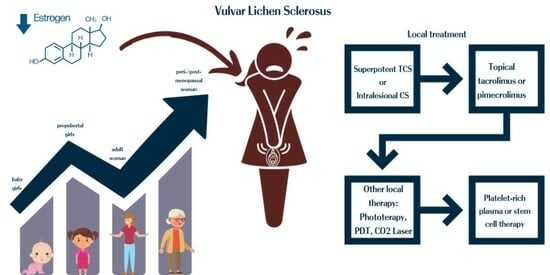
Vulvar Lichen Sclerosus: Navigating Sex Hormone Dynamics and Pioneering Personalized Treatment Paradigm
Abstract
1. Introduction
1.1. Unraveling the Complexities: Exploring the Role of Sex Hormones in Vulvar Lichen Sclerosus
1.2. Interplay of Autoimmunity and Genetics in VLS
1.3. VLS: Current Treatment Paradigm
1.4. Aim
2. Methods
3. Results
3.1. Dynamics of Sexual Hormones throughout Puberty and Beyond in VLS
3.2. VLS: Insights into Conventional Therapeutic Approaches
| First Author Year Type of Study [Reference] | Studied Population | Results | Conclusions |
|---|---|---|---|
| Krause E 2023 Prospective, randomized, double-blinded, dose-controlled [92] | N1 = 29 women with VLS, LDG (aged 54–80.5) N2 = 34 women with VLS, NDG (aged 55–81.5) | BT: N2 vs. N1 VAS 4.3 (±2.4) vs. 5.1 (±2.6); AT: N2 vs. N1 VAS −2.4 (±2.3) vs. −2.7 (±2.8) AT N1 + N2 vs. BT N1 + N2 p < 0.0001 | Microablative CO2 laser results in a notable enhancement of symptoms associated with VLS; however, the reduction of symptoms after 18 weeks between NDG and LDG was not statistically significant (p = 0.6244). |
| Borghi A 2023 Observational [99] | N = 101 women with VLS treated with mometasone furoate 0.1% | 35.8% GSS = 0; 25.7% GOS = 0; 11.5% GSS and GOS = 0 | Clearance of VLS corresponds to a significant improvement in the QoL of patients, making it an ideal therapeutic goal. |
| Salgado HC 2023 Randomized, prospective [90] | N = 20 VLS women; N1 = 11 N treated with tCP; N2 = 9 N treated with fCO2 | µ(SD) at 3 m
| fCO2 laser emerges as a promising therapeutic option, particularly for patients who exhibit minimal or partial responsiveness to CP. |
| García-Souto F 2022 Retrospective, observational [82] | N1 = 62 women with VLS (46.33 ± 2.33 y); N2 = 86 women with ODVA (41.01 ± 1.29 y) | T N1 vs. N2:
| These findings underscore the nuanced T landscape for N1 and N2; furthermore, adjuvant T, such as topical ketoconazole, demonstrated significant disparities between the two groups, emphasizing the need for tailored therapeutic approaches in managing VLS. |
| Günthert AR 2022 Randomized, double-blinded, 2-armed [83] | N1= 17 VLS receiving tP 8%; N2= 20 VLS receiving tCP 0.05% |
81.3% N2 N2 vs. N1 OR (0.35; 95% CI 0.06 to 2.06, p = 0.234) | tCP superior efficacy in improving clinical VLS scores and symptom severity, as well as in achieving complete remission, when compared to tP. |
| Mitchell L 2021 Randomized, prospective, double-blinded [89] | N1 = 19 VLS women randomized to 5SLT; N2 = 18 VLS women randomized to 5fCO2 | N2: 0.20 reduction in HPsS (95% CI −1.1, 0.80, p = 0.74); N1: 0.1 increase in HPsS (95% CI −0.90, 1.0, p = 0.91) | N1 vs. N2 HPsS (95% CI −1.14, 1.06, p = 0.76) not statistically significant. |
| Corazza M 2021 Retrospective, open-label, comparative [98] | N = 61 VLS women; N1 = 29 N treated 24 w (aged 42–86 y); N2 = 32 N treated 12 w (aged 40–86 y) |
| 24 w duration of corticosteroid treatment does not confer significant therapeutic advantages compared to standard 12 w duration courses concerning clearance of VLS. |
| Burkett LS 2021 Randomized, controlled [91] | N1 = 27 women with VLS randomized in the fCO2 arm (mean age 67.6 ± 11.0), N2 = 24 women with VLS randomized in the CP arm (mean age 61.5 ± 8.9) |
| At 6 m:
|
| Wijaya M 2021 Prospective, cross-sectional [101] | N1 = 68 new pretreatment women with VLS; N2 = 136 treated women with VLS > 2 y |
| Long-term, individualized topical corticosteroid treatment is deemed safe and effective in maintaining disease remission and enhancing the quality of life for VLS patients. |
| Borghi A 2020 Prospective [99] | N = 63 women with VLS receiving MMF 0.1%, 12 w |
| PRISM may be more reliable than DLQI in capturing changes in disease-related burden post-treatment, as well as accurately quantifying baseline burden. |
| Pagano T 2020 Prospective, longitudinal [93] | N = 40 women with VLS treated with 2 cycles fCO2 |
| fCO2 is a safe option and may serve as an effective rescue procedure for VLS patients who do not respond to extended ultra-potent TCSs. |
| Kohn JR 2020 Prospective, observational [102] | N = 64 women (aged 41.5 ± 13.1 y) with VLS treated with MBmc |
| T with MBmc effectively alleviated itching in VLS patients, and SHAP decreased significantly. |
| Bizjak Ogrinc U 2019 Randomized, controlled [94] | N1 = 20 women with VLS, mean age 59, SD 10; N2 = 18 women with VLS, mean age 57, SD 14 | 6 m visit:
| N1 demonstrated significant improvements in burning, itching, pain, and overall symptom sum compared to N2, with notable effect sizes favoring the laser treatment. |
| Gajewska M 2018 Longitudinal [84] | N = 11 VLS receiving tCP (aged: 18–77 y) |
| TCSs effectively control lesions in most cases. |
| Corazza M 2018 Observational [103] | N1 = 17 women (mean age: 66.76, SD 11.09) with VLS receiving MMF 0.1% + T 0.005%, 12 w; N2 = 15 women (mean age: 63, SD 9.85) with VLS receiving MMF 0.1% + CC, 12 w |
| Addition of T to the corticosteroid regimen did not provide an observable advantage in terms of dermoscopic outcomes in VLS patients. |
| Maździarz A 2017 Longitudinal [85] | N = 102 VLS receiving 5-ALA + 2-DMSO and PDT (aged: 19–85 y) |
| Favorable outcomes and well-tolerated with PDT. |
| Olejek A 2017 Longitudinal [87] | N = 100 with VLS receiving PDT N1 = 40 N with cAD N2 = 60 N without aAD N3 = 23 N1 with ANA+ |
| PDT may have an impact on the immune status of patients with VLS. |
| Osiecka BJ 2017 Longitudinal [88] | N = 11 women (aged 30–66 y) with VLS receiving 5-ALA-PDT + GL | 2 m AT: itching subsiding in 81.8% N; 4 m AT: no itching in 72.7%; 6 m AT: no itching in 63.6%, weak itching 27.27%, moderate itching 9% | PDT + GL is well-tolerated and can effectively alleviate itching in woman with VLS. |
| Wu C 2017 Retrospective, observational [95] | N1 = 44 women with VLS, treated with FUT; N2 = 85 women with vLSC, treated with FUT; N3 = 7 women with vLP, treated with FUT, mean age 41.5 ± 12.0 y | N1 vs. N2 vs. N3
| FUT is an effective therapeutic option for VLS. |
3.3. VLS: Platelet-Rich Plasma and Stem Cell Therapy
| First Author Year Type of Study [Reference] | Studied Population | Results | Conclusions |
|---|---|---|---|
| Gutierrez-Ontalvilla P 2022 Prospective, randomized [107] | N = 19 women with VLS, N1 = 9 N randomized nanofat–PRP, N2 = 10 N randomized tCP 0.05% | N: R2 AT p = 0.473, R5 AT p = 0.461; N1 AT vs. N2 AT
| In comparison to tCP, these results point to a possible benefit of the nanofat–PRP intervention. |
| Tedesco M 2020 Longitudinal [109] | N = 40 patients with GLS (24 M, 16 F; aged 18–78 y), N1 = 20 N randomized to AD-SVF arm, N2 = 20 N randomized to AD-SVF + PRP |
| Both groups N1 and N2 experienced a reduction in symptoms and improvements in skin and mucosal elasticity, hydration, and atrophy: 32.5% complete disappearance of symptoms; 57.5% significant improvement; 10% no changes; 5% reduction in white lesions. No significant difference in the mean clinical scores between N1 and N2. |
| Goldstein AT 2019 Randomized, double-blinded, placebo-controlled [108] | N = 29 women with VLS (mean age 52.6); N1 = 19 N randomized in PRP arm; N2 = 10 N randomized in placebo arm |
N2: 50% improvement, 40% no change, 10 more inflammation (p= 0.542);
| PRP does not adequately treat VLS. |
4. Discussion
4.1. Dynamics of Sexual Hormones in VLS Individuals
4.2. Insights into VLS Treatment Approaches: Examining the Evolution of Approaches and the Lessons from Varied Therapeutic Strategies over Time
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD-SVF | Adipose-derived stromal vascular fraction |
| CC | Cold cream |
| CP | Clobetasol propionate |
| CV | Coefficient of variation |
| DLQI | Dermatology life quality index |
| GOS | Global objective score |
| GSS | Global subjective score |
| ITT | Intention-to-treat |
| LDG | Low dose group |
| LP | Lichen planus |
| LS | Lichen sclerosus |
| LSC | Lichen simplex chronicus |
| MMF | Mometasone furoate |
| Nd:YAG | Neodymium-doped yttrium aluminum garnet |
| NDG | Normal dose group |
| PRISM | Pictorial representation of illness and self-measure |
| PRP | Platelet-rich plasma |
| QoL | Quality of life |
| SCC | Squamous cell carcinoma |
| SCT | Stem cell therapy |
| SHAP | Squamous hyperplasia area percentage |
| T | Tretinoin |
| TCS | Potent topical corticosteroids |
| UV | Ultraviolet radiation |
| VAS | Visual analogue scale |
| VLS | Vulvar lichen sclerosus |
| VSE | Vulvar self-examination |
| VQLI | Vulvar quality of life index |
References
- Fistarol, S.K.; Itin, P.H. Diagnosis and treatment of lichen sclerosus: An update. Am. J. Clin. Dermatol. 2013, 14, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Fergus, K.B.; Lee, A.W.; Baradaran, N.; Cohen, A.J.; Stohr, B.A.; Erickson, B.A.; Mmonu, N.A.; Breyer, B.N. Pathophysiology, Clinical Manifestations, and Treatment of Lichen Sclerosus: A Systematic Review. Urology 2020, 135, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Krapf, J.M.; Mitchell, L.; Holton, M.A.; Goldstein, A.T. Vulvar Lichen Sclerosus: Current Perspectives. Int. J. Women’s Health 2020, 12, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pope, R.; Lee, M.H.; Myers, A.; Song, J.; Abou Ghayda, R.; Kim, J.Y.; Hong, S.H.; Lee, S.B.; Koyanagi, A.; Jacob, L.; et al. Lichen Sclerosus and Sexual Dysfunction: A Systematic Review and Meta-Analysis. J. Sex. Med. 2022, 19, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.M.; Singh, V.; Nadkarni, N.J.; Patil, S.P. Anogenital lichen sclerosus. Indian J. Sex. Transm. Dis. 2020, 41, 1–9. [Google Scholar] [CrossRef]
- Moyal-Barracco, M.; Wendling, J. Vulvar dermatosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Shah, T.T.; Minhas, S. Recent advances in understanding and managing Lichen Sclerosus. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Leis, M.; Singh, A.; Li, C.; Ahluwalia, R.; Fleming, P.; Lynde, C.W. Risk of Vulvar Squamous Cell Carcinoma in Lichen Sclerosus and Lichen Planus: A Systematic Review. J. Obstet. Gynaecol. Can. 2022, 44, 182–192. [Google Scholar] [CrossRef]
- Williams, A.; Syed, S.; Velangi, S.; Ganesan, R. New Directions in Vulvar Cancer Pathology. Curr. Oncol. Rep. 2019, 21, 88. [Google Scholar] [CrossRef]
- Vieira-Baptista, P.; Lima-Silva, J.; Cavaco-Gomes, J.; Beires, J.; Martinez-de-Oliveira, J. What differentiates symptomatic from asymptomatic women with lichen sclerosus? Gynecol. Obstet. Investig. 2015, 79, 263–268. [Google Scholar] [CrossRef]
- Tran, D.A.; Tan, X.; Macri, C.J.; Goldstein, A.T.; Fu, S.W. Lichen Sclerosus: An autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int. J. Biol. Sci. 2019, 15, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ghatage, P. Etiology, Clinical Features, and Diagnosis of Vulvar Lichen Sclerosus: A Scoping Review. Obstet. Gynecol. Int. 2020, 2020, 7480754. [Google Scholar] [CrossRef] [PubMed]
- Tremaine, R.D.; Miller, R.A. Lichen sclerosus et atrophicus. Int. J. Dermatol. 1989, 28, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.T.; Marinoff, S.C.; Christopher, K.; Srodon, M. Prevalence of vulvar lichen sclerosus in a general gynecology practice. J. Reprod Med. 2005, 50, 477–480. [Google Scholar] [PubMed]
- Leibovitz, A.; Kaplun, V.V.; Saposhnicov, N.; Habot, B. Vulvovaginal examinations in elderly nursing home women residents. Arch. Gerontol. Geriatr. 2000, 31, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Melnick, L.E.; Steuer, A.B.; Bieber, A.K.; Wong, P.W.; Pomeranz, M.K. Lichen sclerosus among women in the United States. Int. J. Women’s Dermatol. 2020, 6, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.; Wojnarowska, F. Childhood vulvar lichen sclerosus: An increasingly common problem. J. Am. Acad. Dermatol. 2001, 44, 803–806. [Google Scholar] [CrossRef]
- Neill, S.M.; Tatnall, F.M.; Cox, N.H. British Association of Dermatologists. Guidelines for the management of lichen sclerosus. Br. J. Dermatol. 2002, 147, 640–649. [Google Scholar] [CrossRef]
- Powell, J.J.; Wojnarowska, F. Lichen sclerosus. Lancet 1999, 353, 1777–1783. [Google Scholar] [CrossRef]
- Bleeker, M.C.; Visser, P.J.; Overbeek, L.I.; van Beurden, M.; Berkhof, J. Lichen Sclerosus: Incidence and Risk of Vulvar Squamous Cell Carcinoma. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 1224–1230. [Google Scholar] [CrossRef]
- Micheletti, L.; Preti, M.; Radici, G.; Boveri, S.; Di Pumpo, O.; Privitera, S.S.; Ghiringhello, B.; Benedetto, C. Vulvar Lichen Sclerosus and Neoplastic Transformation: A Retrospective Study of 976 Cases. J. Low. Genit. Tract Dis. 2016, 20, 180–183. [Google Scholar] [CrossRef]
- Lee, A.; Bradford, J.; Fischer, G. Long-term Management of Adult Vulvar Lichen Sclerosus: A Prospective Cohort Study of 507 Women. JAMA Dermatol. 2015, 151, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Friedrich EGJr Kalra, P.S. Serum levels of sex hormones in vulvar lichen sclerosus, and the effect of topical testosterone. N. Engl. J. Med. 1984, 310, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Clifton, M.M.; Garner, I.B.; Kohler, S.; Smoller, B.R. Immunohistochemical evaluation of androgen receptors in genital and extragenital lichen sclerosus: Evidence for loss of androgen receptors in lesional epidermis. J. Am. Acad. Dermatol. 1999, 41, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, M.B.; Spike, R.C.; Mackie, R.M.; MacLean, A.B. An immunohistochemical study of androgen, oestrogen and progesterone receptors in the vulva and vagina. Br. J. Obstet. Gynaecol. 1998, 105, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.A.; Murphy, M. Androgen receptors and lichen sclerosus. J. Am. Acad. Dermatol. 2000, 43, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Günthert, A.R.; Faber, M.; Knappe, G.; Hellriegel, S.; Emons, G. Early onset vulvar Lichen Sclerosus in premenopausal women and oral contraceptives. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 56–60. [Google Scholar] [CrossRef]
- Murphy, R. Lichen sclerosus. Dermatol. Clin. 2010, 28, 707–715. [Google Scholar] [CrossRef]
- Oyama, N.; Hasegawa, M. Lichen Sclerosus: A Current Landscape of Autoimmune and Genetic Interplay. Diagnostics 2022, 12, 3070. [Google Scholar] [CrossRef]
- Lee, A.; Fischer, G. Diagnosis and Treatment of Vulvar Lichen Sclerosus: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 695–706. [Google Scholar] [CrossRef]
- Cooper, S.M.; Ali, I.; Baldo, M.; Wojnarowska, F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: A case-control study. Arch. Dermatol. 2008, 144, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.I.; Dunsmore, I.R. An investigation into the incidence of auto-immune disorders in patients with lichen sclerosus and atrophicus. Br. J. Dermatol. 1981, 104, 563–566. [Google Scholar] [CrossRef]
- Kyriakis, K.P.; Emmanuelides, S.; Terzoudi, S.; Palamaras, I.; Damoulaki, E.; Evangelou, G. Gender and age prevalence distributions of morphea en plaque and anogenital lichen sclerosus. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.; Gilliam, A.; Khavari, N.; Bass, D. Association between lichen sclerosus and celiac disease: A report of three pediatric cases. Pediatr. Dermatol. 2014, 31, e128–e131. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.D.; Meeks, J.J.; Mehdiratta, N.; Granieri, M.A.; Cashy, J.; Gonzalez, C.M. Lichen sclerosus in men is associated with elevated body mass index, diabetes mellitus, coronary artery disease and smoking. World J. Urol. 2014, 32, 105–108. [Google Scholar] [CrossRef]
- Simpkin, S.; Oakley, A. Clinical review of 202 patients with vulval lichen sclerosus: A possible association with psoriasis. Australas. J. Dermatol. 2007, 48, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Chan, I.; Neill, S.M.; Hamada, T.; South, A.P.; Wessagowit, V.; Wojnarowska, F.; D’Cruz, D.; Hughes, G.J.; Black, M.M.; et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet 2003, 362, 118–123. [Google Scholar] [CrossRef]
- Hamada, T.; Wessagowit, V.; South, A.P.; Ashton, G.H.; Chan, I.; Oyama, N.; Siriwattana, A.; Jewhasuchin, P.; Charuwichitratana, S.; Thappa, D.M.; et al. Extracellular matrix protein 1 gene (ECM1) mutations in lipoid proteinosis and genotype-phenotype correlation. J. Investig. Dermatol. 2003, 120, 345–350. [Google Scholar] [CrossRef]
- Regauer, S. Immune dysregulation in lichen sclerosus. Eur. J. Cell Biol. 2005, 84, 273–277. [Google Scholar] [CrossRef]
- Wright, A.J. Lichen sclerosus thyroid disease. J. Reprod. Med. 1998, 43, 240. [Google Scholar]
- Birenbaum, D.L.; Young, R.C. High prevalence of thyroid disease in patients with lichen sclerosus. J. Reprod. Med. 2007, 52, 28–30. [Google Scholar] [PubMed]
- Lynch, P.J. Lichen sclerosus and thyroid disease. J. Reprod. Med. 1999, 44, 315–316. [Google Scholar] [PubMed]
- De Luca, D.A.; Papara, C.; Vorobyev, A.; Staiger, H.; Bieber, K.; Thaçi, D.; Ludwig, R.J. Lichen sclerosus: The 2023 update. Front. Med. 2023, 10, 1106318. [Google Scholar] [CrossRef] [PubMed]
- Marren, P.; Yell, J.; Charnock, F.M.; Bunce, M.; Welsh, K.; Wojnarowska, F. The association between lichen sclerosus and antigens of the HLA system. Br. J. Dermatol. 1995, 132, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, K.; Chang, J. Screening differential circular RNAs expression profiles in Vulvar Lichen Sclerosus. Biomed. Eng. Online 2022, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, F.M.; Marques, M.T.; Matos, H.J.; Pontes, L.F.; Porto, L.C.; Azevedo, L.M.; Filgueira, A.L. HLA markers in familial Lichen sclerosus. J. Dtsch. Dermatol. Ges. 2006, 4, 842–847, (In English, Germany). [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Terras, S.; Kreuter, A.; Skrygan, M. Altered global methylation and hydroxymethylation status in vulvar lichen sclerosus: Further support for epigenetic mechanisms. Br. J. Dermatol. 2014, 170, 687–693. [Google Scholar] [CrossRef]
- Lewis, F.M.; Tatnall, F.M.; Velangi, S.S.; Bunker, C.B.; Kumar, A.; Brackenbury, F.; Mohd Mustapa, M.F.; Exton, L.S. British Association of Dermatologists guidelines for the management of lichen sclerosus, 2018. Br. J. Dermatol. 2018, 178, 839–853. [Google Scholar] [CrossRef]
- van der Meijden, W.I.; Boffa, M.J.; Ter Harmsel, W.A.; Kirtschig, G.; Lewis, F.M.; Moyal-Barracco, M.; Tiplica, G.S.; Sherrard, J. 2016 European guideline for the management of vulval conditions. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 925–941. [Google Scholar] [CrossRef]
- Dalziel, K.L.; Millard, P.R.; Wojnarowska, F. The treatment of vulval lichen sclerosus with a very potent topical steroid (clobetasol propionate 0.05%) cream. Br. J. Dermatol. 1991, 124, 461–464. [Google Scholar] [CrossRef]
- Renaud-Vilmer, C.; Cavelier-Balloy, B.; Porcher, R.; Dubertret, L. Vulvar lichen sclerosus: Effect of long-term topical application of a potent steroid on the course of the disease. Arch. Dermatol. 2004, 140, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Bradford, J.; Fischer, G. Long-term management of vulval lichen sclerosus in adult women. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Corazza, M.; Borghi, A.; Minghetti, S.; Toni, G.; Virgili, A. Clobetasol propionate vs. mometasone furoate in 1-year proactive maintenance therapy of vulvar lichen sclerosus: Results from a comparative trial. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Urman, B.; Yüce, K.; Ayhan, A.; Gököz, A. Topical testosterone for lichen sclerosus. Int. J. Gynaecol. Obstet. 1989, 30, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Yüce, K.; Urman, B.; Ayhan, A.; Gököz, A. Vulvar dystrophies: An evaluation. Aust. N. Z. J. Obstet. Gynaecol. 1989, 29 Pt 1, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Funaro, D. Lichen sclerosus: A review and practical approach. Dermatol. Ther. 2004, 17, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Tasker, G.L.; Wojnarowska, F. Lichen sclerosus. Clin. Exp. Dermatol. 2003, 28, 128–133. [Google Scholar] [CrossRef]
- Val, I.; Almeida, G. An overview of lichen sclerosus. Clin. Obstet. Gynecol. 2005, 48, 808–817. [Google Scholar] [CrossRef]
- Kirtschig, G.; Cooper, S.; Aberer, W.; Günthert, A.; Becker, K.; Jasaitiene, D.; Chi, C.C.; Kreuter, A.; Rall, K.; Riechardt, S.; et al. Evidence-based (S3) Guideline on (anogenital) Lichen sclerosus. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e81–e83. [Google Scholar] [CrossRef]
- Luqman, T.S.; Kalavant, A.; Sunil Kumar, K.S.; Swathy, A.R. Vulvar Lichen Sclerosus Et Atrophicus: Refractory to Standard Therapy. J. Indian Assoc. Pediatr. Surg. 2023, 28, 345–347. [Google Scholar] [CrossRef]
- Funaro, D.; Lovett, A.; Leroux, N.; Powell, J. A double-blind, randomized prospective study evaluating topical clobetasol propionate 0.05% versus topical tacrolimus 0.1% in patients with vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2014, 71, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Krause, W.; Hofmann, H.; Stadler, R.; Gross, G.; Meurer, M.; Brinkmeier, T.; Frosch, P.; Moll, I.; Fritsch, P.; et al. Multicentre, phase II trial on the safety and efficacy of topical tacrolimus ointment for the treatment of lichen sclerosus. Br. J. Dermatol. 2006, 155, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Bradford, J. Topical immunosuppressants, genital lichen sclerosus and the risk of squamous cell carcinoma: A case report. J. Reprod. Med. 2007, 52, 329–331. [Google Scholar] [PubMed]
- Borghi, A.; Corazza, M.; Minghetti, S.; Virgili, A. Topical tretinoin in the treatment of vulvar lichen sclerosus: An advisable option? Eur. J. Dermatol. 2015, 25, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Colmenero, C.; Martínez-Peinado, C.M.; Galán-Gutiérrez, M.; Barranco-Millán, V.; Ruiz-Villaverde, R. Successful response of vulvar lichen sclerosus with NB-UVB. Dermatol. Ther. 2021, 34, e14801. [Google Scholar] [CrossRef]
- Reichrath, J.; Reinhold, U.; Tilgen, W. Treatment of genito-anal lesions in inflammatory skin diseases with PUVA cream photochemotherapy: An open pilot study in 12 patients. Dermatology 2002, 205, 245–248. [Google Scholar] [CrossRef]
- Rombold, S.; Lobisch, K.; Katzer, K.; Grazziotin, T.C.; Ring, J.; Eberlein, B. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol. Photoimmunol. Photomed. 2008, 24, 19–23. [Google Scholar] [CrossRef]
- Terras, S.; Gambichler, T.; Moritz, R.K.; Stücker, M.; Kreuter, A. UV-A1 phototherapy vs clobetasol propionate, 0.05%, in the treatment of vulvar lichen sclerosus: A randomized clinical trial. JAMA Dermatol. 2014, 150, 621–627. [Google Scholar] [CrossRef]
- Kirtschig, G.; Becker, K.; Günthert, A.; Jasaitiene, D.; Cooper, S.; Chi, C.C.; Kreuter, A.; Rall, K.K.; Aberer, W.; Riechardt, S.; et al. Evidence-based (S3) Guideline on (anogenital) Lichen sclerosus. J. Eur. Acad. Dermatol. Venereol. 2015, 29, e1–e43. [Google Scholar] [CrossRef]
- Gerkowicz, A.; Szczepanik-Kułak, P.; Krasowska, D. Photodynamic Therapy in the Treatment of Vulvar Lichen Sclerosus: A Systematic Review of the Literature. J. Clin. Med. 2021, 10, 5491. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, J.; Liu, J. High-frequency ultrasound assessment of vulvar lichen sclerosus treated with photodynamic therapy. Photodiagnosis Photodyn. Ther. 2023, 41, 103277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, D.; Shi, L.; Gu, Y.; Xu, Y. 5-ALA-photodynamic therapy in refractory vulvar lichen sclerosus et atrophicus. Int. J. Clin. Exp. Pathol. 2020, 13, 3100–3110. [Google Scholar] [PubMed]
- Declercq, A.; Güvenç, C.; De Haes, P. Proposition of standardized protocol for photodynamic therapy for vulvar lichen sclerosus. J. Dermatol. Treat. 2022, 33, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Zawislak, A.A.; McCluggage, W.G.; Donnelly, R.F.; Maxwell, P.; Price, J.H.; Dobbs, S.P.; McClelland, H.R.; Woolfson, A.D.; Mccarron, P.A. Response of vulval lichen sclerosus and squamous hyperplasia to photodynamic treatment using sustained topical delivery of aminolevulinic acid from a novel bioadhesive patch system. Photodermatol. Photoimmunol. Photomed. 2009, 25, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Topal, I.O.; Tülay Sayilgan, A.; Kalçin, S. An uncommon cause of vulval pruritus in childhood: Lichen sclerosus. Turk. Arch. Pediatr. 2014, 89, 86–87. [Google Scholar] [CrossRef]
- Kumar, K.S.; Morrel, B.; van Hees, C.L.M.; van der Toorn, F.; van Dorp, W.; Mendels, E.J. Comparison of lichen sclerosus in boys and girls: A systematic literature review of epidemiology, symptoms, genetic background, risk factors, treatment, and prognosis. Pediatr. Dermatol. 2022, 39, 400–408. [Google Scholar] [CrossRef]
- Cooper, S.M.; Gao, X.H.; Powell, J.J.; Wojnarowska, F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch. Dermatol. 2004, 140, 702–706. [Google Scholar] [CrossRef]
- Nerantzoulis, I.; Grigoriadis, T.; Michala, L. Genital lichen sclerosus in childhood and adolescence—A retrospective case series of 15 patients: Early diagnosis is crucial to avoid long-term sequelae. Eur. J. Pediatr. 2017, 176, 1429–1432. [Google Scholar] [CrossRef]
- Morrel, B.; van Eeersel, R.; Burger, C.W.; Bramer, W.M.; Kate-Booij, M.J.T.; van der Avoort, I.A.; Pasmans, S.G. The long-term clinical consequences of juvenile vulvar lichen sclerosus: A systematic review. J. Am. Acad. Dermatol. 2020, 82, 469–477. [Google Scholar] [CrossRef]
- Winfrey, O.K.; Fei, Y.F.; Dendrinos, M.L.; Rosen, M.W.; Smith, Y.R.; Quint, E.H. Lichen Sclerosus throughout Childhood and Adolescence: Not Only a Premenarchal Disease. J. Pediatr. Adolesc. Gynecol. 2022, 35, 624–628. [Google Scholar] [CrossRef]
- Boero, V.; Cavalli, R.; Caia, C.; Berrettini, A.; Cetera, G.E.; Monti, E.; Barbara, G.; Albertini, G.C.; Restelli, E.; Libutti, G.; et al. Pediatric vulvar lichen sclerosus: Does it resolve or does it persist after menarche? Pediatr. Dermatol. 2023, 40, 472–475. [Google Scholar] [CrossRef] [PubMed]
- García-Souto, F.; Lorente-Lavirgen, A.I.; Ildefonso Mendonça, F.M.; García-de-Lomas, M.; Hoffner-Zuchelli, M.V.; Rodriguez-Ojeda, D.; Pozo, E.; Bernabéu-Wittel, J. Vulvar dermatoses: A cross-sectional 5-year study. Experience in a specialized vulvar unit. An. Bras. Dermatol. 2022, 97, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Günthert, A.R.; Limacher, A.; Beltraminelli, H.; Krause, E.; Mueller, M.D.; Trelle, S.; Bobos, P.; Jüni, P. Efficacy of topical progesterone versus topical clobetasol propionate in patients with vulvar Lichen sclerosus—A double-blind randomized phase II pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 272, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, M.; Jagodzinska, A.; Wielgos, M. Evaluation of the effectiveness of treatment of vulvar lichen sclerosus et atrophicus. Analysis of own material and review of the literature. Neuro Endocrinol. Lett. 2018, 39, 417–421. [Google Scholar] [PubMed]
- Maździarz, A.; Osuch, B.; Kowalska, M.; Nalewczyńska, A.; Śpiewankiewicz, B. Photodynamic therapy in the treatment of vulvar lichen sclerosus. Photodiagnosis Photodyn. Ther. 2017, 19, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.A.; Schipani, C.; Cannizzaro, M.V.; Messinese, S.; Chimenti, S.; Piccione, E.; Saraceno, R. New therapeutic approaches in the treatment of anogenital lichen sclerosus: Does photodynamic therapy represent a novel option? G. Ital. Dermatol. Venereol. 2017, 152, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Olejek, A.; Gabriel, I.; Bilska-Janosik, A.; Kozak-Darmas, I.; Kawczyk-Krupka, A. ALA-Photodynamic treatment in Lichen sclerosus-clinical and immunological outcome focusing on the assesment of antinuclear antibodies. Photodiagnosis Photodyn. Ther. 2017, 18, 128–132. [Google Scholar] [CrossRef]
- Osiecka, B.J.; Jurczyszyn, K.; Nockowski, P.; Murawski, M.; Ziółkowski, P. Photodynamic therapy with green light for the treatment of vulvar lichen sclerosus-Preliminary results. Photodiagnosis Photodyn. Ther. 2017, 17, 185–187. [Google Scholar] [CrossRef]
- Mitchell, L.; Goldstein, A.T.; Heller, D.; Mautz, T.; Thorne, C.; Joyce Kong, S.Y.; Sophocles, M.E.; Tolson, H.; Krapf, J.M. Fractionated Carbon Dioxide Laser for the Treatment of Vulvar Lichen Sclerosus: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 979–987. [Google Scholar] [CrossRef]
- Salgado, H.C.; Drumond, D.G.; Pannain, G.D.; de Melo ECosta, L.G.; Sampaio, F.S.; Leite, I.C.G. Randomized clinical trial with fractional CO2 laser and Clobetasol in the treatment of Vulvar Lichen Sclerosus: A clinic study of feasibility. BMC Res. Notes 2023, 16, 33. [Google Scholar] [CrossRef]
- Burkett, L.S.; Siddique, M.; Zeymo, A.; Brunn, E.A.; Gutman, R.E.; Park, A.J.; Iglesia, C.B. Clobetasol Compared With Fractionated Carbon Dioxide Laser for Lichen Sclerosus: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.; Neumann, S.; Maier, M.; Imboden, S.; Knabben, L.; Mueller, M.D.; Kuhn, A. LASER treatment in gynaecology—A randomized controlled trial in women with symptomatic lichen sclerosus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 287, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Pagano, T.; Conforti, A.; Buonfantino, C.; Schettini, F.; Vallone, R.; Gallo, A.; Avino, L.; Alviggi, C.; De Placido, G.; Sopracordevole, F. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: A prospective longitudinal study. Menopause 2020, 27, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Bizjak Ogrinc, U.; Senčar, S.; Luzar, B.; Lukanović, A. Efficacy of Non-ablative Laser Therapy for Lichen Sclerosus: A Randomized Controlled Trial. J. Obstet. Gynaecol. Can. 2019, 41, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zou, M.; Xiong, Y.; Wang, L.; Chen, H.; Fan, Y.; Li, C. Short- and long-term efficacy of focused ultrasound therapy for non-neoplastic epithelial disorders of the vulva. BJOG 2017, 124 (Suppl. S3), 87–92. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.; Flacco, M.E.; Zedde, P.; Toni, G.; Schettini, N.; Corazza, M. Does Clearance of Vulvar Lichen Sclerosus after a Corticosteroid Treatment Correspond to a Decrease in Disease-Related Burden? Results from a Cohort Study Using Pictorial Representation of Illness and Self-Measure and the Dermatology Life Quality Index. Dermatology 2023, 239, 81–90. [Google Scholar] [CrossRef]
- Corazza, M.; Toni, G.; Valpiani, G.; Morotti, C.; Borghi, A. Does longer duration of corticosteroid treatment improve clearance in vulvar lichen sclerosus? Results from a single centre, comparative, open label study. Dermatol. Ther. 2021, 34, e14955. [Google Scholar] [CrossRef]
- Corazza, M.; Virgili, A.; Toni, G.; Valpiani, G.; Morotti, C.; Borghi, A. Pictorial Representation of Illness and Self-Measure to assess the perceived burden in patients with chronic inflammatory vulvar diseases: An observational study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2645–2651. [Google Scholar] [CrossRef]
- Borghi, A.; Odorici, G.; Scuderi, V.; Valpiani, G.; Morotti, C.; Corazza, M. Measuring perceived benefit and disease-related burden in patients affected with vulvar lichen sclerosus after a standard topical corticosteroid treatment. Results from a cohort study using Pictorial Representation of Illness and Self-measure and Dermatology Life Quality Index. Dermatol. Ther. 2020, 33, e14334. [Google Scholar] [CrossRef]
- Saunderson, R.B.; Harris, V.; Yeh, R.; Mallitt, K.A.; Fischer, G. Vulvar quality of life index (VQLI)—A simple tool to measure quality of life in patients with vulvar disease. Australas. J. Dermatol. 2020, 61, 152–157. [Google Scholar] [CrossRef]
- Wijaya, M.; Lee, G.; Fischer, G.; Lee, A. Quality of Life in Vulvar Lichen Sclerosus Patients Treated with Long-Term Topical Corticosteroids. J. Low. Genit. Tract. Dis. 2021, 25, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.R.; Connors, T.M.; Chan, W.; Liang, C.S.; Dao, H., Jr.; Vyas, A. Clinical outcomes and adherence to topical corticosteroid therapy in women with vulvar lichen sclerosus: A retrospective cohort study. J. Am. Acad. Dermatol. 2020, 83, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Corazza, M.; Maietti, E.; Toni, G.; Virgili, A.; Borghi, A. Combining topical tretinoin with mometasone furoate in the treatment of vulvar lichen sclerosus: Results of dermoscopic assessment. Dermatol. Ther. 2018, 31, e12735. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, J.; Tan, W.; Ma, Q.; Wu, X.; Gao, H. Prospective observational study of the efficacy of mixed methylene blue compound injection for treatment of vulvar non-neoplastic epithelial disorders. Int. J. Gynaecol. Obstet. 2020, 148, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Boero, V.; Brambilla, M.; Sipio, E.; Liverani, C.A.; Di Martino, M.; Agnoli, B.; Libutti, G.; Cribiù, F.; Del Gobbo, A.; Ragni, E.; et al. Vulvar lichen sclerosus: A new regenerative approach through fat grafting. Gynecol. Oncol. 2015, 139, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Casabona, F.; Priano, V.; Vallerino, V.; Cogliandro, A.; Lavagnino, G. New surgical approach to lichen sclerosus of the vulva: The role of adipose-derived mesenchymal cells and platelet-rich plasma in tissue regeneration. Plast. Reconstr. Surg. 2010, 126, 210e–211e. [Google Scholar] [CrossRef]
- Goldstein, A.T.; Mitchell, L.; Govind, V.; Heller, D. A randomized double-blind placebo-controlled trial of autologous platelet-rich plasma intradermal injections for the treatment of vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2019, 80, 1788–1789. [Google Scholar] [CrossRef]
- Gutierrez-Ontalvilla, P.; Giner, F.; Vidal, L.; Iborra, M. The Effect of Lipofilling and Platelet-Rich Plasma on Patients with Moderate-Severe Vulvar Lichen Sclerosus who were Non-Responders to Topical Clobetasol Propionate: A Randomized Pilot Study. Aesthetic Plast. Surg. 2022, 46, 2469–2479. [Google Scholar] [CrossRef]
- Tedesco, M.; Bellei, B.; Garelli, V.; Caputo, S.; Latini, A.; Giuliani, M.; Cota, C.; Chichierchia, G.; Romani, C.; Foddai, M.L.; et al. Adipose tissue stromal vascular fraction and adipose tissue stromal vascular fraction plus platelet-rich plasma grafting: New regenerative perspectives in genital lichen sclerosus. Dermatol. Ther. 2020, 33, e14277. [Google Scholar] [CrossRef]
- Corazza, M.; Schettini, N.; Zedde, P.; Borghi, A. Vulvar Lichen Sclerosus from Pathophysiology to Therapeutic Approaches: Evidence and Prospects. Biomedicines 2021, 9, 950. [Google Scholar] [CrossRef]
- Taylor, A.H.; Guzail, M.; Al-Azzawi, F. Differential expression of oestrogen receptor isoforms and androgen receptor in the normal vulva and vagina compared with vulval lichen sclerosus and chronic vaginitis. Br. J. Dermatol. 2008, 158, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Bracco, G.L.; Carli, P.; Sonni, L.; Maestrini, G.; De Marco, A.; Taddei, G.L.; Cattaneo, A. Clinical and histologic effects of topical treatments of vulval lichen sclerosus. A critical evaluation. J. Reprod. Med. 1993, 38, 37–40. [Google Scholar] [PubMed]
- Elchalal, U.; Gilead, L.; Vardy, D.A.; Ben-shachar, I.; Anteby, S.O.; Schenker, J.G. Treatment of vulvar lichen sclerosus in the elderly: An update. Obstet. Gynecol. Surv. 1995, 50, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Heymann, W.R. Juvenile lichen sclerosus: A loss of innocence. J. Am. Acad. Dermatol. 2020, 82, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Wines, N.; Willsteed, E. Menopause and the skin. Australas. J. Dermatol. 2001, 42, 149–158; quiz 159. [Google Scholar] [CrossRef] [PubMed]
- Onnis, A.; Nardelli, G.B.; Lamaina, V.; Mozzanega, B.; Becagli, L.; Fais, G.F. Hormonal receptors in vulvar tissues. Eur. J. Gynaecol. Oncol. 1985, 6, 125–128. [Google Scholar] [PubMed]
- Nardelli, G.B. Vulvar hormonal receptor modifications during topical steroid treatment. Clin. Exp. Obstet. Gynecol. 1988, 15, 170–173. [Google Scholar]
- Dendrinos, M.L.; Quint, E.H. Lichen sclerosus in children and adolescents. Curr. Opin. Obstet. Gynecol. 2013, 25, 370–374. [Google Scholar] [CrossRef]
- Halonen, P.; Jakobsson, M.; Heikinheimo, O.; Riska, A.; Gissler, M.; Pukkala, E. Lichen sclerosus and risk of cancer. Int. J. Cancer 2017, 140, 1998–2002. [Google Scholar] [CrossRef]
- Spekreijse, J.J.; Streng, B.M.M.; Vermeulen, R.F.M.; Voss, F.O.; Vermaat, H.; van Beurden, M. The risk of developing squamous cell carcinoma in patients with anogenital lichen sclerosis: A systematic review. Gynecol. Oncol. 2020, 157, 671–677. [Google Scholar] [CrossRef]
- Vieira-Baptista, P.; Pérez-López, F.R.; López-Baena, M.T.; Stockdale, C.K.; Preti, M.; Bornstein, J. Risk of Development of Vulvar Cancer in Women with Lichen Sclerosus or Lichen Planus: A Systematic Review. J. Low. Genit. Tract. Dis. 2022, 26, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.; Scurry, J.; Bradford, J.; Lee, G.; Fischer, G. Association of Topical Corticosteroids with Reduced Vulvar Squamous Cell Carcinoma Recurrence in Patients with Vulvar Lichen Sclerosus. JAMA Dermatol. 2020, 156, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Meyrick Thomas, R.H.; Ridley, C.M.; McGibbon, D.H.; Black, M.M. Lichen sclerosus et atrophicus and autoimmunity: A study of 350 women. Br. J. Dermatol. 1988, 118, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Kryvosheyeva, Y.; Terras, S.; Moritz, R.; Möllenhoff, K.; Altmeyer, P.; Scola, N.; Gambichler, T. Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta. Derm. Venereol. 2013, 93, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Carli, P.; Cattaneo, A.; Giannotti, B. Clobetasol propionate 0.05% cream in the treatment of vulvar lichen sclerosus: Effect on the immunohistological profile. Br. J. Dermatol. 1992, 127, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Marfatia, Y.; Surani, A.; Baxi, R. Genital lichen sclerosus et atrophicus in females: An update. Indian J. Sex. Transm. Dis. 2019, 40, 6–12. [Google Scholar] [CrossRef]
- Virgili, A.; Borghi, A.; Toni, G.; Minghetti, S.; Corazza, M. First randomized trial on clobetasol propionate and mometasone furoate in the treatment of vulvar lichen sclerosus: Results of efficacy and tolerability. Br. J. Dermatol. 2014, 171, 388–396. [Google Scholar] [CrossRef]
- Cattaneo, A.; De Magnis, A.; Botti, E.; Sonni, L.; Carli, P.; Taddei, G.L. Topical mometasone furoate for vulvar lichen sclerosus. J. Reprod. Med. 2003, 48, 444–448. [Google Scholar]
- Murina, F.; Rehman, S.; Di Francesco, S.; Mantegazza, V.; Felice, R.; Bianco, V. Vulvar Lichen Sclerosus. J. Low. Genit. Tract. Dis. 2015, 19, 149–151. [Google Scholar] [CrossRef]
- Mautz, T.T.; Krapf, J.M.; Goldstein, A.T. Topical Corticosteroids in the Treatment of Vulvar Lichen Sclerosus: A Review of Pharmacokinetics and Recommended Dosing Frequencies. Sex. Med. Rev. 2022, 10, 42–52. [Google Scholar] [CrossRef]
- Notay, M.; Fazel, N.; Awasthi, S. Cushing Syndrome Induced by Topical Corticosteroids for the Treatment of Lichen Sclerosus. J. Pediatr. Adolesc. Gynecol. 2019, 32, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Carli, P.; De Marco, A.; Sonni, L.; Bracco, G.; De Magnis, A.; Taddei, G.L. Testosterone maintenance therapy: Effects on vulvar lichen sclerosus treated with clobetasol propionate. J. Reprod. Med. 1996, 41, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Sideri, M.; Origoni, M.; Spinaci, L.; Ferrari, A. Topical testosterone in the treatment of vulvar lichen sclerosus. Int. J. Gynaecol. Obstet. 1994, 46, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, Y.; Eijkemans, M.J.; Coelingh Bennink, H.J.; Blankenstein, M.A.; Fauser, B.C. The effect of combined oral contraception on testosterone levels in healthy women: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 76–105. [Google Scholar] [CrossRef] [PubMed]
- Kohlberger, P.D.; Joura, E.A.; Bancher, D.; Gitsch, G.; Breitenecker, G.; Kieback, D.G. Evidence of androgen receptor expression in lichen sclerosus: An immunohistochemical study. J. Soc. Gynecol. Investig. 1998, 5, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Zarcone, R.; Vicinanza, G.; Bellini, P.; Cardone, A. Il trattamento medico nel lichen scleroso vulvare [Drug treatment in vulvar lichen sclerosus]. Minerva Ginecol. 1996, 48, 441–444. [Google Scholar] [PubMed]
- Prodromidou, A.; Chatziioannou, E.; Daskalakis, G.; Stergios, K.; Pergialiotis, V. Photodynamic Therapy for Vulvar Lichen Sclerosus-A Systematic Review. J. Low. Genit. Tract. Dis. 2018, 22, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mauskar, M.M. Fractionated Carbon Dioxide Laser for the Treatment of Vulvar Lichen Sclerosus. Obstet. Gynecol. 2021, 137, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Marnach, M.L.; Torgerson, R.R. Therapeutic Interventions for Challenging Cases of Vulvar Lichen Sclerosus and Lichen Planus. Obstet. Gynecol. 2021, 138, 374–378. [Google Scholar] [CrossRef]
- Farrell, A.M.; Dean, D.; Millard, P.R.; Charnock, F.M.; Wojnarowska, F. Cytokine alterations in lichen sclerosus: An immunohistochemical study. Br. J. Dermatol. 2006, 155, 931–940. [Google Scholar] [CrossRef]
- Farrell, A.M.; Dean, D.; Millard, P.R.; Charnock, F.M.; Wojnarowska, F. Alterations in fibrillin as well as collagens I and III and elastin occur in vulval lichen sclerosus. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, S.; Lombardo, G.A.; Tarico, M.S.; Perrotta, R.E. The Role of Nanofat Grafting in Vulvar Lichen Sclerosus: A Preliminary Report. Arch. Plast. Surg. 2016, 43, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Eshtiaghi, P.; Sadownik, L.A. Fact or Fiction? Adipose-Derived Stem Cells and Platelet-Rich Plasma for the Treatment of Vulvar Lichen Sclerosus. J. Low. Genit. Tract. Dis. 2019, 23, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Monreal, J. Safety and Efficacy of Stromal Vascular Fraction Enriched Fat Grafting Therapy for Vulvar Lichen Sclerosus. Cureus 2020, 12, e7096. [Google Scholar] [CrossRef] [PubMed]
- Boero, V.; Brambilla, M.; Di Loreto, E.; Cetera, G.E.; Cipriani, S.; Boggio, F.; Monti, E.; Libutti, G.; Caia, C.; Parazzini, F. Fat Grafting in Vulvar Lichen Sclerosus: Long Term Follow-Up. J. Low. Genit. Tract. Dis. 2023, 27, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Casabona, F.; Gasparini, G.; Cozzani, E.; Barbazza, A.; Casabona, F.; Carmisciano, L.; Parodi, A. Improvement in quality of life and sexual function in patients affected by vulvar lichen sclerosus treated with combined autologous platelet-rich plasma and fat grafting. Eur. J. Dermatol. 2023, 33, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- van der Sluis, N.; Scheers, E.C.A.H.; Krenning, G.; van der Lei, B.; Oonk, M.H.M.; van Dongen, J.A. Autologous lipoaspirate as a new treatment of vulvar lichen sclerosus: A review on literature. Exp. Dermatol. 2022, 31, 689–699. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Wang, M.; Tian, W.; Wang, H. Concentrated Growth Factor Enhanced Fat Graft Survival: A Comparative Study. Dermatol. Surg. 2018, 44, 976–984. [Google Scholar] [CrossRef]
- Selk, A.; Elangainesan, P.; Tannenbaum, E.; Wong, K. “Check Your Vulva”—A Patient Education and Virtual Vulva Care Pilot Project. J. Low. Genit. Tract. Dis. 2023, 27, 390–394. [Google Scholar] [CrossRef]
- Preti, M.; Selk, A.; Stockdale, C.; Bevilacqua, F.; Vieira-Baptista, P.; Borella, F.; Gallio, N.; Cosma, S.; Melo, C.; Micheletti, L.; et al. Knowledge of Vulvar Anatomy and Self-examination in a Sample of Italian Women. J. Low. Genit. Tract. Dis. 2021, 25, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, F.; Selk, A.; Stockdale, C.; Vieira-Baptista, P.; Adedipe, T.; Bohl, T.; Marozio, L.; Borella, F.; Gallio, N.; Pollano, B.; et al. The International Society for the Study of Vulvovaginal Disease (ISSVD) Vulvar Awareness Day Campaign: Knowledge of Vulvovaginal Diseases Among Italian Obstetrics and Gynecology Residents. J. Low. Genit. Tract. Dis. 2024, 28, 91–94. [Google Scholar] [CrossRef] [PubMed]
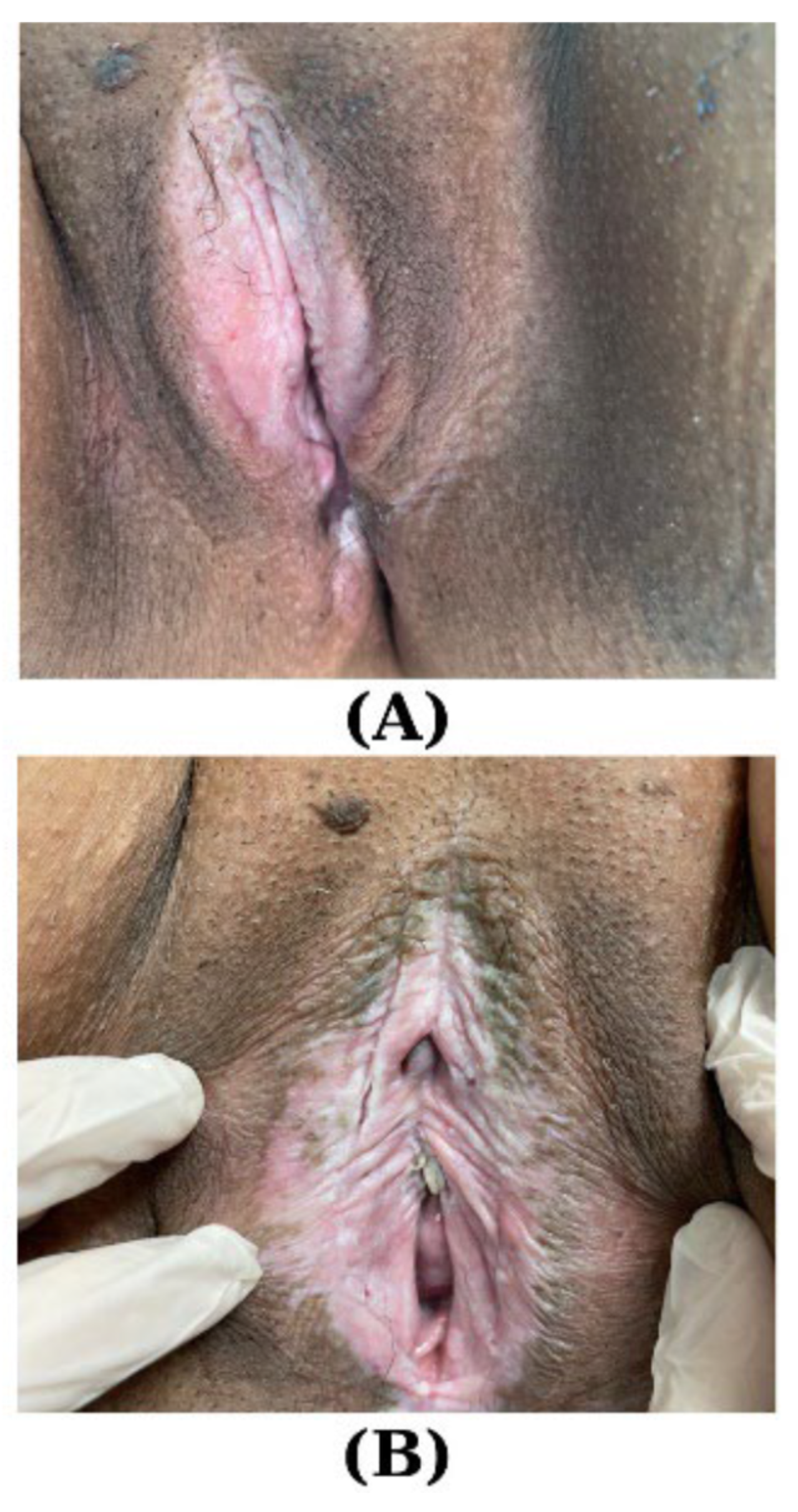

| First Author Year Type of Study [Reference] | Studied Population | Results | Conclusions |
|---|---|---|---|
| Boero V 2023 Observational retrospective study [81] | N = 31 pVLP (mean age: 6.3 years; SD ± 2.58) | N at re-examination:
| At re-examination: 58.1% were considered still affected by VLS, 16.1% achieved complete remission, and 25.8% were asymptomatic despite clinical signs. |
| Winfrey OK 2022 Retrospective [80] | N1 = 141 premenarchal women with VLS N2 = 36 postmenarchal women with VLS N3 = 26 premenarchal women with VLS followed through the pubertal transition | 38.5% in the N3 continued to experience VLS symptoms
| VLS can persist after menarche in approximately 40% of adolescents and may also initially develop in postmenarchal adolescents, with differences in initial symptoms and examination findings based on menarchal status. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.; Dumitrascu, M.C.; Petca, A.; Petca, R.-C.; Sandru, F. Vulvar Lichen Sclerosus: Navigating Sex Hormone Dynamics and Pioneering Personalized Treatment Paradigm. J. Pers. Med. 2024, 14, 76. https://doi.org/10.3390/jpm14010076
Popa A, Dumitrascu MC, Petca A, Petca R-C, Sandru F. Vulvar Lichen Sclerosus: Navigating Sex Hormone Dynamics and Pioneering Personalized Treatment Paradigm. Journal of Personalized Medicine. 2024; 14(1):76. https://doi.org/10.3390/jpm14010076
Chicago/Turabian StylePopa, Adelina, Mihai Cristian Dumitrascu, Aida Petca, Razvan-Cosmin Petca, and Florica Sandru. 2024. "Vulvar Lichen Sclerosus: Navigating Sex Hormone Dynamics and Pioneering Personalized Treatment Paradigm" Journal of Personalized Medicine 14, no. 1: 76. https://doi.org/10.3390/jpm14010076
APA StylePopa, A., Dumitrascu, M. C., Petca, A., Petca, R.-C., & Sandru, F. (2024). Vulvar Lichen Sclerosus: Navigating Sex Hormone Dynamics and Pioneering Personalized Treatment Paradigm. Journal of Personalized Medicine, 14(1), 76. https://doi.org/10.3390/jpm14010076