Abstract
Background: Noninvasive respiratory support (NRS), including high-flow nasal oxygen therapy (HFNOT), noninvasive ventilation (NIV) and continuous positive airway pressure (CPAP), are routinely used in the perioperative period. Objectives: This narrative review provides an overview on the perioperative use of NRS. Preoperative, intraoperative, and postoperative respiratory support is discussed, along with potential future areas of research. Results: During induction of anesthesia, in selected patients at high risk of difficult intubation, NIV is associated with improved gas exchange and reduced risk of postoperative respiratory complications. HFNOT demonstrated an improvement in oxygenation. Evidence on the intraoperative use of NRS is limited. Compared with conventional oxygenation, HFNOT is associated with a reduced risk of hypoxemia during procedural sedation, and recent data indicate a possible role for HFNOT for intraoperative apneic oxygenation in specific surgical contexts. After extubation, “preemptive” NIV and HFNOT in unselected cohorts do not affect clinical outcome. Postoperative “curative” NIV in high-risk patients and among those exhibiting signs of respiratory failure can reduce reintubation rate, especially after abdominal surgery. Data on postoperative “curative” HFNOT are limited. Conclusions: There is increasing evidence on the perioperative use of NRS. Use of NRS should be tailored based on the patient’s specific characteristics and type of surgery, aimed at a personalized cost-effective approach.
1. Introduction
Perioperative care, also known as perioperative medicine, is the practice of patient-centered, multidisciplinary, and integrated medical care from the preoperative phase to the postoperative recovery [1]. “Anesthesiomics” [2] embeds this holistic and personalized approach intended to provide high-quality care, minimize complications, and reduce requirement of postoperative intensive care unit (ICU) admission and hospital length of stay (LOS).
Perioperative respiratory complications are a leading cause of morbidity and mortality. General anesthesia induces a loss of muscle tone and a cephalic displacement of the diaphragm, yielding reduction of functional residual capacity due to atelectasis and postoperative hypoxemia. These mechanisms lead to perioperative respiratory dysfunction and predispose to postoperative pulmonary complications (PPCs) [3,4]. These include postoperative hypoxemia, atelectasis, pneumonia, acute respiratory failure, and acute respiratory distress syndrome, which may prompt the use of mechanical ventilation [5]. PPCs are a prominent cause of prolonged hospitalization and mortality [6]. Several scores have been developed to predict the occurrence of PPCs [7,8], the emergence of which is related to surgical factors (intraoperative positional changes, diaphragmatic splinting, direct surgical injury to the lungs, pleura, diaphragm, chest wall, abdominal wall, or to the nerves supplying the respiratory muscles) and patient factors and comorbidities (advanced age, smoking, cardiovascular and respiratory comorbidities, obesity, pregnancy).
Current research is aimed at optimizing perioperative oxygenation and mechanical ventilation for specific cohorts of patients in different surgical subspecialties [9,10].
Thus, efforts have been made to adopt personalized strategies, with the aim to reduce perioperative respiratory complications [11] and improve postoperative patients’ outcome and recovery.
The incidence of acute respiratory failure after surgery is variable: the need for reintubation and invasive mechanical ventilation among unselected surgical patients is approximatively 5–10% but can be as high as 30–50% in high-risk patients undergoing abdominal and cardiac surgery [12,13].
Preserving perioperative respiratory function is a mainstay for ensuring safe anesthesia administration, success of surgical procedures, and uneventful recovery. The perioperative use of noninvasive respiratory support (NRS) may be associated with improved respiratory function and reduced PPCs. The term NRS is used in reference to noninvasive ventilation (NIV), continuous positive airway pressure (CPAP), and high-flow nasal oxygen therapy (HFNOT) [14,15].
This narrative review’s aim is to give an overview (Figure 1) on the perioperative use of NRS across different surgical subspecialties and patient populations. Each of the three perioperative periods (preoperative, intraoperative, and postoperative) is discussed, highlighting the rationale and evidence (Table 1, Figure 2) on perioperative NRS use and the future areas of research in terms of personalized care.
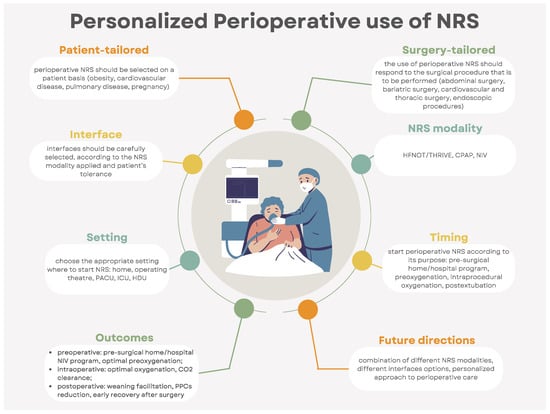
Figure 1.
Infographic on the personalized perioperative use of noninvasive respiratory support (CPAP: continuous positive airway pressure; CO2: carbon dioxide; HDU: high-dependency care unit; ICU: intensive care unit; NIV: noninvasive ventilation; NRS: noninvasive respiratory support, PACU: postanesthesia care unit; PPCs: postoperative pulmonary complications; THRIVE: transnasal humidified rapid-insufflation ventilatory exchange).

Table 1.
Landmark studies on NRS use in the perioperative period (ARF: acute respiratory failure; COPD: chronic obstructive pulmonary disease; COT: conventional oxygen therapy; ICU: intensive care unit; GA: general anesthesia; NRS: noninvasive respiratory support; HFNOT: high-flow nasal oxygen therapy; THRIVE: transnasal humidified rapid-insufflation ventilatory exchange; NIV: noninvasive ventilation; PACU: postanesthesia care unit; RM: recruitment maneuver).
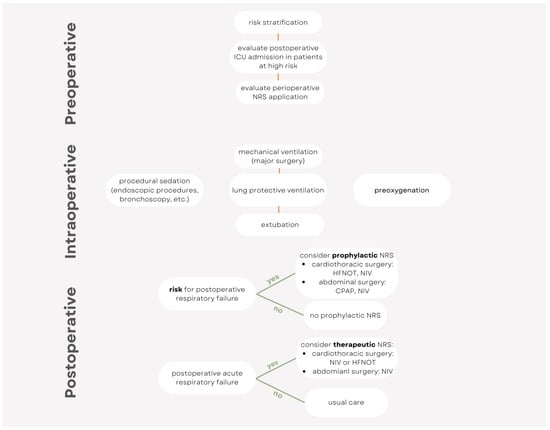
Figure 2.
Simplified algorithm for the perioperative use of noninvasive respiratory support.
2. Preoperative Use of Noninvasive Ventilation and CPAP
Preoxygenation before general anesthesia induction is a well-established procedure designed to increase oxygen reserves, in the attempt to delay oxyhemoglobin desaturation and prolong apnea time. The term apneic oxygenation refers to continuous oxygen flowing from upper airways to alveoli after anesthesia induction and during the apneic phase [31].
Preoxygenation with NIV limits alveolar collapse and atelectasis formation, which are responsible for hypoventilation, low perfusion–ventilation coupling and hypoxemia [31,32]. Through these mechanisms, NIV delays hypoxemia onset and lengthens the apneic period, with a prominent clinical effect in high-risk patients, such as those with difficult airways, patients undergoing bariatric surgeries, or emergency anesthesia for the critically ill [33].
Futier et al. [16] showed that the combination of NIV followed by early recruitment maneuvers can lead to an average increase in end-expiratory lung volume of approximately 700 mL when compared to conventional bag-mask preoxygenation. This increase in end-expiratory lung volume after NIV-based preoxygenation might be attributed to the recruitment of collapsed alveoli, consequently boosting oxygen reserves [34].
In the subgroup of obese patients, NIV significantly increases functional residual capacity, improves ventilation–perfusion ratio, and improves cardiac output [35]. When compared to spontaneous breathing, NIV can achieve a higher oxygenation within a shorter time frame [16] and increase duration of nonhypoxic apnea [36], making it particularly valuable in morbidly obese patients. Of note, a 5 min trial of CPAP during preoxygenation in obese patients has been shown to improve arterial partial pressure of oxygen (PaO2) after tracheal intubation compared to controls [17].
Preoperative CPAP may benefit patients with obstructive sleep apnea (OSA), a condition often undiagnosed before surgery and posing a substantial risk, including hypoxemia, hypercapnia, arrhythmias, myocardial ischemia, delirium, and unplanned ICU admissions [37]. Robust evidence for perioperative NIV use in this patient group is limited, but preoperative CPAP may control disease severity, reduce postoperative pulmonary dysfunction, and increase patient familiarity with the therapy [38].
Only few studies evaluated presurgical home/hospital NIV programs aiming at improving ventilatory status and familiarizing the patients with the NIV technique that will be applied after surgery. Perrin et al. [18] found that oxygenation and lung volumes were significantly better in patients using preoperative home NIV for 7 days before surgery than patients randomized to usual preoperative care. Of note, the incidence of major atelectasis was 14% in the NIV group and 39% in the control group. When considering cardiovascular and thoracic surgery, atelectasis are more commonly observed as postoperative complications, dramatically heightening the risk of respiratory distress. Prophylactic preoperative use of NIV has been proposed with the aim to reduce major PPCs after elective aortic surgery [39], demonstrating a reduction of early postoperative atelectasis and shortening ICU and hospital LOS.
The preOVNI study [40] investigated whether preoperative NIV could reduce PPCs after lung cancer surgery. In this randomized controlled trial (RCT), adult patients undergoing lung cancer resection were randomized to preoperative NIV (at least 7 days and 4 h/day) or conventional preoperative treatment. The authors concluded that there was no difference in PPCs between groups (42.6% in NIV group and 44.8% in no-NIV group). The rate of pneumonia was greater in the no-NIV group, but statistical significance was not achieved (28.0 vs. 37.7%, respectively; p = 0.08).
In conclusion, NIV represents a safe and effective tool for preoperative oxygenation, with a relevant clinical effect in patients prone to anesthesia-induced functional residual capacity loss and at high risk of difficult intubation with prolonged apnea following anesthesia induction.
3. Preoperative and Intraprocedural Use of High-Flow Nasal Oxygen Therapy
High-flow nasal oxygenation therapy (HFNOT) is an NRS technique that delivers high flows (60–80 L/min) of a heated, humidified, and blended air/oxygen mixture via specifically designed nasal cannulas [41].
A recent systematic review and meta-analysis on peri-intubation use of HFNOT revealed that apneic time and PaO2 measured after preoxygenation or after intubation were similar with HFNOT and conventional oxygen therapy (COT). In addition, the use of HFNOT also conferred no advantage in terms of peri-intubation hypoxemia, serious complications, or 28-day mortality [42]. However, it might be misleading to draw any conclusions for patients at higher risk of peri-intubation hypoxemia, as the mean apnea time in the selected studies was only <2 min.
In 2015, Patel et al. [19] coined the term transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) to indicate the delivery of 100% oxygen at 70 L/min using the Optiflow THRIVETM apparatus (Fisher and Paykel Healthcare Ltd., Auckland, New Zealand) to adult patients with difficult airways undergoing otorhinolaryngologic surgery. The authors reported a median apnea time of 14 min and no episode of oxygen desaturation (SpO2 < 90%), proving that this approach represents an efficient way to mitigate the risk of hypoxemia following anesthesia induction and neuromuscular blockade [19]. Since then, a large body of evidence coming from studies conducted in the surgical population and during procedural sedation has elucidated the physiological mechanisms of this approach, as well as the important differences when the technique is applied to apneic or unparalyzed patients [43,44,45,46].
Provision of effective and extended oxygenation by HFNOT during rapid-sequence induction of anesthesia and during apnea has been examined in several settings [43,47,48,49,50,51], with some evidence also coming from morbidly obese patients, where HFNOT allowed prolonging of apnea time to 75 s compared to conventional facemask ventilation [52].
Tremey et al. [53] also described a “without the hands” technique for general anesthesia induction using HFNOT for preoxygenation and periprocedural oxygenation until tracheal intubation.
Hypercapnia is the most common complication with HFNOT and affects the overall apnea time. In an RCT including 118 patients undergoing laryngeal microsurgery under neuromuscular blockade, Min et al. [20] showed that, despite providing prolonged periods of apnea time, incidence of rescue interventions because of hypercapnia, desaturations, and acidosis was more frequent for HFNOT compared to tracheal intubation. The pattern of carbon dioxide (CO2) rise (linear or nonlinear) as well as exact mechanisms regarding the extent of CO2 removal during HFNOT still need to be fully characterized. Some of the mechanisms involved were investigated in bench models: under apneic conditions, enhanced CO2 clearance may be explained by an interaction between entrained and highly turbulent supraglottic flow vortices created by HFNOT and cardiogenic oscillations that favor gas mixing [44,46].
Forsberg et al. [49] hypothesized that the increased difference in arterial and end-tidal CO2 in combination with high arterial oxygen levels, that were noted during apnea in patients undergoing laryngeal surgery and neuromuscular blockade during THRIVE [43], could be attributed to increased oxygen absorption and compression atelectasis, with subsequent shunt and loss of lung volume. However, lung volume changes assessed over time by electrical impedance tomography, albeit indicating an increased ventilation–perfusion mismatch, resulted in comparable reductions in lung volume during application of THRIVE and in mechanically ventilated patients. Comparable decreases in lung volumes estimated by electrical impedance tomography were also observed by Riedel et al. [21] when applying flow rates between 0.25 L/min and 70 L/min of humidified 100% oxygen in anesthetized and paralyzed adults, thus demonstrating that the induced increase in functional residual capacity occurring during spontaneous breathing [54] could not be replicated under general anesthesia [49]. Nevertheless, those conclusions cannot be prescinded from the consideration that respiratory mechanics differs significantly between paralyzed and unparalyzed states. With paralysis, dead space gas mixing and microventilation induced by pharyngeal pressure variations are inevitably altered [55]. However, a recent five-arm RCT [56] observed that differing flow rates of humidified 100% oxygen during apnea induced comparable arterial CO2 increases, thus questioning the existence of an additional ventilatory effect attributable to HFNOT [57].
Hypercapnia and desaturation are common concerns highlighted in most of the reported studies on procedural sedation. Other complications include gastric insufflation and airway ignition, the latter being theoretically possible in the case of concomitant use of lasers for surgical procedures. As a consequence, the need for cautious monitoring and a backup airway management plan, in particular when neuromuscular blockade is applied, is of primary importance [58].
Transcutaneous CO2 monitoring could be applied during procedural sedation. It is an accurate noninvasive alternative to arterial blood sampling, which provides clinically acceptable accuracy particularly when the sensor is applied to the earlobe [59].
The safety profile of HFNOT in procedural sedation, or whenever neuromuscular blockade is not needed, is less controversial [60]. A systematic review and meta-analysis including 15 RCTs (4451 patients) published in 2022 [22] showed with moderate-quality evidence that application of HFNOT compared to COT was associated with improved oxygenation, decreased need for airway intervention, and reduced procedure interruption in patients undergoing bronchoscopy and gastrointestinal endoscopic procedures. Those results were confirmed by another meta-analysis [61] evaluating the same outcome measures in 19 RCTs (4121 patients). Although 11 of the included studies coincided with those analyzed by Tao et al. [22], the latter included pediatric, cardiology, dental, and endovascular settings.
The effectiveness and safety of THRIVE as a stand-alone airway and breathing technique for airway management were recently evaluated during brief operative hysteroscopies under general anesthesia. In their study, Yuanyuan et al. [62] showed a reduced incidence of hypoxia, airway-related interventions, and involuntary movements during surgery in the THRIVE group when compared to COT, with high satisfaction scores both for anesthesiologists and gynecologists.
Frassanito et al. [63], in a pilot study including 20 patients undergoing operative hysteroscopies with the use of THRIVE apparatus, reported adequate gas exchange with acceptable short-term mean maximum transcutaneous CO2 levels of 51 ± 7 mmHg, no need for rescue airway interventions, and optimal comfort for the patients.
4. Postoperative Use of Noninvasive Ventilation (NIV)
Noninvasive ventilation (NIV) is widely used in the postoperative period to both treat (“therapeutic” or “curative” approach) and prevent (“preemptive” or “prophylactic” approach) postoperative acute respiratory failure [64]. There is a growing interest in its use in weaning strategies, bolstered by recent trials on early extubation practices (“facilitative” approach) [65].
The rationale behind its postoperative use resides on the improvement of alveolar ventilation due to reversal of atelectasis, which improves oxygenation and, partially, work of breathing. In addition, NIV does not require an artificial airway and it is achieved through interfaces which may be customized to improve adherence to treatment [66].
Current guidelines recommend NIV use for preventing postextubation respiratory failure in high-risk patients (moderate certainty of evidence), but not in low-risk patients (conditional recommendation, very low certainty of evidence) [67]. Regarding surgical patients, NIV finds a possible therapeutic application in the case of postextubation established respiratory failure (conditional recommendation, moderate certainty of evidence). However, there is no clear recommendation on the preventive use of NIV in the postoperative period.
The joint ESA/ESICM guidelines on NRS in the hypoxemic peri-operative/periprocedural patient [68] suggest using noninvasive positive pressure ventilation or CPAP immediately after extubation for hypoxemic patients at risk of developing acute respiratory failure after abdominal surgery (Grade 1B).
Patients requiring postoperative NIV should be admitted in predefined areas, such as postanesthesia care units (PACUs), ICUs, or high-dependency care units (HDUs), where a close monitoring of vital parameters, blood gas exchanges, and multidisciplinary evaluation can be performed [69,70] to avoid delayed intubation in case of deterioration of their condition.
4.1. NIV as Preventive Strategy in the Postoperative Period
The use of NRS after extubation in the postoperative period aims to mitigate the occurrence of acute respiratory failure and PPCs. So far, several risk scores have been validated to allow early identification of patients at higher risk for deterioration, thus preventing extubation failure [71]. Several trials have been performed with the purpose to analyze the effectiveness of preventive early NIV after major surgery, finding beneficial effects on PPC prevention [12,72].
Despite these findings, conflicting evidence emerges from larger randomized trials and meta-analysis. These inconsistencies are possibly explained by distinct target populations for whom postoperative NIV is used, suggesting that different modes of NRS may be successful when applied following specific types of surgery.
Zayed et al. [23] conducted a pairwise meta-analysis of nine RCTs on HFNOT, NIV, and COT after major surgery in patients at high risk for or with established postoperative acute respiratory failure. They found that neither HFNOT nor NIV influenced mortality rates. However, lower postoperative reintubation rates were observed with NIV or HFNOT over COT. Subgroup analysis showed lower reintubation rates with NIV after abdominal surgery (odds ratio (OR) 0.51 95% CI [0.26–0.87], whereas after cardiothoracic surgery reintubation rates were lower with both HFNO and NIV when compared to COT (OR 0.08 95% CI [0.03–0.21] and OR 0.08 95% CI [0.03–0.19], respectively).
The PRISM trial [73], a pragmatic clinical effectiveness trial investigating the real-world implementation of CPAP in PACUs, found that preventive CPAP started early after major open abdominal surgery does not reduce the incidence of postoperative pneumonia, reintubation rates, or death at 30 days. Therefore, its adoption as a preventive measure against PPCs could not be supported.
A recent systematic review and meta-analysis on the routine use of postoperative NRS after elective surgery [74], including 38 trials and 9782 patients, found that compared with standard care, the use of CPAP, NIV, or HFNOT did not reduce the incidence of pneumonia. In accordance with the previous findings, an open, multicenter RCT including patients at high risk for PPCs (Assess Respiratory Risk in Surgical Patients in Catalonia [7] (ARISCAT) score ≥ 45) showed that the preventive use of NIV has no beneficial effects over usual care in the incidence of postoperative acute respiratory failure. In this study, patients were randomly assigned to intermittent prophylactic face-mask NIV for 6–8 h per day or usual postoperative care, with the primary outcome being in-hospital acute respiratory failure within 7 days after surgery [75].
4.2. NIV as Therapeutic Strategy in the Postoperative Period
Therapeutic strategies are applied with the aim of avoiding reintubation and invasive mechanical ventilation in case of an already established postoperative acute respiratory failure. According to ERS/ATS 2017 clinical practice guidelines [67], NIV should be applied in these circumstances (conditional recommendation, moderate certainty of evidence) as a therapeutic strategy.
Several studies have shown that therapeutic NIV is able to improve clinical outcomes of patients undergoing major abdominal [12,76,77] and thoracic [24] surgery. Beneficial effects of postoperative therapeutic NIV have also been found in transplant procedures [78].
In their systematic review and meta-analysis, Faria et al. [72] found that compared to COT, CPAP or NIV may reduce the rate of tracheal intubation (risk ratio (RR) 0.25; 95% confidence interval (CI) 0.08 to 0.83; low-quality evidence). However, no differences were found for mortality (low-quality evidence) and hospital LOS. In addition, there was insufficient evidence to prove a potential link between postoperative NRS and complications such as anastomotic leakages, sepsis, and pneumonia-related sequalae. It is worth remembering that after abdominal surgery, the risk of gastric insufflation rises when pressure reaches values higher than 25 cmH2O. Hence, in upper-gastrointestinal surgical procedures, the risk of anastomotic leakage should be reduced by maintaining the pressure support level below 6–8 cmH2O [64].
Squadrone et al. [25] studied 209 consecutive patients who had undergone elective major abdominal surgery and developed postoperative hypoxemia. The patients were randomized to receive either COT or helmet CPAP in the recovery room. Patients receiving helmet CPAP had a lower reintubation rate (1% vs. 10%; p = 0.005) and a lower occurrence of pneumonia (2% vs. 10%, p = 0.02), infection (3% vs. 10%, p = 0.03), and sepsis (2% vs. 9%; p = 0.03) than patients treated with COT. When considering patients readmitted to an ICU with postoperative acute respiratory failure, NIV successfully prevented reintubation in 67% of cases.
In a multicenter RCT conducted among patients with acute respiratory failure after abdominal surgery, NIV use reduced the need for reintubation and for invasive mechanical ventilation and was associated with fewer healthcare-related infections when compared to COT [77].
4.3. Use of NIV after Specific Major Surgery Settings
The use of NIV after major cardiac and thoracic surgical procedures is a very attractive issue. Patients undergoing cardiac and thoracic surgeries are at higher risk of developing PPCs because of underlying pulmonary and cardiovascular dysfunctions. In these cases, preventive NIV may reduce PPC occurrence by reducing left ventricular preload and afterload, followed by an improvement of cardiac and respiratory functions [69]. Contradictory results emerge from studies conducted so far.
In their single randomized trial, Auriant et al. [24] found that NIV after lung resection decreased the frequency of intubation from 50.0% to 20.8% (p = 0.035) and mortality (13% vs. 38%; p = 0.045). In contrast with these findings, an RCT aiming to investigate whether prophylactic postoperative NIV could prevent acute respiratory postoperative conditions in COPD patients who had undergone lung resection surgery did not find any advantage on postoperative outcome. The authors found that acute respiratory failure rate was 18.8% in the prophylactic NIV group and 24.5% in controls (p = 0.20), reintubation rates were similar in the prophylactic NIV and control group (10/181 (5.5%) and 13/179 (7.2%), respectively, p = 0.53), and mortality rates were 5 and 2.2% in the control and prophylactic NIV groups, respectively (p = 0.16) [79].
The Bilevel Positive Airway Pressure Versus Optiflow study [26] was the first large, multicenter randomized controlled noninferiority trial comparing HFNOT and NIV in 830 patients developing hypoxemia during the spontaneous breathing trial or after extubation following cardiothoracic surgery. Treatment failure, defined as reintubation, switch to the other treatment, or premature discontinuation of the assigned treatment, was similar in both groups (21% for HFNOT and 21.9% for NIV, for the three strategies combined; absolute risk difference 0.9%, 95% CI −4.9% to 6.6%, p = 0.003), as were reintubations (14% in both groups). A post hoc analysis showed that there were no differences in the proportion of patients with treatment failure between HFNOT and NIV when these techniques were used as a weaning strategy or as a therapeutic strategy (27% for HFNOT vs. 28% for NIV, p = 0.93). When considering a preventive strategy for high-risk patients, treatment failure was lower in the HFNOT group than in the NIV group.
Interestingly, a recent network meta-analysis of 16 trials enrolling 3011 patients [80] found that NIV significantly reduced the incidence of PPCs when compared to standard of care (RR 0.67, 95% CI: 0.49 to 0.93; absolute risk reduction (ARR) 7.6%, 95% CI: 1.6–11.8%; low certainty) and the incidence of atelectasis (RR 0.65, 95% CI: 0.45 to 0.93; ARR 19.3%, 95% CI: 3.9–30.4%; moderate certainty); however, prophylactic NIV was not associated with a decreased reintubation rate or reduced short-term mortality. Further analysis showed that the highest ranked treatment for reducing the incidence of PPCs was NIV (83.0%), followed by HFNOT (62.5%), CPAP (44.3%), and standard of care treatment (10.2%).
Due to a restrictive syndrome and decreased chest wall compliance, obese patients are at higher risk of hypoxemia and PPCs. Increased body mass index is directly correlated with a loss of functional residual capacity by up to 50% of the preoperative values, with morbidly obese patients having significantly more atelectasis than nonobese patients, before induction, after tracheal extubation, and 24 h following laparoscopic surgery [81]. In addition, different comorbidities such as OSA, obesity hypoventilation syndrome, and cardiovascular and metabolic diseases are to be considered as predisposing factors for PPCs [12].
The obese patient, with or without OSA, might not respond to supplemental oxygen administration, being prone to postoperative oxygen desaturation and hypoxic episodes. Postoperative NIV could, therefore, mitigate the risk of extubation failure and the incidence of PPCs. However, evidence on the use of NIV after bariatric surgery is still limited.
A recent retrospective analysis evaluated the efficacy of short-term NIV use in reducing postextubation acute respiratory failure after biliointestinal bypass in obese patients in the PACU setting. The authors found an improvement of PaO2 (67.91 ± 3.03 vs. 60.14 ± 4.17, p < 0.001) and SpO2 (93.95 ± 2.09, p < 0.001) values in the NIV group, with usual postoperative care (oxygen therapy via a Venturi mask) being significantly associated with postoperative acute respiratory failure (RR 0.51, CI: 0.27 to 0.96, p < 0.05) [82].
Huerta et al. [83] adopted NIV in morbidly obese patients undergoing sleeve gastrectomy and open bariatric surgery, demonstrating that NIV increases PaO2 during the postoperative period (PaO2: 78.87 ± 8.31 in NIV group vs. 64.27 ± 6.33 in oxygen group). In addition, they found that only 15 out of more 1000 patients presented major anastomotic leaks, with only two cases related to postoperative NIV use.
The perioperative application of NIV in obese patients with positive airway pressure < 20 cmH2O is rarely a cause of NIV-related anastomotic leakage [84].
According to the meta-analysis conducted by Carron et al. [27], the postoperative use of NIV among obese patients was associated with a decreased risk of respiratory complications (RR 0.33, 95% CI: 0.16 to 0.66, p = 0.002) but not of reintubation after tracheal extubation (RR 0.41, 95% CI: 0.09 to 1.82, p = 0.3657) and unplanned ICU admission (RR 0.43, 95% CI: 0.16 to 1.15, p = 0.0937).
5. Postoperative Use of High-Flow Nasal Oxygen Therapy
The use of HFNOT has been widely studied and adopted after extubation of critically ill patients [85,86], particularly as a prophylactic measure to reduce the need for respiratory support escalation [68,87,88]. A series of RCTs evaluated the use of HFNOT with respect to COT [89] and NIV [90,91,92] in critically ill patients. Although initial trials demonstrated that HFNOT prevents reintubation in unselected cohorts of critically ill patients and performs as well as NIV in high-risk patients [93,94], recent data indicate that prophylactic HFNOT in all patients does not reduce the reintubation rate if patients treated with COT can receive a trial of rescue NRS (either HFNOT or NIV) before intubation [89,95]. In high-risk or obese patients, prophylactic NIV combined with HFNOT performs better than HFNOT alone [28,41]. This evidence indicates that HFNOT, even if not alternated with NIV, plays a crucial role in improving weaning outcome. Whether this evidence can be translated to patients undergoing general anesthesia has to be demonstrated by ongoing investigations.
The use of HFNOT has recently been proposed as part of an enhanced recovery program after major surgeries in patients at high risk for respiratory complications. This approach could improve functional recovery and reduce hospital LOS, with potential implications for reduced healthcare costs [96,97].
The effectiveness of preemptive HFNOT has been studied after cardiothoracic and abdominal surgery [26,98,99]. While data suggest that preemptive HFNOT can prevent respiratory support escalation in postcardiac surgery patients, with HFNOT being noninferior to NIV in hypoxemic patients [26], results in thoracic surgery may vary, likely due to differing control strategies or patient population [98,100]. In a randomized trial conducted after lung resection, patients receiving HFNOT showed a lower arterial CO2 and a reduced risk of postoperative hypercapnia than those treated with a Venturi mask [98]. In a meta-analysis by Chaudhuri et al. [29], prophylactic HFNOT reduced the rate of reintubation and respiratory support escalation when compared to COT in the immediate postoperative period after cardiothoracic surgery, an effect likely driven by patients at high risk and/or obesity. Positive pressure effect given by HFNOT application reverses atelectasis by increasing end-expiratory lung volume, finally reducing alveolar shunt fraction and enhancing arterial oxygenation [54,101,102]. Consistently with this, a recent randomized trial [30] involving patients undergoing major gynecological surgery in the Trendelenburg position showed that a preemptive two-hour HFNOT session following extubation improved postoperative oxygenation if compared to conventional Venturi mask oxygen therapy. This was primarily attributed to a reduction in atelectasis in the dorsal lung regions. Additionally, a quarter of patients in the HFNOT group received the assigned treatment with no supplemental oxygen, emphasizing that the beneficial effects of HFNOT were mainly due to high flow rather than supplemental oxygen provision [103,104]. However, HFNOT after extubation did not reduce the incidence of postoperative hypoxemia nor improved any other clinical outcome in patients at intermediate-to-high risk of PPCs [99]. The difference in outcomes between these studies may arise from population characteristics: patients undergoing laparoscopy in the Trendelenburg position had a higher incidence of postoperative hypoxemia compared to those of the OPERA trial [99], and patients undergoing lung resection may be at especially high risk of postoperative hypercapnia. Nevertheless, data from a recent meta-analysis did not support the routine use of postoperative CPAP, NIV, or HFNOT to prevent the occurrence of PPCs or pneumonia after surgery in adults [74].
Preemptive HFNOT may provide physiological benefits in specific patient subgroups but may not have a big impact in terms of patient-centered outcomes [99], especially when NIV use before reintubation is possible [89,95]. Therefore, preemptive postextubation HFNOT in surgical patients at low risk of PPCs necessitates a meticulous personalized assessment.
6. Future Perspectives: Artificial Intelligence and Machine Learning
Artificial intelligence and machine learning have the potential to personalize perioperative management and mechanical ventilation strategies for patients needing respiratory support [105]. Machine-learning models are complex mathematical constructs which could help the perioperative identification of patients at higher risk for deterioration while pointing out factors which could be optimized.
Optimization of perioperative respiratory support could be reached with the use of these models, and future research might focus on these issues in order to curb PPCs in high-risk patients undergoing major surgery. A recent study [106] validated a machine-learning model to predict and identify patients at high risk of NIV failure. The timely assessment of NIV efficacy and subsequent clinical decision are particularly crucial: if a patient is predicted to have a high risk of NIV failure, strict clinical monitoring could be provided and earlier reintubation considered, thus reducing mortality.
Several studies on critically ill patients have shown that machine-learning models may accurately predict extubation failure with remarkable accuracy [107] and identify patients in whom duration of mechanical ventilation might be longer [108]. However, there is a substantial paucity of data regarding the application of these tools in subjects undergoing general anesthesia. Therefore, development of new algorithms should be promoted to improve confidence and translate these approaches to everyday clinical practice.
Genomic data are increasingly applied in medicine, and the most difficult challenge to overcome is the huge amount of data generated and the difficulties related to translating these findings to clinical practice. With special reference to mechanical ventilation, there is no doubt that it has a direct impact on patients’ lung physiology. While ventilator-induced lung injury has been extensively explored, only a few experimental studies report that vigorous breathing effort during spontaneous ventilation can worsen lung injury and cause a phenomenon that has been termed patient self-inflicted lung injury [109]. The genetic determinants for these respiratory complications are unknown. However, the identification of novel therapeutic targets and the optimal NRS to apply is essential for progress in the understanding of these respiratory complications and for limiting their occurrence.
7. Conclusions
This narrative review revise much of the existing evidence on perioperative NRS use, reporting the most influential studies on the topic. What emerges is that personalized medicine is progressively shaping anesthesia practice and perioperative medicine, also for NRS. The evidence gathered so far brings profound implications for current clinical practice and should promote a careful reappraisal of guidelines on the perioperative use of NRS, especially when high-risk patients are considered.
Use of NRS should be tailored based on the patient’s specific characteristics and type of surgery, aiming at a personalized cost-effective approach.
Author Contributions
G.M.: conceptualization, methodology, resources, writing—original draft, writing—review and editing; L.F.: methodology, writing—original draft, writing—review and editing, resources, supervision, project administration; R.S.: methodology, resources, writing—original draft, visualization; T.R.: writing—original draft, writing—review and editing; D.L.G.: writing—original draft, resources; A.P.: writing—original draft, resources; E.D.R.: conceptualization, writing—review and editing, supervision; C.G.: conceptualization, writing—review and editing, supervision, project administration; G.M. and L.F. equally contributed as first authors. All the authors approved the submitted version and agree to be personally accountable for their own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Fisher & Paykel Healthcare (Auckland, New Zealand).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Giovanni Misseri declares a patent in association with the University of Palermo, Italy (No 102019000020532, Italian Ministry of Economic Development); Luciano Frassanito received honoraria from Edwards Lifesciences Ltd. for scientific advice; Domenico Luca Grieco received honoraria from GE Healthcare, Intersurgical, Fisher&Paykel, Gilead, and Pfizer for lectures, and discloses research grants by GE Healthcare and Fisher&Paykel. Cesare Gregoretti received honoraria for lectures or consultancies from Vivisol, Philips, Mindray, and Air Liquid, and declares a patent in association with the University of Palermo, Italy (No 102019000020532, Italian Ministry of Economic Development). Edoardo De Robertis received honoraria from Drager, GE, Baxter, MSD, Fresenius, and Fisher&Paykel for lectures or consultancies. All the other authors declare no conflicts of interest. All other authors declare no conflict of interest.
Abbreviations
| ARF | acute respiratory failure |
| ARISCAT (score) | Assess Respiratory Risk in Surgical Patients in Catalonia (score) |
| ARR | absolute risk reduction |
| CI | confidence interval |
| CO2 | carbon dioxide |
| COPD | chronic obstructive pulmonary disease |
| COT | conventional oxygen therapy |
| CPAP | continuous positive airway pressure |
| GA | general anesthesia |
| HDU | high-dependency care unit |
| HFNOT | high-flow nasal oxygen therapy |
| ICU | intensive care unit |
| LOS | length of stay |
| NIV | noninvasive ventilation |
| NRS | noninvasive respiratory support |
| OR | odds ratio |
| OSA | obstructive sleep apnea |
| PACU | postanesthesia care unit |
| PaO2 | arterial partial pressure of oxygen |
| PPCs | postoperative pulmonary complications |
| RCT | randomized controlled trial |
| RM | recruitment maneuver |
| RR | risk ratio |
| THRIVE | transnasal humidified rapid-insufflation ventilatory exchange |
References
- Wall, J.; Dhesi, J.; Snowden, C.; Swart, M. Perioperative medicine. Future Healthc. J. 2022, 9, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, A. Anesthesiomics: Could a New Name Be Coined for Anesthesia? Anesthesiol. Pain Med. 2020, 10, e100988. [Google Scholar] [CrossRef]
- Bigatello, L.; Pesenti, A. Respiratory Physiology for the Anesthesiologist. Anesthesiology 2019, 130, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Michelet, P.; Blayac, D.; Jaber, S.; Riou, B. Case scenario: Management of postesophagectomy respiratory failure with noninvasive ventilation. Anesthesiology 2010, 113, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Hemmes, S.N.; Neto, A.S.; Binnekade, J.M.; Canet, J.; Hedenstierna, G.; Jaber, S.; Hiesmayr, M.; Hollmann, M.W.; Mills, G.H.; et al. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS—An observational study in 29 countries. Eur. J. Anaesthesiol. 2017, 34, 492–507. [Google Scholar] [CrossRef]
- Neto, A.S.; Hemmes, S.N.; Barbas, C.S.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Hollmann, M.W.; Jaber, S.; Kozian, A.; Licker, M.; et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: A systematic review and meta-analysis. Lancet Respir. Med. 2014, 2, 1007–1015, Erratum in Lancet Respir. Med. 2014, 2, e23. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J.; et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.K.; Teng, A.; Lee, D.Y.; Rose, K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J. Surg. Res. 2015, 198, 441–449. [Google Scholar] [CrossRef]
- Ferrando, C.; Soro, M.; Unzueta, C.; Suarez-Sipmann, F.; Canet, J.; Librero, J.; Pozo, N.; Peiró, S.; Llombart, A.; León, I.; et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): A randomised controlled trial. Lancet Respir. Med. 2018, 6, 193–203. [Google Scholar] [CrossRef]
- Tartler, T.M.; Ahrens, E.C.M.; Munoz-Acuna, R.; Azizi, B.A.C.M.; Chen, G.; Suleiman, A.; Wachtendorf, L.J.C.M.; Costa, E.L.; Talmor, D.S.; Amato, M.B.; et al. High Mechanical Power and Driving Pressures are Associated with Postoperative Respiratory Failure Independent from Patients’ Respiratory System Mechanics. Crit. Care Med. 2023, 52, 68–79. [Google Scholar] [CrossRef]
- O’gara, B.; Talmor, D. Perioperative lung protective ventilation. BMJ 2018, 362, k3030. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Chevallard, G.; Gregoretti, C. Non-invasive ventilation in postoperative patients: A systematic review. Intensive Care Med. 2011, 37, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.-O.; Brotons, F.; Briant, A.R.; Suehiro, K.; Gozdzik, W.; Sponholz, C.; Kirkeby-Garstad, I.; Joosten, A.; Neto, C.N.; Kunstyr, J.; et al. Postoperative Pulmonary Complications after Cardiac Surgery: The VENICE International Cohort Study. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Einav, S.; Lakbar, I.; Leone, M. Non-Invasive Respiratory Support for Management of the Perioperative Patient: A Narrative Review. Adv. Ther. 2021, 38, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Simonte, R.; De Robertis, E. Comfort during Non-invasive Ventilation. Front. Med. 2022, 9, 874250, Erratum in Front. Med. 2023, 10, 1193466. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.-M.; Pelosi, P.; Chanques, G.; Massone, A.; Petit, A.; Kwiatkowski, F.; Bazin, J.-E.; Jaber, S. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: A randomized controlled study. Anesthesiology 2011, 114, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Coussa, M.; Proietti, S.; Schnyder, P.; Frascarolo, P.; Suter, M.; Spahn, D.R.; Magnusson, L. Prevention of atelectasis formation during the induction of general anesthesia in morbidly obese patients. Anesth. Analg. 2004, 98, 1491–1495. [Google Scholar] [CrossRef]
- Perrin, C.; Jullien, V.; Vénissac, N.; Berthier, F.; Padovani, B.; Guillot, F.; Coussement, A.; Mouroux, J. Prophylactic use of noninvasive ventilation in patients undergoing lung resectional surgery. Respir. Med. 2007, 101, 1572–1578. [Google Scholar] [CrossRef]
- Patel, A.; Nouraei, S.A.R. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): A physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015, 70, 323–329. [Google Scholar] [CrossRef]
- Min, S.-H.; Yoon, H.; Huh, G.; Kwon, S.K.; Seo, J.-H.; Cho, Y.J. Efficacy of high-flow nasal oxygenation compared with tracheal intubation for oxygenation during laryngeal microsurgery: A randomised non-inferiority study. Br. J. Anaesth. 2022, 128, 207–213. [Google Scholar] [CrossRef]
- Riedel, T.; Bürgi, F.; Greif, R.; Kaiser, H.; Riva, T.; Theiler, L.; Nabecker, S. Changes in lung volume estimated by electrical impedance tomography during apnea and high-flow nasal oxygenation: A single-center randomized controlled trial. PLoS ONE 2022, 17, e0273120. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Sun, M.; Miao, M.; Han, Y.; Yang, Y.; Cong, X.; Zhang, J. High flow nasal cannula for patients undergoing bronchoscopy and gastrointestinal endoscopy: A systematic review and meta-analysis. Front. Surg. 2022, 9, 949614. [Google Scholar] [CrossRef] [PubMed]
- Zayed, Y.; Kheiri, B.; Barbarawi, M.; Rashdan, L.; Gakhal, I.; Ismail, E.; Kerbage, J.; Rizk, F.; Shafi, S.; Bala, A.; et al. Effect of oxygenation modalities among patients with postoperative respiratory failure: A pairwise and network meta-analysis of randomized controlled trials. J. Intensive Care 2020, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Auriant, I.; Jallot, A.; Hervé, P.; Cerrina, J.; Ladurie, F.L.R.; Fournier, J.L.; Lescot, B.; Parquin, F. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am. J. Respir. Crit. Care Med. 2001, 164, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, V.; Coha, M.; Cerutti, E.; Schellino, M.M.; Biolino, P.; Occella, P.; Belloni, G.; Vilianis, G.; Fiore, G.; Cavallo, F.; et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: A randomized controlled trial. JAMA 2005, 293, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Stéphan, F.; Barrucand, B.; Petit, P.; Rézaiguia-Delclaux, S.; Médard, A.; Delannoy, B.; Cosserant, B.; Flicoteaux, G.; Imbert, A.; Pilorge, C.; et al. High-Flow Nasal Oxygen vs. Noninvasive Positive Airway Pressure in Hypoxemic Patients after Cardiothoracic Surgery: A Randomized Clinical Trial. JAMA 2015, 313, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Zarantonello, F.; Tellaroli, P.; Ori, C. Perioperative noninvasive ventilation in obese patients: A qualitative review and meta-analysis. Surg. Obes. Relat. Dis. 2016, 12, 681–691. [Google Scholar] [CrossRef]
- Thille, A.W.; Coudroy, R.; Nay, M.-A.; Gacouin, A.; Decavèle, M.; Sonneville, R.; Beloncle, F.; Girault, C.; Dangers, L.; Lautrette, A.; et al. Beneficial Effects of Noninvasive Ventilation after Extubation in Obese or Overweight Patients: A Post Hoc Analysis of a Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 440–449. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Granton, D.; Wang, D.X.; Burns, K.E.; Helviz, Y.; Einav, S.; Trivedi, V.; Mauri, T.; Ricard, J.-D.; Mancebo, J.; et al. High-Flow Nasal Cannula in the Immediate Postoperative Period: A Systematic Review and Meta-analysis. Chest 2020, 158, 1934–1946. [Google Scholar] [CrossRef]
- Frassanito, L.; Grieco, D.L.; Zanfini, B.A.; Catarci, S.; Rosà, T.; Settanni, D.; Fedele, C.; Scambia, G.; Draisci, G.; Antonelli, M. Effect of a pre-emptive 2-hour session of high-flow nasal oxygen on postoperative oxygenation after major gynaecologic surgery: A randomised clinical trial. Br. J. Anaesth. 2023, 131, 775–785. [Google Scholar] [CrossRef]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Gregoretti, C.; Giarratano, A. Respiratory support techniques to avoid desaturation in critically ill patients requiring endotracheal intubation: A systematic review and meta-analysis. J. Crit. Care 2017, 41, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Rusca, M.; Proietti, S.; Schnyder, P.; Frascarolo, P.; Hedenstierna, G.; Spahn, D.R.; Magnusson, L. Prevention of atelectasis formation during induction of general anesthesia. Obstet. Anesth. Dig. 2003, 97, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Frerk, C.; Mitchell, V.; McNarry, A.; Mendonca, C.; Bhagrath, R.; Patel, A.; O’sullivan, E.; Woodall, N.; Ahmad, I.; Difficult Airway Society Intubation Guidelines Working Group. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br. J. Anaesth. 2015, 115, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Baillard, C.; Fosse, J.-P.; Sebbane, M.; Chanques, G.; Vincent, F.; Courouble, P.; Cohen, Y.; Eledjam, J.-J.; Adnet, F.; Jaber, S. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am. J. Respir. Crit. Care Med. 2006, 174, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Amraoui, J.; Lefrant, J.-Y.; Arich, C.; Cohendy, R.; Landreau, L.; Calvet, Y.; Capdevila, X.; Mahamat, A.; Eledjam, J.-J. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: A prospective, multiple-center study. Crit. Care Med. 2006, 34, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Gander, S.; Frascarolo, P.; Suter, M.; Spahn, D.R.; Magnusson, L. Positive end-expiratory pressure during induction of general anesthesia increases duration of nonhypoxic apnea in morbidly obese patients. Anesth. Analg. 2005, 100, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, A.O.; Lee, W.; Greilich, N.B.; Joshi, G.P. Perioperative management of obstructive sleep apnea. Chest 2010, 138, 1489–1498, Erratum in Chest 2011, 140, 1393. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: An updated report by the american society of anesthesiologists task force on perioperative management of patients with obstructive sleep apnea. Anesthesiology 2014, 120, 268–286. [Google Scholar] [CrossRef]
- Bagan, P.; Bouayad, M.; Benabdesselam, A.; Landais, A.; Mentec, H.; Couffinhal, J.-C. Prevention of pulmonary complications after aortic surgery: Evaluation of prophylactic noninvasive perioperative ventilation. Ann. Vasc. Surg. 2011, 25, 920–922. [Google Scholar] [CrossRef]
- Paleiron, N.; Grassin, F.; Lancelin, C.; Tromeur, C.; Margery, J.; Natale, C.; Couturaud, F.; GFPC Group. Assessment of preoperative noninvasive ventilation before lung cancer surgery: The preOVNI randomized controlled study. J. Thorac. Cardiovasc. Surg. 2020, 160, 1050–1059. [Google Scholar] [CrossRef]
- Rochwerg, B.; Einav, S.; Chaudhuri, D.; Mancebo, J.; Mauri, T.; Helviz, Y.; Goligher, E.C.; Jaber, S.; Ricard, J.-D.; Rittayamai, N.; et al. The role for high flow nasal cannula as a respiratory support strategy in adults: A clinical practice guideline. Intensive Care Med. 2020, 46, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, D.; Granton, D.B.; Wang, D.X.B.; Einav, S.; Helviz, Y.; Mauri, T.; Ricard, J.-D.; Mancebo, J.; Frat, J.-P.; Jog, S.; et al. Moderate Certainty Evidence Suggests the Use of High-Flow Nasal Cannula Does Not Decrease Hypoxia When Compared with Conventional Oxygen Therapy in the Peri-Intubation Period: Results of a Systematic Review and Meta-Analysis. Crit. Care Med. 2020, 48, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, I.M.; Lodenius, Å.; Tunelli, J.; Ullman, J.; Fagerlund, M.J. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE)—A physiological study. Br. J. Anaesth. 2017, 118, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Hermez, L.A.; Spence, C.J.; Payton, M.J.; Nouraei, S.A.R.; Patel, A.; Barnes, T.H. A physiological study to determine the mechanism of carbon dioxide clearance during apnoea when using transnasal humidified rapid insufflation ventilatory exchange (THRIVE). Anaesthesia 2019, 74, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.; Callaghan, M. Uses and mechanisms of apnoeic oxygenation: A narrative review. Anaesthesia 2019, 74, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Laviola, M.; Das, A.; Chikhani, M.; Bates, D.; Hardman, J. Computer simulation clarifies mechanisms of carbon dioxide clearance during apnoea. Br. J. Anaesth. 2019, 122, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.; Patel, A.; Iqbal, R.; Cecconi, M.; Nouraei, S.A.R. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia 2017, 72, 439–443. [Google Scholar] [CrossRef]
- Lodenius, Å.; Piehl, J.; Östlund, A.; Ullman, J.; Fagerlund, M.J. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) vs. facemask breathing pre-oxygenation for rapid sequence induction in adults: A prospective randomised non-blinded clinical trial. Anaesthesia 2018, 73, 564–571. [Google Scholar] [CrossRef]
- Forsberg, I.; Ullman, J.; Hoffman, A.; Eriksson, L.I.; Lodenius, A.; Fagerlund, M.J. Lung volume changes in Apnoeic Oxygenation using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) compared to mechanical ventilation in adults undergoing laryngeal surgery. Acta Anaesthesiol. Scand. 2020, 64, 1491–1498. [Google Scholar] [CrossRef]
- To, K.; Harding, F.; Scott, M.; Milligan, P.; Nixon, I.; Adamson, R.; McNarry, A. The use of transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in 17 cases of subglottic stenosis. Clin. Otolaryngol. 2017, 42, 1407–1410. [Google Scholar] [CrossRef]
- Raineri, S.M.; Cortegiani, A.; Accurso, G.; Procaccianti, C.; Vitale, F.; Caruso, S.; Giarrjatano, A.; Gregoretti, C. Efficacy and Safety of Using High-Flow Nasal Oxygenation in Patients Undergoing Rapid Sequence Intubation. Turk. J. Anesth. Reanim. 2017, 45, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Dallaire, A.; Singh, K.P.; Madhusudan, P.; Jackson, T.; Singh, M.; Wong, J.; Chung, F. High-Flow Nasal Oxygen Improves Safe Apnea Time in Morbidly Obese Patients Undergoing General Anesthesia: A Randomized Controlled Trial. Anesth. Analg. 2019, 129, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Tremey, B.; Squara, P.; De Labarre, H.; Ma, S.; Fischler, M.; Lawkoune, J.-D.; Le Guen, M. Hands-free induction of general anesthesia: A randomised pilot study comparing usual care and high-flow nasal oxygen. Minerva Anestesiol. 2020, 86, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Corley, A.; Caruana, L.; Barnett, A.; Tronstad, O.; Fraser, J. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br. J. Anaesth. 2011, 107, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Karim, H.M.R.; Esquinas, A.M.; O’brien, B. Flow Effects of High-flow Nasal Oxygenation: Comment. Anesthesiology 2023, 138, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Riva, T.; Greif, R.; Kaiser, H.; Riedel, T.; Huber, M.; Theiler, L.; Nabecker, S. Carbon dioxide changes during high-flow nasal oxygenation in apneic patients: A single-center randomized controlled noninferiority trial. Anesthesiology 2022, 136, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Simpao, A.F.; London, M.J. Can We Finally Take the “VE” Out of THRIVE? Anesthesiology 2022, 136, 1–3. [Google Scholar] [CrossRef]
- Kim, H.J.; Asai, T. High-flow nasal oxygenation for anesthetic management. Korean J. Anesthesiol. 2019, 72, 527–547. [Google Scholar] [CrossRef]
- Conway, A.; Tipton, E.; Liu, W.-H.; Conway, Z.; Soalheira, K.; Sutherland, J.; Fingleton, J. Accuracy and precision of transcutaneous carbon dioxide monitoring: A systematic review and meta-analysis. Thorax 2019, 74, 157–163. [Google Scholar] [CrossRef]
- Mazzeffi, M.A.; Petrick, K.M.; Magder, L.; Greenwald, B.D.; Darwin, P.; Goldberg, E.M.; Bigeleisen, P.; Chow, J.H.; Anders, M.; Boyd, C.M.; et al. High-Flow Nasal Cannula Oxygen in Patients Having Anesthesia for Advanced Esophagogastroduodenoscopy: HIFLOW-ENDO, a Randomized Clinical Trial. Anesth. Analg. 2021, 132, 743–751. [Google Scholar] [CrossRef]
- Thiruvenkatarajan, V.; Sekhar, V.; Wong, D.T.; Currie, J.; Van Wijk, R.; Ludbrook, G.L. Effect of high-flow nasal oxygen on hypoxaemia during procedural sedation: A systematic review and meta-analysis. Anaesthesia 2023, 78, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Yuanyuan, C.; Ke, D.; Jing, H.; Wenwen, Z.; Hongguang, B.; Xiaoliang, W. Application effect of transnasal humidified rapid-insufflation ventilatory exchange in hysteroscopic surgery under intravenous anesthesia. J. Nanjing Med. Univ. 2021, 41, 10. [Google Scholar]
- Frassanito, L.; Piersanti, A.; Vassalli, F.; Zanfini, B.A.; Catarci, S.; Ciano, F.; Scorzoni, M.; Draisci, G. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) as unique technique for airway management during operative hysteroscopy under general anesthesia: A registered feasibility pilot cohort study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6208–6214. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Chanques, G.; Jung, B.; Riou, B. Postoperative Noninvasive Ventilation. Anesthesiology 2010, 112, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Vaschetto, R.; Longhini, F.; Persona, P.; Ori, C.; Stefani, G.; Liu, S.; Yi, Y.; Lu, W.; Yu, T.; Luo, X.; et al. Early extubation followed by immediate noninvasive ventilation vs. standard extubation in hypoxemic patients: A randomized clinical trial. Intensive Care Med. 2019, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, P.; Portacci, A.; Carpagnano, G.E.; Banfi, P.; Crimi, C.; Misseri, G.; Gregoretti, C. The right interface for the right patient in noninvasive ventilation: A systematic review. Expert Rev. Respir. Med. 2022, 16, 931–944. [Google Scholar] [CrossRef]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef]
- Leone, M.; Contributors, G.; Einav, S.; Chiumello, D.; Constantin, J.-M.; De Robertis, E.; De Abreu, M.G.; Gregoretti, C.; Jaber, S.; Maggiore, S.M.; et al. Noninvasive respiratory support in the hypoxaemic peri-operative/periprocedural patient: A joint ESA/ESICM guideline. Intensive Care Med. 2020, 46, 697–713. [Google Scholar] [CrossRef]
- Maggiore, S.M.; Battilana, M.; Serano, L.; Petrini, F. Ventilatory support after extubation in critically ill patients. Lancet Respir. Med. 2018, 6, 948–962. [Google Scholar] [CrossRef]
- Misseri, G.; Cortegiani, A.; Gregoretti, C. How to communicate between surgeon and intensivist? Curr. Opin. Anaesthesiol. 2020, 33, 170–176. [Google Scholar] [CrossRef]
- Ball, L.; Battaglini, D.; Pelosi, P. Postoperative respiratory disorders. Curr. Opin. Crit. Care 2016, 22, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Faria, D.A.; da Silva, E.M.; Atallah, N.; Vital, F.M. Noninvasive positive pressure ventilation for acute respiratory failure following upper abdominal surgery. Cochrane Database Syst. Rev. 2015, 2015, CD009134. [Google Scholar] [CrossRef] [PubMed]
- Pearse, R.; Ranieri, M.; Abbott, T.; Pakats, M.L.; Piervincenzi, E.; Patel, A.; Kahan, B.; Rhodes, A.; Dias, P.; Hewson, R.; et al. Postoperative continuous positive airway pressure to prevent pneumonia, re-intubation, and death after major abdominal surgery (PRISM): A multicentre, open-label, randomised, phase 3 trial. Lancet Respir. Med. 2021, 9, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Fowler, A.J.; Cashmore, R.M.; Fisher, T.J.; Schlautmann, J.; Body, S.; Lan-Pak-Kee, V.; Webb, M.; Kyriakides, M.; Ng, J.Y.; et al. Routine postoperative noninvasive respiratory support and pneumonia after elective surgery: A systematic review and meta-analysis of randomised trials. Br. J. Anaesth. 2022, 128, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Abrard, S.; Rineau, E.; Seegers, V.; Lebrec, N.; Sargentini, C.; Jeanneteau, A.; Longeau, E.; Caron, S.; Callahan, J.-C.; Chudeau, N.; et al. Postoperative prophylactic intermittent noninvasive ventilation versus usual postoperative care for patients at high risk of pulmonary complications: A multicentre randomised trial. Br. J. Anaesth. 2023, 130, e160–e168. [Google Scholar] [CrossRef]
- Ireland, C.J.; Chapman, T.M.; Mathew, S.F.; Herbison, G.P.; Zacharias, M. Continuous positive airway pressure (CPAP) during the postoperative period for prevention of postoperative morbidity and mortality following major abdominal surgery. Cochrane Database Syst. Rev. 2014, 2014, CD008930. [Google Scholar] [CrossRef]
- Jaber, S.; Lescot, T.; Futier, E.; Paugam-Burtz, C.; Seguin, P.; Ferrandiere, M.; Lasocki, S.; Mimoz, O.; Hengy, B.; Sannini, A.; et al. Effect of Noninvasive Ventilation on Tracheal Reintubation Among Patients with Hypoxemic Respiratory Failure Following Abdominal Surgery: A Randomized Clinical Trial. JAMA 2016, 315, 1345–1353. [Google Scholar] [CrossRef]
- Antonelli, M.; Conti, G.; Bufi, M.; Costa, M.G.; Lappa, A.; Rocco, M.; Gasparetto, A.; Meduri, G.U. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: A randomized trial. JAMA 2000, 283, 235–241. [Google Scholar] [CrossRef]
- Lorut, C.; Lefebvre, A.; Planquette, B.; Quinquis, L.; Clavier, H.; Santelmo, N.; Hanna, H.A.; Bellenot, F.; Regnard, J.-F.; Riquet, M.; et al. Early postoperative prophylactic noninvasive ventilation after major lung resection in COPD patients: A randomized controlled trial. Intensive Care Med. 2014, 40, 220–227, Erratum in Intensive Care Med. 2014, 40, 469. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, J.; Wang, H.; Xu, Z.; Zhao, L.; Chen, B. Prophylactic noninvasive respiratory support in the immediate postoperative period after cardiac surgery—A systematic review and network meta-analysis. BMC Pulm. Med. 2023, 23, 233. [Google Scholar] [CrossRef]
- Zoremba, M.; Kalmus, G.; Begemann, D.; Eberhart, L.; Zoremba, N.; Wulf, H.; Dette, F. Short term non-invasive ventilation post-surgery improves arterial blood-gases in obese subjects compared to supplemental oxygen delivery—A randomized controlled trial. BMC Anesthesiol. 2011, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, F.; Gritti, F.; Esposito, R.; del Giudice, C.; Cafora, C.; Pennacchio, F.; Maglione, F.; Catauro, A.; Pace, M.C.; Docimo, L.; et al. Non-Invasive Ventilation Reduces Postoperative Respiratory Failure in Patients Undergoing Bariatric Surgery: A Retrospective Analysis. Medicina 2023, 59, 1457. [Google Scholar] [CrossRef] [PubMed]
- Huerta, S.; DeShields, S.; Shpiner, R.; Li, Z.; Liu, C.; Sawicki, M.; Arteaga, J.; Livingston, E.H. Safety and efficacy of postoperative continuous positive airway pressure to prevent pulmonary complications after roux-en-y gastric bypass. J. Gastrointest. Surg. 2002, 6, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, T.L.; Hoddinott, K. A potential complication of bi-level positive airway pressure after gastric bypass surgery. Obes. Surg. 2004, 14, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Tonetti, T.; Navalesi, P.; Nava, S.; Antonelli, M.; Pesenti, A.; Grasselli, G.; Grieco, D.L.; Menga, L.S.; Pisani, L.; et al. High-Flow Nasal Oxygen for Severe Hypoxemia: Oxygenation Response and Outcome in Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2022, 205, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Rittayamai, N.; Grieco, D.L.; Brochard, L. Noninvasive respiratory support in intensive care medicine. Intensive Care Med. 2022, 48, 1211–1214, Erratum in Intensive Care Med. 2022, 48, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.M.; Tran, A.; Sadeghirad, B.; Burns, K.E.A.; Fan, E.; Brodie, D.; Munshi, L.; Goligher, E.C.; Cook, D.J.; Fowler, R.A.; et al. Noninvasive respiratory support following extubation in critically ill adults: A systematic review and network meta-analysis. Intensive Care Med. 2022, 48, 137–147. [Google Scholar] [CrossRef]
- Yasuda, H.; Okano, H.; Mayumi, T.; Narita, C.; Onodera, Y.; Nakane, M.; Shime, N. Post-extubation oxygenation strategies in acute respiratory failure: A systematic review and network meta-analysis. Crit. Care 2021, 25, 135. [Google Scholar] [CrossRef]
- Maggiore, S.M.; Jaber, S.; Grieco, D.L.; Mancebo, J.; Zakynthinos, S.; Demoule, A.; Ricard, J.-D.; Navalesi, P.; Vaschetto, R.; Hraiech, S.; et al. High-Flow Versus VenturiMask Oxygen Therapy to Prevent Reintubation in Hypoxemic Patients after Extubation: A Multicenter Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 206, 1452–1462. [Google Scholar] [CrossRef]
- Thille, A.W.; Muller, G.; Gacouin, A.; Coudroy, R.; Decavèle, M.; Sonneville, R.; Beloncle, F.; Girault, C.; Dangers, L.; Lautrette, A.; et al. Effect of Postextubation High-Flow Nasal Oxygen with Noninvasive Ventilation vs. High-Flow Nasal Oxygen Alone on Reintubation among Patients at High Risk of Extubation Failuree: A Randomized Clinical Trial. JAMA 2019, 322, 1465–1475, Erratum in JAMA 2020, 323, 793. [Google Scholar] [CrossRef]
- Hernández, G.; Paredes, I.; Moran, F.; Buj, M.; Colinas, L.; Rodríguez, M.L.; Velasco, A.; Rodríguez, P.; Pérez-Pedrero, M.J.; Suarez-Sipmann, F.; et al. Effect of postextubation noninvasive ventilation with active humidification vs. high-flow nasal cannula on reintubation in patients at very high risk for extubation failure: A randomized trial. Intensive Care Med. 2022, 48, 1751–1759, Erratum in Intensive Care Med. 2023, 49, 385. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Walline, J.H.; Ling, B.; Xu, Y.; Sun, J.; Wang, B.; Shan, X.; Wang, Y.; Cao, P.; Zhu, Q.; et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: A multicenter, randomized controlled trial. Crit. Care 2020, 24, 489. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Vaquero, C.; Colinas, L.; Cuena, R.; González, P.; Canabal, A.; Sanchez, S.; Rodriguez, M.L.; Villasclaras, A.; Fernández, R. Effect of Postextubation High-Flow Nasal Cannula vs. Noninvasive Ventilation on Reintubation and Postextubation Respiratory Failure in High-Risk Patients: A Randomized Clinical Trial. JAMA 2016, 316, 1565–1574, Erratum in JAMA 2016, 316, 2047–2048; Erratum in JAMA 2017, 317, 858. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Vaquero, C.; González, P.; Subira, C.; Frutos-Vivar, F.; Rialp, G.; Laborda, C.; Colinas, L.; Cuena, R.; Fernández, R. Effect of Postextubation High-Flow Nasal Cannula vs. Conventional Oxygen Therapy on Reintubation in Low-Risk Patients. JAMA 2016, 315, 1354–1361. [Google Scholar] [CrossRef]
- Casey, J.D.; Vaughan, E.M.; Lloyd, B.D.; Billas, P.A.; Jackson, K.E.; Hall, E.J.; Toporek, A.H.; Buell, K.G.; Brown, R.M.; Richardson, R.K.; et al. Protocolized Postextubation Respiratory Support to Prevent Reintubation: A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 294–302. [Google Scholar] [CrossRef]
- Ansari, B.M.; Hogan, M.P.; Collier, T.J.; Baddeley, R.A.; Scarci, M.; Coonar, A.S.; Bottrill, F.E.; Martinez, G.C.; Klein, A.A. A randomized controlled trial of high-flow nasal oxygen (Optiflow) as part of an enhanced recovery program after lung resection surgery. Ann. Thorac. Surg. 2016, 101, 459–464. [Google Scholar] [CrossRef]
- Earwaker, M.; Villar, S.; Fox-Rushby, J.; Duckworth, M.; Dawson, S.; Steele, J.; Chiu, Y.-D.; Litton, E.; Kunst, G.; Murphy, G.; et al. The effect of high-flow nasal oxygen on hospital length of stay in cardiac surgical patients at high risk for respiratory complications: A randomized controlled trial. Trials 2022, 23, 232. [Google Scholar] [CrossRef]
- Pennisi, M.A.; Bello, G.; Congedo, M.T.; Montini, L.; Nachira, D.; Ferretti, G.M.; Meacci, E.; Gualtieri, E.; De Pascale, G.; Grieco, D.L.; et al. Early nasal high-flow versus Venturi mask oxygen therapy after lung resection: A randomized trial. Crit. Care 2019, 23, 68. [Google Scholar] [CrossRef]
- Futier, E.; Paugam-Burtz, C.; Godet, T.; Khoy-Ear, L.; Rozencwajg, S.; Delay, J.-M.; Verzilli, D.; Dupuis, J.; Chanques, G.; Bazin, J.E.; et al. Effect of early postextubation high-flow nasal cannula vs. conventional oxygen therapy on hypoxaemia in patients after major abdominal surgery: A French multicentre randomised controlled trial (OPERA). Intensive Care Med. 2016, 42, 1888–1898. [Google Scholar] [CrossRef]
- Yu, Y.; Qian, X.; Liu, C.; Zhu, C. Effect of High-Flow Nasal Cannula versus Conventional Oxygen Therapy for Patients with Thoracoscopic Lobectomy after Extubation. Can. Respir. J. 2017, 2017, 7894631. [Google Scholar] [CrossRef]
- Basile, M.C.; Mauri, T.; Spinelli, E.; Corte, F.D.; Montanari, G.; Marongiu, I.; Spadaro, S.; Galazzi, A.; Grasselli, G.; Pesenti, A. Nasal high flow higher than 60 L/min in patients with acute hypoxemic respiratory failure: A physiological study. Crit. Care 2020, 24, 654. [Google Scholar] [CrossRef] [PubMed]
- Corley, A.; Bull, T.; Spooner, A.J.; Barnett, A.G.; Fraser, J.F. Direct extubation onto high-flow nasal cannulae post-cardiac surgery versus standard treatment in patients with a BMI ≥30: A randomised controlled trial. Intensive Care Med. 2015, 41, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Spinelli, E.; Pavlovsky, B.; Grieco, D.L.; Ottaviani, I.; Basile, M.C.; Corte, F.D.; Pintaudi, G.; Garofalo, E.; Rundo, A.; et al. Respiratory Drive in Patients with Sepsis and Septic Shock: Modulation by High-flow Nasal Cannula. Anesthesiology 2021, 135, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Riera, J.; Pérez, P.; Cortés, J.; Roca, O.; Masclans, J.R.; Rello, J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: A cohort study using electrical impedance tomography. Respir. Care 2013, 58, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Gallifant, J.; Zhang, J.; Lopez, M.d.P.A.; Zhu, T.; Camporota, L.; Celi, L.A.; Formenti, F. Artificial intelligence for mechanical ventilation: Systematic review of design, reporting standards, and bias. Br. J. Anaesth. 2022, 128, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Q.-Y.; Luo, J.-C.; Liu, K.; Yu, S.-J.; Ma, J.-F.; Luo, M.-H.; Hao, G.-W.; Su, Y.; Zhang, Y.-J.; et al. Early prediction of noninvasive ventilation failure after extubation: Development and validation of a machine-learning model. BMC Pulm. Med. 2022, 22, 304. [Google Scholar] [CrossRef]
- Meyer, A.; Zverinski, D.; Pfahringer, B.; Kempfert, J.; Kuehne, T.; Sündermann, S.H.; Stamm, C.; Hofmann, T.; Falk, V.; Eickhoff, C. Machine learning for real-time prediction of complications in critical care: A retrospective study. Lancet Respir Med. 2018, 6, 905–914. [Google Scholar] [CrossRef]
- Schwager, E.; Liu, X.; Nabian, M.; Feng, T.; French, R.M.; Amelung, P.; Atallah, L.; Badawi, O. Machine learning prediction of the total duration of invasive and non-invasive ventilation During ICU Stay. PLoS Digit. Health 2023, 2, e0000289. [Google Scholar] [CrossRef]
- Battaglini, D.; Robba, C.; Ball, L.; Silva, P.L.; Cruz, F.F.; Pelosi, P.; Rocco, P.R.M. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: A narrative review. Br. J. Anaesth. 2021, 127, 353–364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).