| Postoperative PRP | 8 (36.4%) | 5 (29.4%) | 0.648 |
| Recurrent VH | 7 (31.82%) | 1 (2.56%) | 0.047 |
| Recurrent RD | 6 (27.3%) | 5 (29.4%) | 0.883 |
| Recurrent iris neovascularization | 8 (36.36%) | 1 (2.56%) | 0.025 |
| Final BCVA | 1.46 ± 0.68 | 1.27 ± 0.57 | 0.361 |
| Change in BCVA (Final–Baseline) | −0.52 ± 0.76 | −0.69 ± 0.52 | 0.428 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Lin, Z.; Zhong, Y.; Shen, X. Clinical Efficacy of Preoperative and Intraoperative Intravitreal Ranibizumab as Adjuvant Therapy of Ahmed Glaucoma Valve Implantation Combined with Vitrectomy in the Management of Neovascular Glaucoma with Diabetic Vitreous Hemorrhage. J. Pers. Med. 2024, 14, 18. https://doi.org/10.3390/jpm14010018
Gao S, Lin Z, Zhong Y, Shen X. Clinical Efficacy of Preoperative and Intraoperative Intravitreal Ranibizumab as Adjuvant Therapy of Ahmed Glaucoma Valve Implantation Combined with Vitrectomy in the Management of Neovascular Glaucoma with Diabetic Vitreous Hemorrhage. Journal of Personalized Medicine. 2024; 14(1):18. https://doi.org/10.3390/jpm14010018
Chicago/Turabian StyleGao, Shuang, Zhongjing Lin, Yisheng Zhong, and Xi Shen. 2024. "Clinical Efficacy of Preoperative and Intraoperative Intravitreal Ranibizumab as Adjuvant Therapy of Ahmed Glaucoma Valve Implantation Combined with Vitrectomy in the Management of Neovascular Glaucoma with Diabetic Vitreous Hemorrhage" Journal of Personalized Medicine 14, no. 1: 18. https://doi.org/10.3390/jpm14010018
APA StyleGao, S., Lin, Z., Zhong, Y., & Shen, X. (2024). Clinical Efficacy of Preoperative and Intraoperative Intravitreal Ranibizumab as Adjuvant Therapy of Ahmed Glaucoma Valve Implantation Combined with Vitrectomy in the Management of Neovascular Glaucoma with Diabetic Vitreous Hemorrhage. Journal of Personalized Medicine, 14(1), 18. https://doi.org/10.3390/jpm14010018





