Assessment of the Impact of Bone Quality and Abutment Configuration on the Fatigue Performance of Dental Implant Systems Using Finite Element Analysis (FEA)
Abstract
:1. Introduction
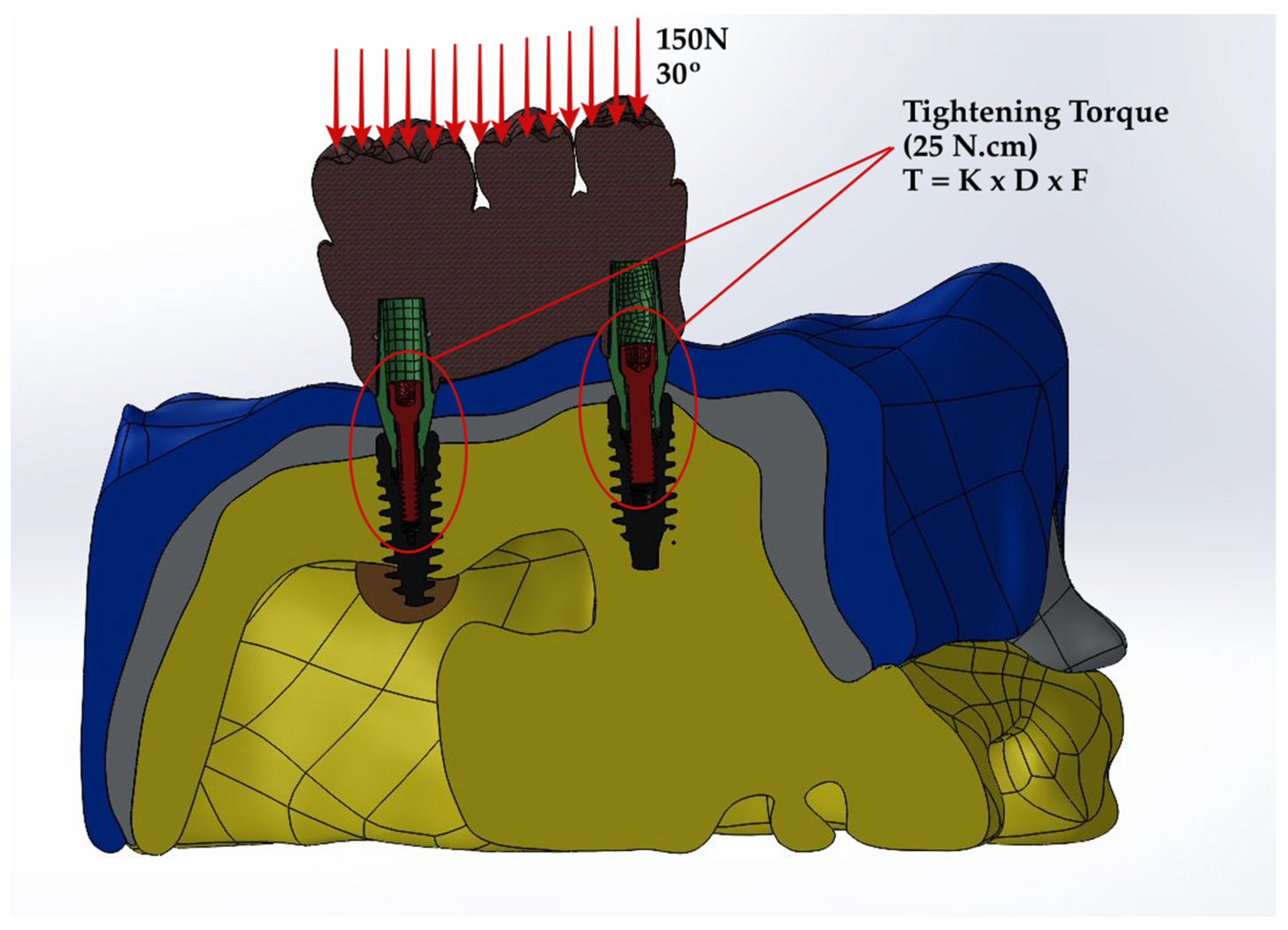
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Prediction of Stress Distribution
2.3. Prediction of Fatigue Performance
3. Results
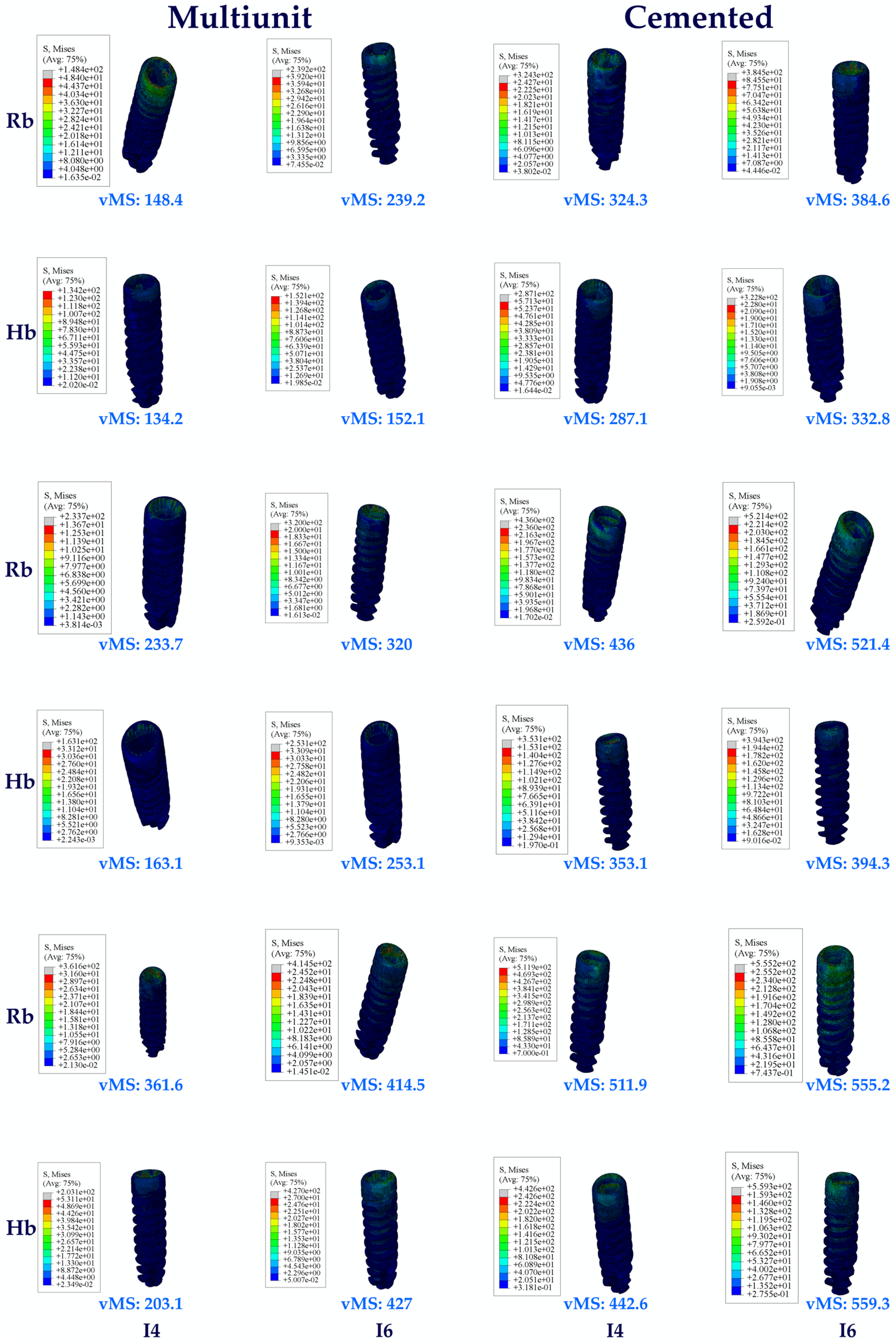
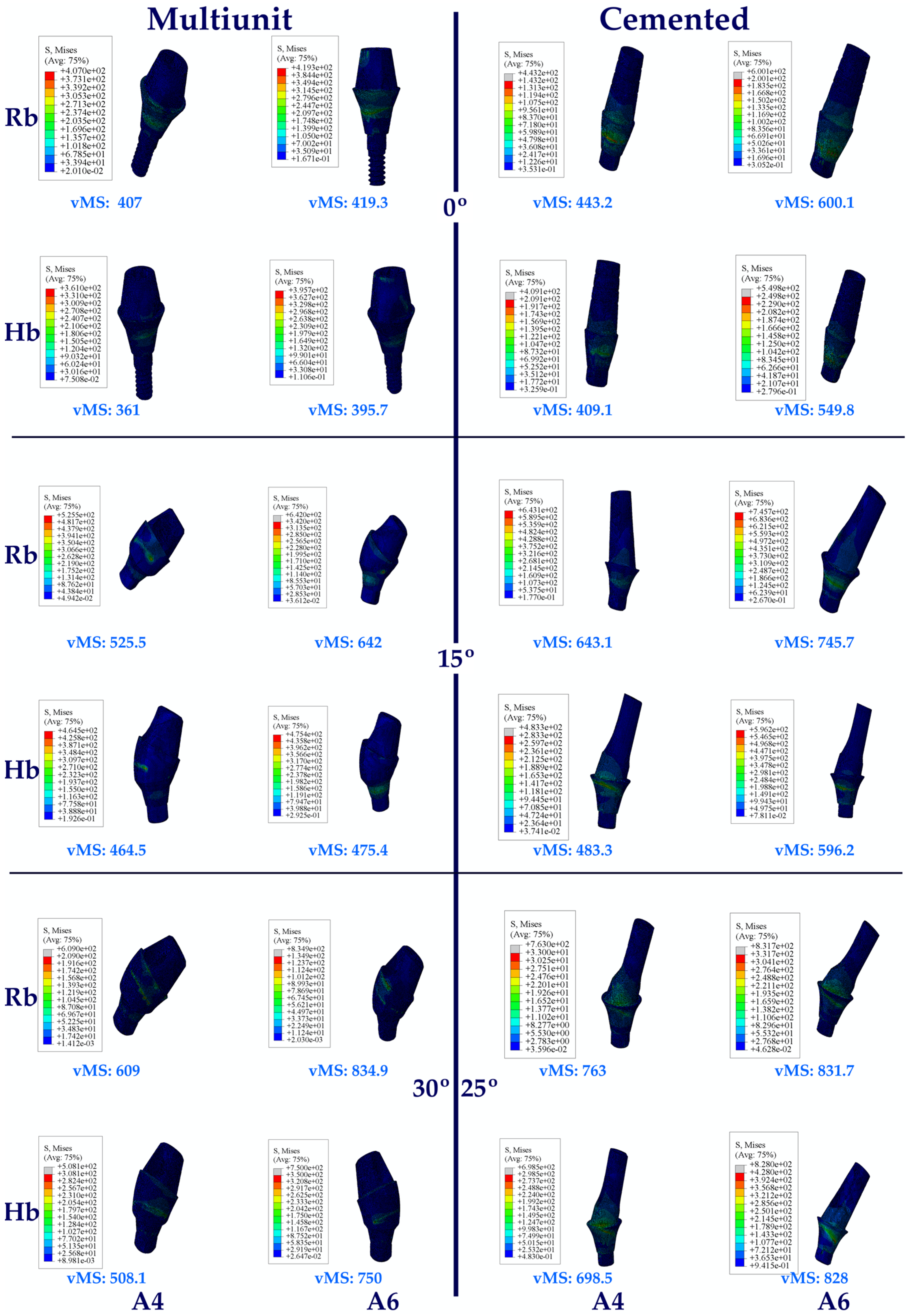
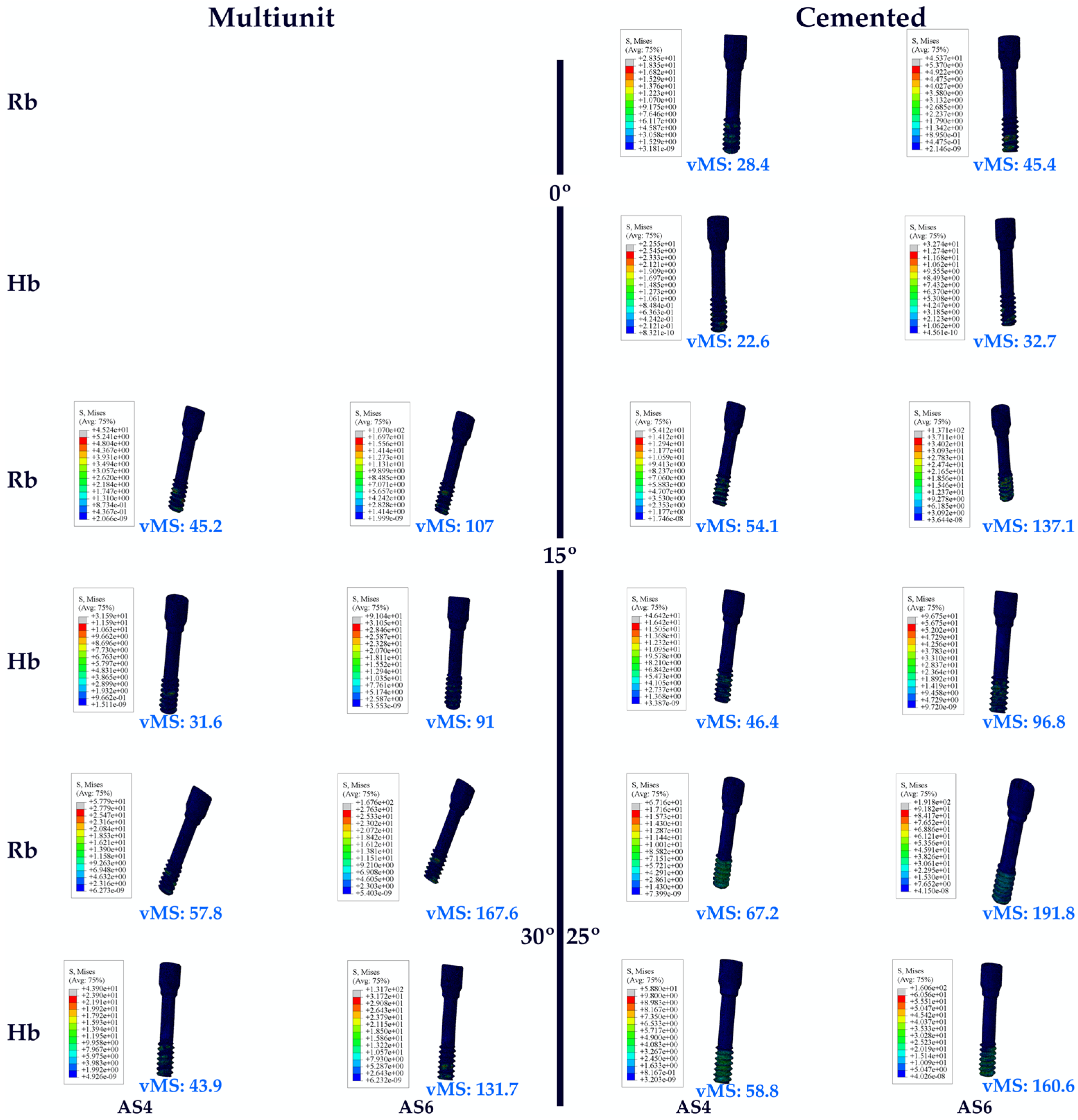
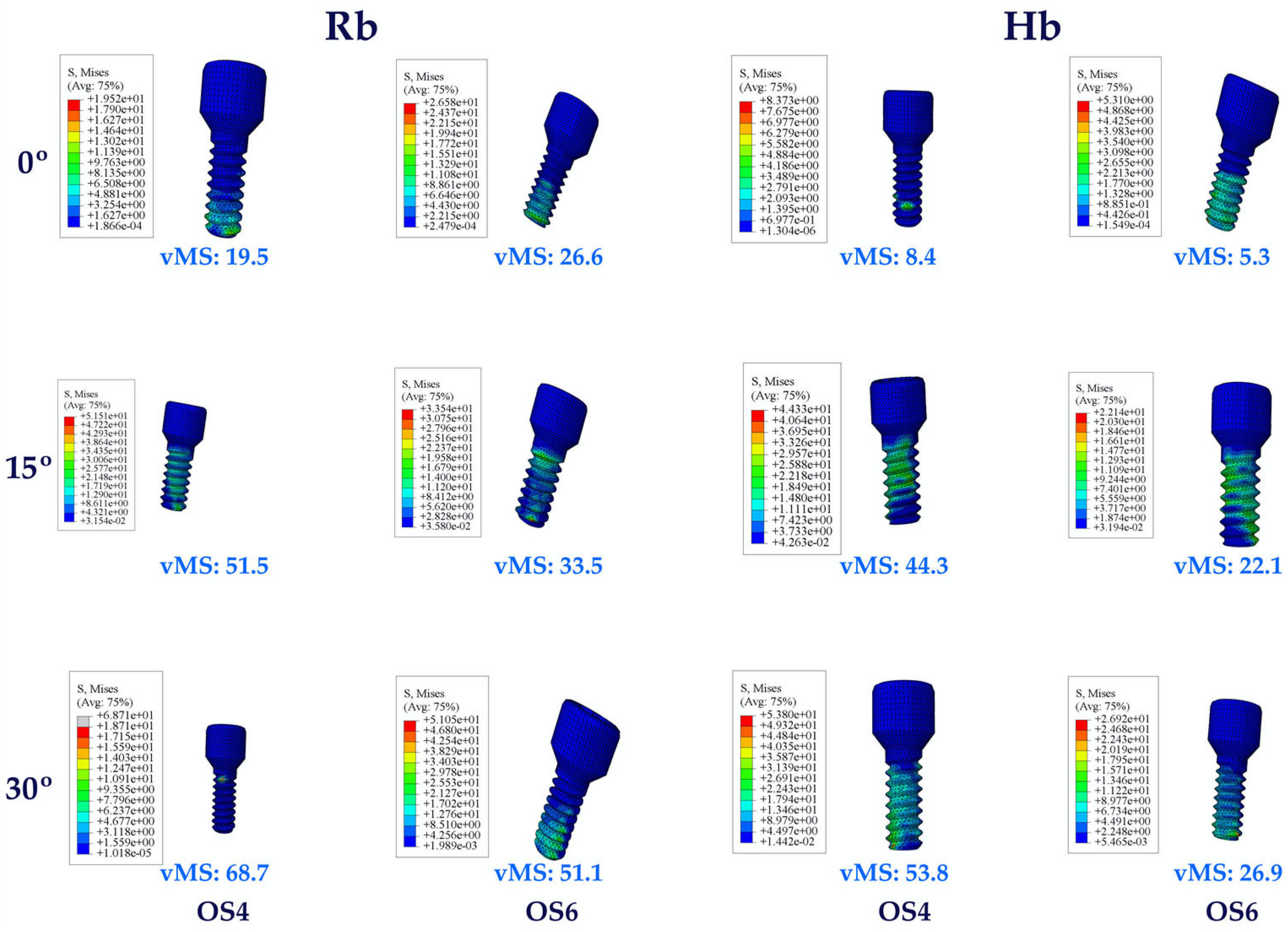
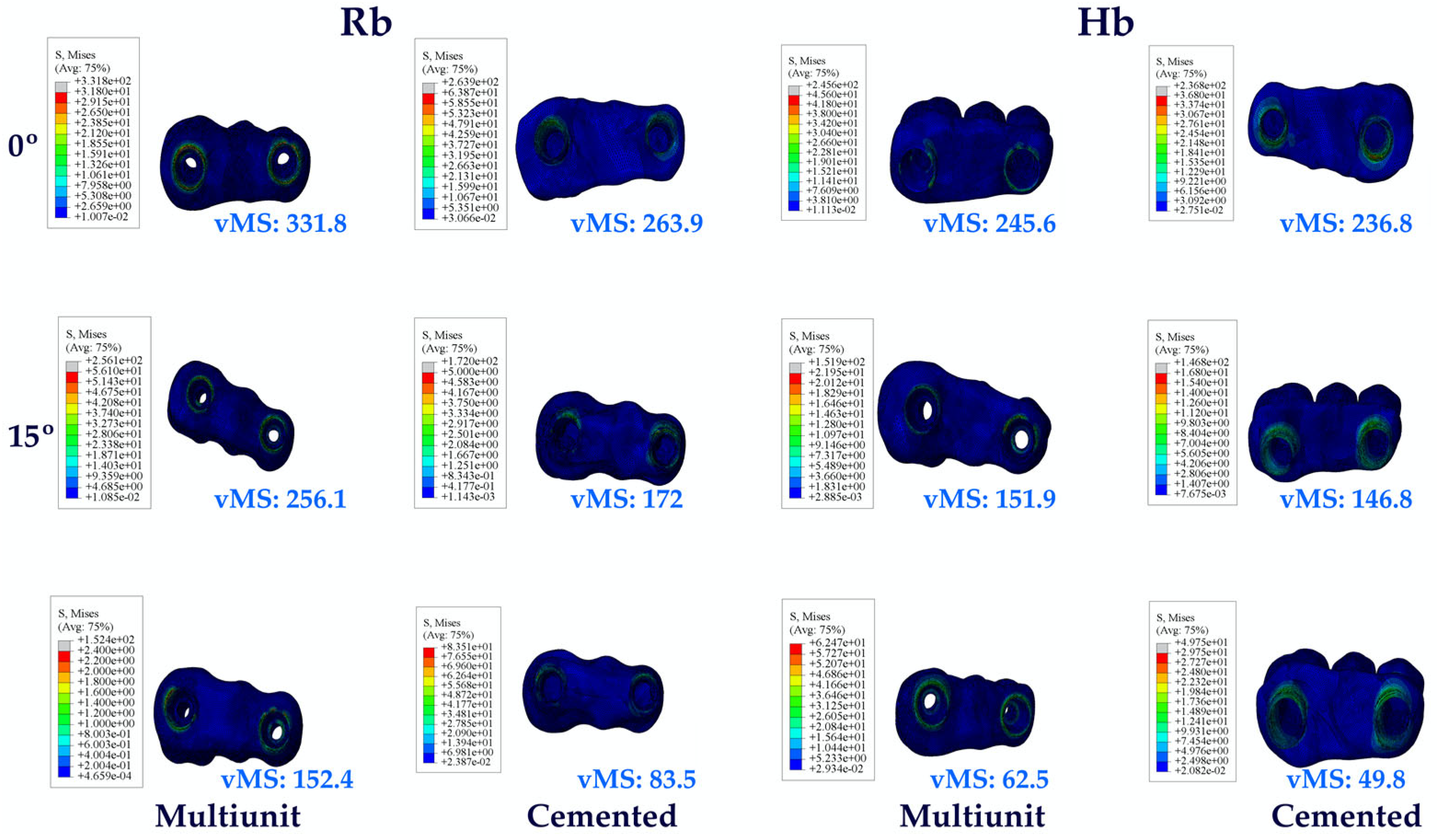
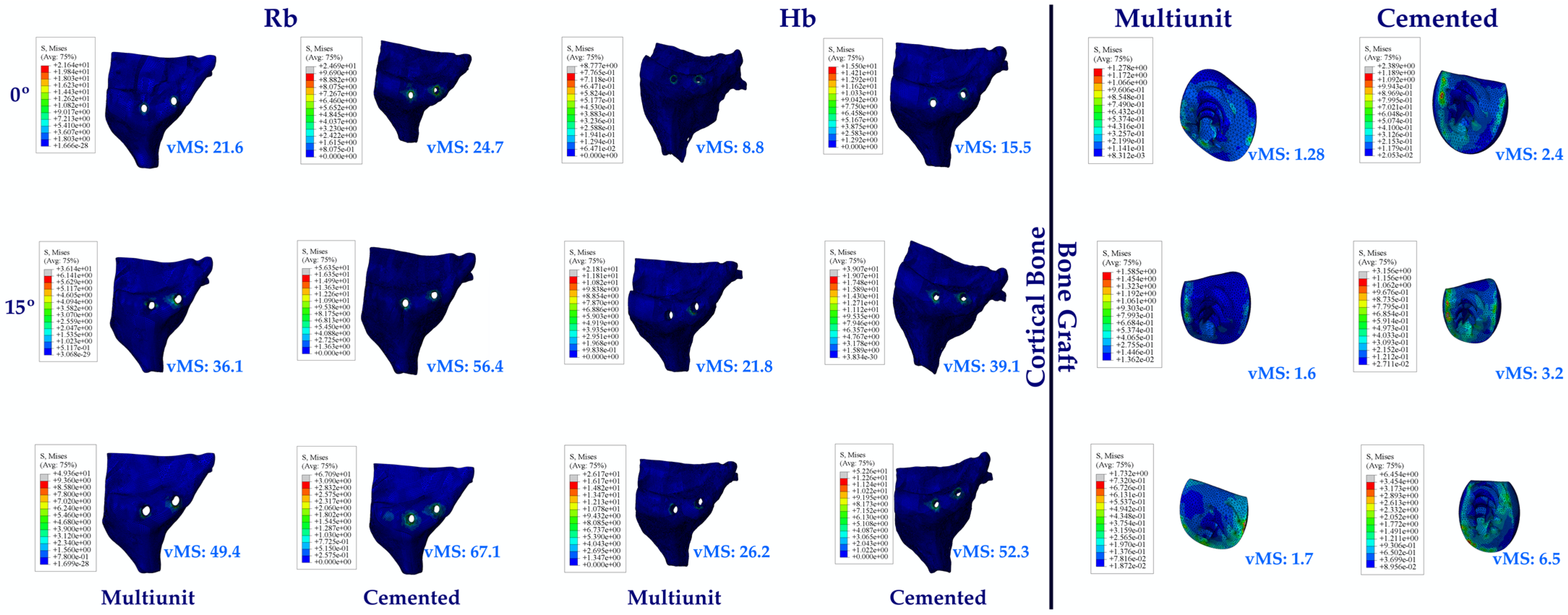
3.1. Stress Distribution
3.1.1. Stress Values on the Implants
3.1.2. Stress Values on the Abutments
3.1.3. Stress Values on the Abutment Screw
3.1.4. Stress Values on the Occlusal Screw
3.1.5. Stress Values on the RESIN Cement
3.1.6. Stress Values on the Prosthetic Restoration
3.1.7. Stress Values on the Cortical Bone and Bone Graft
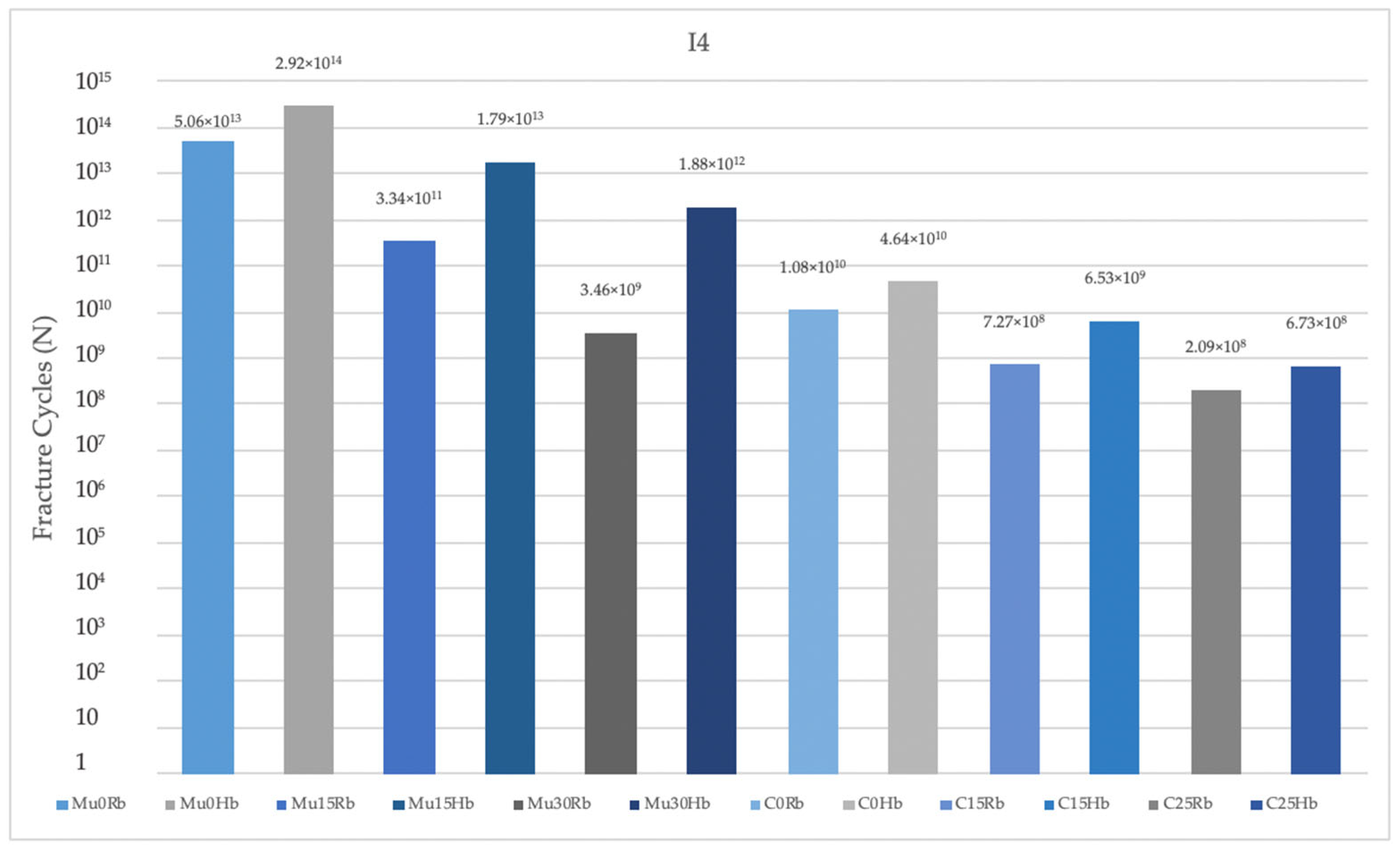
3.2. Fatigue Performances
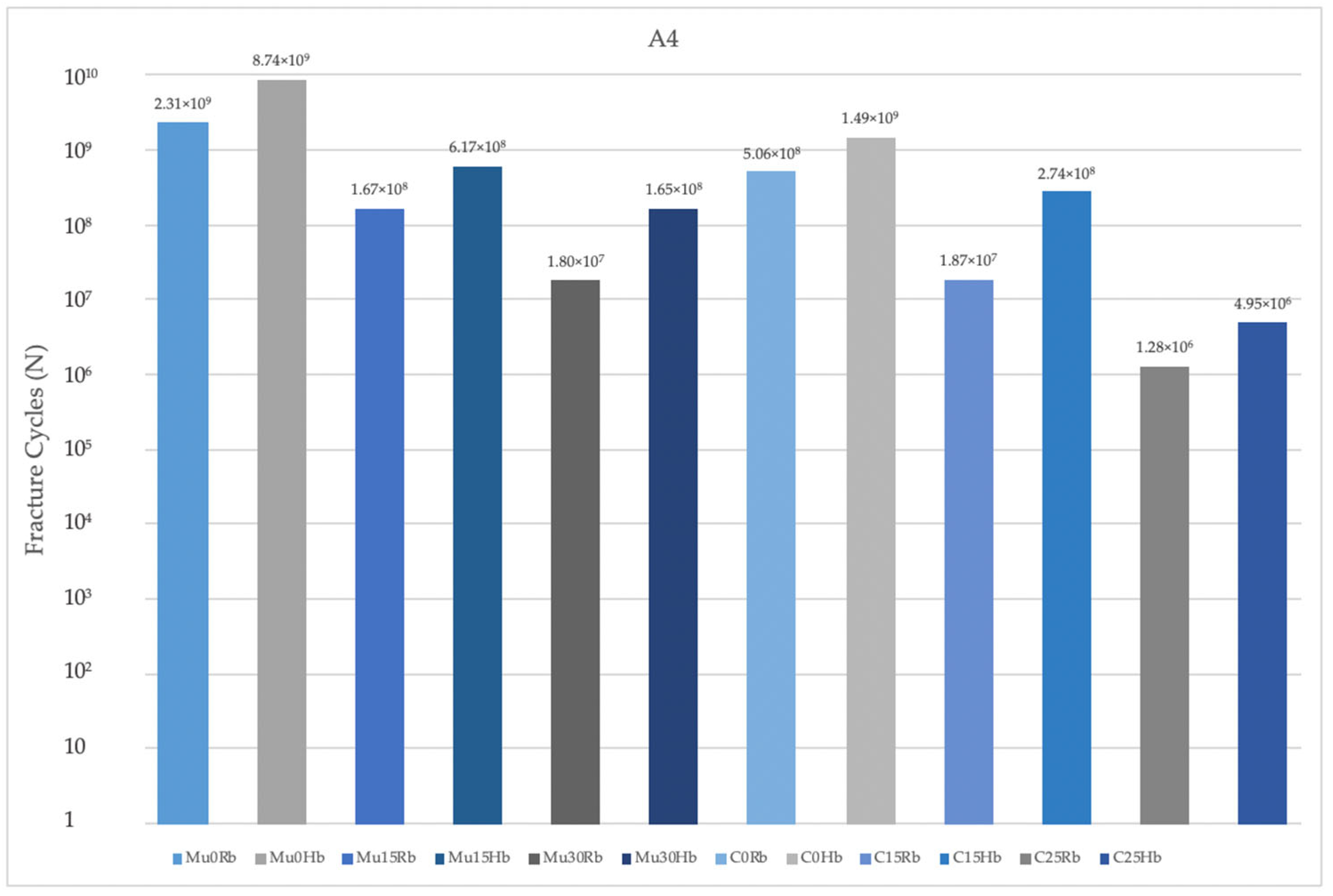
3.2.1. Fatigue Performance of Implant
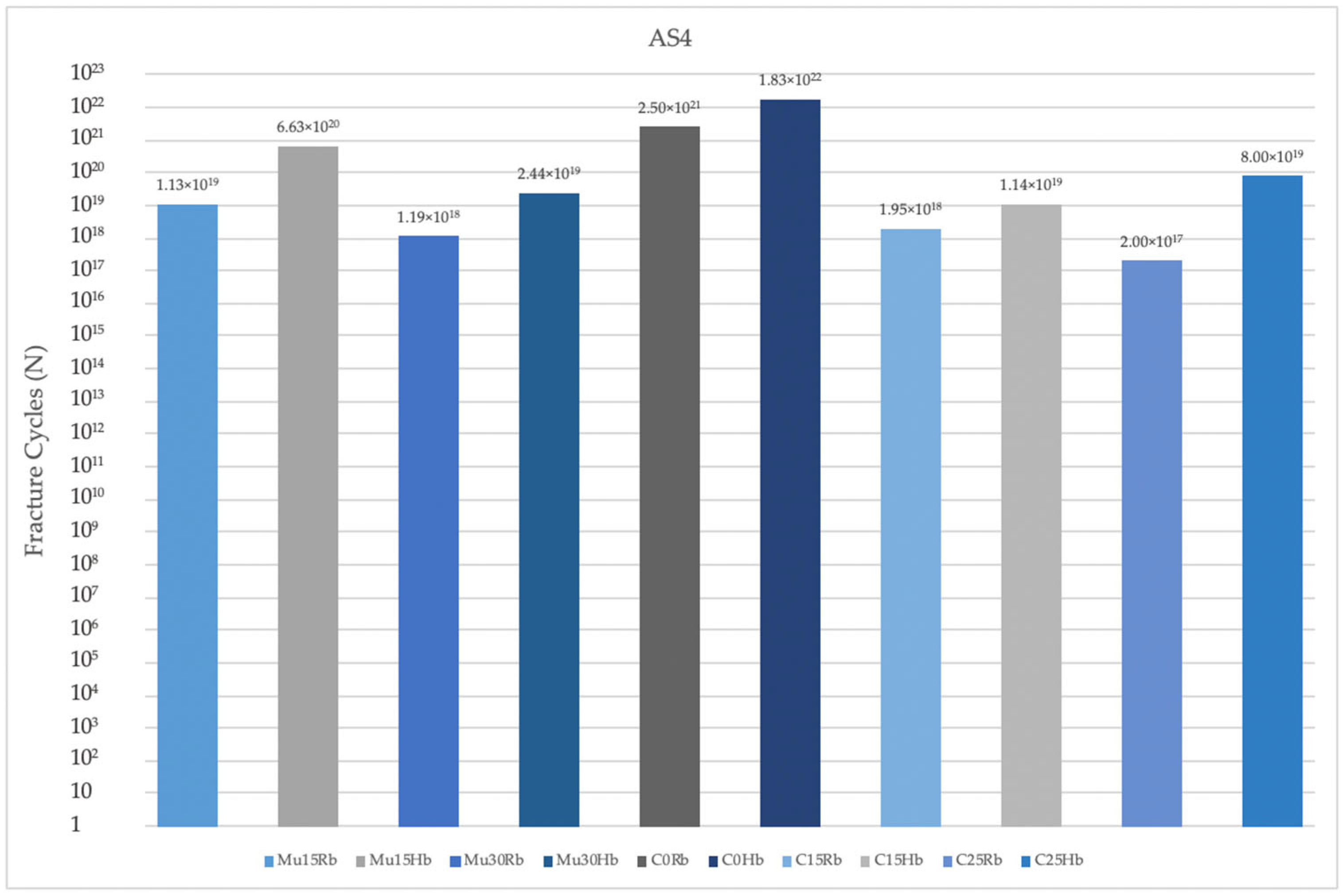
3.2.2. Fatigue Performance of Abutment
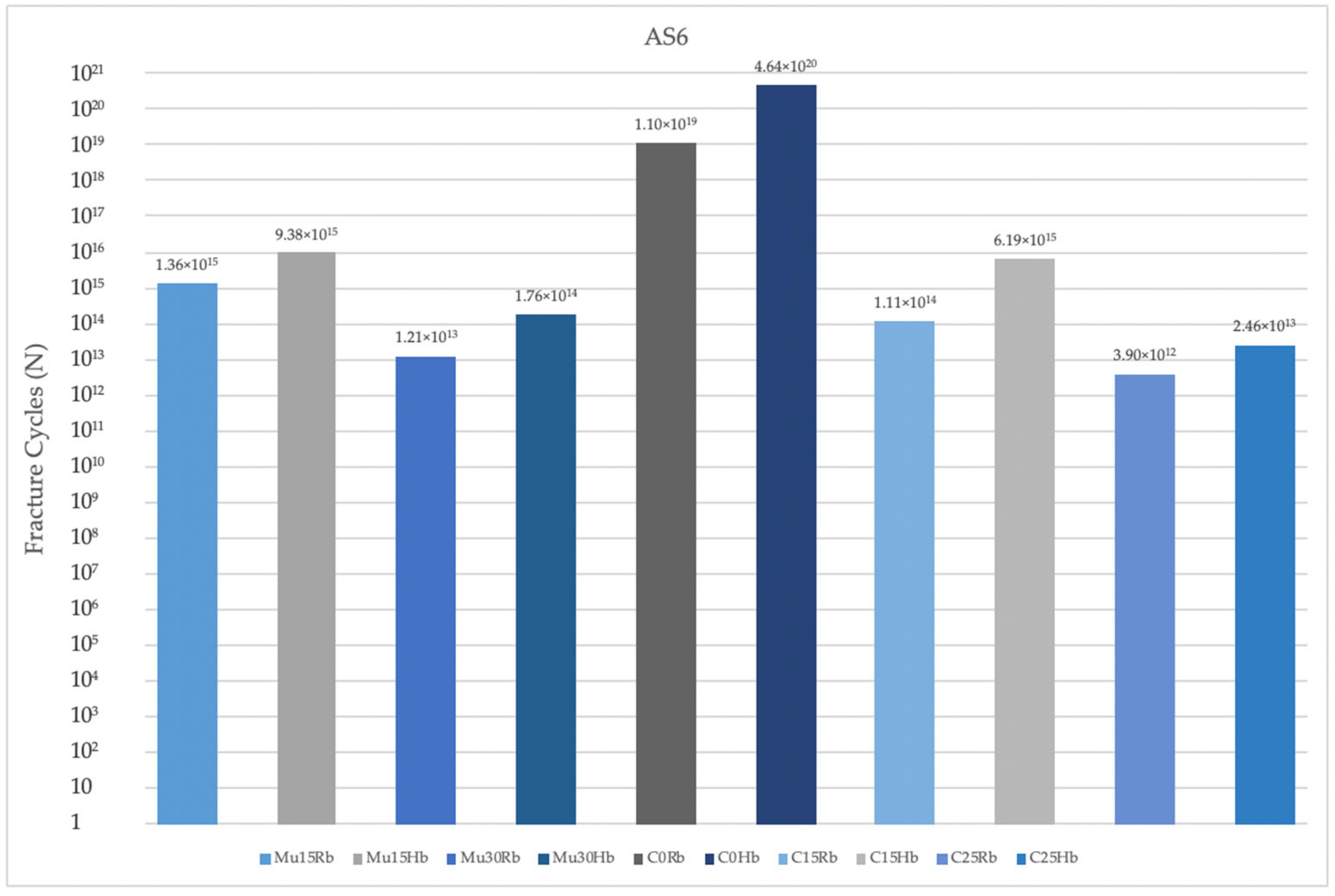
3.2.3. Fatigue Performance of Abutment Screw
3.2.4. Fatigue Performance of Occlusal Screw
4. Discussion
5. Conclusions
- -
- Implants with angulated placements demonstrated a reduction in fatigue performance, which is a critical consideration for clinical practice. Clinicians must be attentive to the angulation of implants, as excessive angulation may exacerbate mechanical complications and lead to reduced overall durability and function. Optimal implant placement, ideally in a more aligned orientation, is crucial to minimize adverse stress effects and enhance the long-term success of the implant.
- -
- Multiunit abutments generally achieved superior fatigue performance compared to cemented abutment types, particularly in scenarios involving implant angulation. Clinicians must therefore pay close attention to the selection of abutment types, especially in cases where implants are not perfectly aligned. Multiunit abutments may provide a more robust solution in these situations by accommodating angulations and mitigating the adverse effects on fatigue performance. This can lead to improved long-term outcomes and reduce the risk of mechanical complications, ultimately contributing to the success and durability of the implant restoration.
- -
- Molar regions exhibited lower fatigue performance compared to premolar regions due to their role as the primary zone for masticatory load. This reduced fatigue performance is primarily attributable to the significantly higher forces exerted during chewing in the molar area. Clinicians must carefully consider these factors when planning implant placements and restorations in the molar area. The elevated masticatory forces in this region necessitate the use of more robust and resilient implant components to withstand the increased stress and reduce the risk of mechanical failure.
- -
- Finally, lower fatigue performance values were observed in cases of bone resorption, likely due to the reduced support from cortical bone surrounding the implants. Clinicians must pay close attention to the implications of bone resorption when planning and placing implants. In cases where significant bone loss is present, it is essential to evaluate the remaining bone structure carefully and consider potential bone augmentation procedures or the use of implants with designs specifically intended for compromised bone conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, K.; Chen, J.; Han, L.; Yang, J.H.; Huang, W.; Wu, D. Angled Abutments Result in Increased or Decreased Stress on Surrounding Bone of Single-Unit Dental Implants: A Finite Element Analysis. Med. Eng. Phys. 2012, 34, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, M.E.I.; Nakhodchi, S.; Kia, D.S. Fatigue Life Prediction and Stress Distribution of Straight and Angled Abutments Using Numerical Techniques. Biomed. Phys. Eng. Express 2018, 4, 35025. [Google Scholar] [CrossRef]
- Sánchez, S.B.; Ciódaro, A.R.; Laverde, D.M.; Cantoral, A.C. Prevalence and Characteristics of Findings Related Implant in Panoramic X-rays. Wearable Technol. 2022, 1, 7. [Google Scholar] [CrossRef]
- Korkmaz, İ.H.; Kul, E. Investigation of the Type of Angled Abutment for Anterior Maxillary Implants: A Finite Element Analysis. J. Prosthodont. 2021, 31, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Butnaru-Moldoveanu, S.A.; Munteanu, F.; Forna, N.C. Virtual Bone Augmentation in Atrophic Mandible to Assess Optimal Implant-Prosthetic Rehabilitation—A Finite Element Study. Appl. Sci. 2020, 10, 401. [Google Scholar] [CrossRef]
- Synnott, S.; Langohr, G.D.G.; Reeves, J.M.; Johnson, J.A.; Athwal, G.S. The Effect of Humeral Implant Thickness and Canal Fill on Interface Contact and Bone Stresses in the Proximal Humerus. JSES Int. 2021, 5, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Gulinelli, J.L.; Dutra, R.A.; Marão, H.F.; Simeão, S.F.P.; Klein, G.; dos Santos, P.L. Maxilla Reconstruction With Autogenous Bone Block Grafts: Computed Tomography Evaluation and Implant Survival in a 5-Year Retrospective Study. Int. J. Oral Maxillofac. Surg. 2017, 46, 1045–1051. [Google Scholar] [CrossRef]
- Forna, D.A.; Forna, N.C.; Moldoveanu, S.A.B. Influence of Implant Dimensions in the Resorbed and Bone Augmented Mandible: A Finite Element Study. Contemp. Clin. Dent. 2020, 11, 336. [Google Scholar] [CrossRef]
- dal Polo, M.R.; Poli, P.P.; Rancitelli, D.; Beretta, M.; Maiorana, C. Alveolar Ridge Reconstruction With Titanium Meshes: A Systematic Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e639–e646. [Google Scholar] [CrossRef]
- Matsumoto, K.; Mano, T.; Inoue, K.; Yamamoto, K.; Imagawa, N.; Takahashi, A.; Ueno, T. Investigation of Implant Stability Quotient Values of Dental Implants Placed in Vascularized Bone Grafts. J. Hard Tissue Biol. 2022, 31, 55–58. [Google Scholar] [CrossRef]
- Arabi, S.R.; Moghimbeigi, A.; Hedayatipanah, M.; Samadi, M. Clinical and Radiographic Assessment of Peri-Implant Tissue in Posterior Areas With and Without the Need for Guided Bone Regeneration. Avicenna J. Dent. Res. 2016, 9, e30457. [Google Scholar] [CrossRef]
- Alqahtani, A.R.; Desai, S.R.; Patel, J.R.; Alqhtani, N.R.; Alqahtani, A.S.; Heboyan, A.; Fernandes, G.V.O.; Mustafa, M.; Karobari, M.I. Investigating the Impact of Diameters and Thread Designs on the Biomechanics of Short Implants Placed in D4 Bone: A 3D Finite Element Analysis. BMC Oral Health 2023, 23, 686. [Google Scholar] [CrossRef]
- Al-Kordy, N.M.T.A.; Al-Saadi, M.H. Finite Element Study of Stress Distribution with Tooth-Supported Mandibular Overdenture Retained by Ball Attachments or Resilient Telescopic Crowns. Eur. J. Dent. 2023, 17, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, A.; Hashtbaran, D.; Moghadam, A.; Aalaei, S. Stress Distribution in Peri-Implant Bone in the Replacement of Molars with One or Two Implants: A Finite Element Analysis. J. Dent. 2023, 24, 132. [Google Scholar]
- Ersöz, M.B.T.; Mumcu, E. Biomechanical Investigation of Maxillary Implant-Supported Full-Arch Prostheses Produced with Different Framework Materials: A Finite Elements Study. J. Adv. Prosthodont. 2022, 14, 346. [Google Scholar] [CrossRef]
- Chen, S.; Hong, X.; Ye, Z.; Wu, M.; Chen, L.; Wu, L.; Wang, Y.; Chen, Y.; Wu, J.; Wang, J. The Effect of Root Canal Treatment and Post-Crown Restorations on Stress Distribution in Teeth with Periapical Periodontitis: A Finite Element Analysis. BMC Oral Health 2023, 23, 973. [Google Scholar] [CrossRef]
- Singh, S.V.; Gupta, S.; Sharma, D.; Pandit, N.; Nangom, A.; Satija, H. Stress Distribution of Posts on the Endodontically Treated Teeth with and without Bone Height Augmentation: A Three-Dimensional Finite Element Analysis. J. Conserv. Dent. 2015, 18, 196–199. [Google Scholar] [CrossRef]
- Brown, M.W.; Miller, K.J. A Theory for Fatigue Failure under Multiaxial Stress-Strain Conditions. Proc. Inst. Mech. Eng. 1973, 187, 745–755. [Google Scholar] [CrossRef]
- Duan, Y.; Gonzalez, J.A.; Kulkarni, P.A.; Nagy, W.W.; Griggs, J.A. Fatigue Lifetime Prediction of a Reduced-Diameter Dental Implant System: Numerical and Experimental Study. Dent. Mater. 2018, 34, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Young, C.S.; Durham, J.C. Industrial Applications of Titanium and Zirconium: Fourth Volume: A Symposium; ASTM International: West Conshohocken, PA, USA, 1986; Volume 4, ISBN 0803104847. [Google Scholar]
- Allum, S.R.; Tomlinson, R.A.; Joshi, R. The Impact of Loads on Standard Diameter, Small Diameter and Mini Implants: A Comparative Laboratory Study. Clin. Oral Implant. Res. 2008, 19, 553–559. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D.; Machtei, E.E.; Levin, L. Effect of Dental Implant Diameter on Fatigue Performance. Part II: Failure Analysis. Clin. Implant Dent. Relat. Res. 2012, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kazarinov, N.A.; Stotskiy, A.; Polyakov, A.; Valiev, R.Z.; Enikeev, N.A. Finite Element Modeling for Virtual Design to Miniaturize Medical Implants Manufactured of Nanostructured Titanium With Enhanced Mechanical Performance. Materials 2022, 15, 7417. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Lv, L.-T.; Cheng, W.; Zhang, J.-L.; Ba, D.; Song, G.; Lin, Z. Effect of Loading Angles and Implant Lengths on the Static and Fatigue Fractures of Dental Implants. Materials 2021, 14, 5542. [Google Scholar] [CrossRef] [PubMed]
- León, P.P.; Bartolomé, J.F.; Lombardía, C.; Pradíes, G. Mechanical Fatigue Behaviour of Different Lengths Screw-retained Restorations Connected to Two Designs Prosthetic Connection Level. J. Oral Rehabil. 2019, 46, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; Stampa, C.; Schweyen, R.; Hey, J.; Boeckler, A.F. Retentive Characteristics of a New Attachment System for Hybrid Dentures. Materials 2020, 13, 3434. [Google Scholar] [CrossRef] [PubMed]
- Teimoori, H.; Shayegh, S.S.; Zavaree, M.A.; Hakimaneh, S.M.R.; Khodadad, F.; Shidfar, S.; Baghani, M.T. Effects of Excessive Implant Angulation on Retention of Two Types of Overdenture Attachments During Cyclic Loading. J. Contemp. Dent. Pract. 2018, 19, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.H.; Memarian, M.; Alikhasi, M.; Zeighami, S. Effect of Angle and Type of Customized Abutment (Castable &Amp; Cast-To) on Torque Loss and Fracture Resistance After Cyclic Loading. Open Dent. J. 2018, 12, 987–994. [Google Scholar] [CrossRef]
- Lopes, D.d.M.; Nishyama, R.; Steagall, W.; Tamaki, R.; Tortamano, P. Impact of Different Scan Strategies and Implant Angulation on Impression Accuracy of Full Arch Multiple Implant: An in Vitro Study. Braz. Dent. Sci. 2022, 25, 1–8. [Google Scholar] [CrossRef]
- Tan, S.; Tan, M.Y.; Wong, K.M.; Maria, R.; Tan, K.-B. Comparison of 3D Positional Accuracy of Implant Analogs in Printed Resin Models Versus Conventional Stone Casts: Effect of Implant Angulation. J. Prosthodont. 2023, 33, 46–53. [Google Scholar] [CrossRef]
- Ebadian, B.; Mosharraf, R.; Abbasi, S.; Pouya, M.A.; Mahmood, F. The Effect of Implant Angulation and Splinting on Stress Distribution in Implant Body and Supporting Bone: A Finite Element Analysis. Eur. J. Dent. 2015, 9, 311–318. [Google Scholar] [CrossRef]
- Brum, J.-R.; Macedo, F.-R.; Oliveira, M.-B.; Paranhos, L.-R.; de Brito-Junior, R.-B.; Ramacciato, J.-C. Assessment of the Stresses Produced on the Bone Implant/Tissue Interface to the Different Insertion Angulations of the Implant—A Three-Dimensional Analysis by the Finite Elements Method. J. Clin. Exp. Dent. 2020, 12, e930–e937. [Google Scholar] [CrossRef]
- Castro, A.P.G.; Completo, A.; Simões, J.A.; Flores, P. Biomechanical Behaviour of Cancellous Bone on Patellofemoral Arthroplasty With Journey Prosthesis: A Finite Element Study. Comput. Methods Biomech. Biomed. Eng. 2014, 18, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.J.; Weber, U.; Rechenberg, B.V.; Mayer, J. Enhancing the Mechanical Integrity of the Implant–Bone Interface With BoneWelding® Technology: Determination of Quasi-static Interfacial Strength and Fatigue Resistance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 77B, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bosco, R.; van den Beucken, J.; Leeuwenburgh, S.C.; Jansen, J.A. Surface Engineering for Bone Implants: A Trend From Passive to Active Surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef]
- Cheong, V.S.; Mumith, A.; Coathup, M.; Blunn, G.; Fromme, P. Bone Remodeling in Additive Manufactured Porous Implants Changes the Stress Distribution. In Health Monitoring of Structural and Biological Systems XIV; SPIE: Ile-de-France, France, 2020; pp. 323–330. [Google Scholar] [CrossRef]
- Arabmotlagh, M.; Bachmaier, S.; Geiger, F.; Rauschmann, M. PMMA-hydroxyapatite Composite Material Retards Fatigue Failure of Augmented Bone Compared to Augmentation with Plain PMMA: In Vivo Study Using a Sheep Model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Baggi, L.; Cappelloni, I.; Girolamo, M.D.; Maceri, F.; Vairo, G. The Influence of Implant Diameter and Length on Stress Distribution of Osseointegrated Implants Related to Crestal Bone Geometry: A Three-Dimensional Finite Element Analysis. J. Prosthet. Dent. 2008, 100, 422–431. [Google Scholar] [CrossRef]
- Lee, W.-T.; Koak, J.; Lim, Y.; Kim, S.-K.; Kwon, H.-B.; Kim, M. Stress Shielding and Fatigue Limits of Poly-ether-ether-ketone Dental Implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1044–1052. [Google Scholar] [CrossRef]
- Glorius, S.; Nies, B.; Jaña, F.; Quadbeck, P.; Hauser, R.; Standke, G.; Rößler, S.; Scharnweber, D.; Stephani, G. Metal Foam—Bone Cement Composites: Mechanical and Biological Properties and Perspectives for Bone Implant Design. Adv. Eng. Mater. 2011, 13, 1019–1023. [Google Scholar] [CrossRef]
- Han, J.; Sun, Y.; Wang, C. Effect of Integration Patterns Around Implant Neck on Stress Distribution in Peri-Implant Bone: A Finite Element Analysis. J. Prosthodont. 2016, 26, 549–558. [Google Scholar] [CrossRef]
- Geng, J.; Tan, K.-B.; Liu, G. Application of Finite Element Analysis in Implant Dentistry: A Review of the Literature. J. Prosthet. Dent. 2001, 85, 585–598. [Google Scholar] [CrossRef]
- Huang, H.; Chang, Y.-Y.; Lin, D.; Li, Y.; Chen, K.-T.; Hsu, J. Initial Stability and Bone Strain Evaluation of the Immediately Loaded Dental Implant: An in Vitro Model Study. Clin. Oral Implant. Res. 2010, 22, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Yuan, X.; Ren, M.; Chen, X.; Luo, L.; Zheng, L.; Liu, Y. Three-Dimensional Finite Element Analysis of Stress Distribution on Short Implants With Different Bone Conditions and Osseointegration Rates. BMC Oral Health 2023, 23, 220. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Yamamoto, K.; Horita, S.; Murakami, K.; Tsutsumi, S.; Kirita, T. The Effects of Bone Density and Crestal Cortical Bone Thickness on Micromotion and Peri-Implant Bone Strain Distribution in an Immediately Loaded Implant: A Nonlinear Finite Element Analysis. J. Periodontal Implant Sci. 2016, 46, 152. [Google Scholar] [CrossRef] [PubMed]
- Chou, I.-C.; Lee, S.Y.; Jiang, C. Effects of Implant Neck Design on Primary Stability and Overload in a Type IV Mandibular Bone. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 1223–1237. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Q.; Sun, Y.; Liao, W.; Bai, X.; Zhang, L.; Du, L.; Jin, Y.; Wang, Q.; Li, Z.; et al. Controlled-release of Bone Morphogenetic Protein-2 from a Microsphere Coating Applied to Acid-etched Ti6AL4V Implants Increases Biological Bone Growth in Vivo. J. Orthop. Res. 2014, 32, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Fuh, L.; Tu, M.-G.; Li, Y.; Chen, K.-T.; Huang, H. The Effects of Cortical Bone Thickness and Trabecular Bone Strength on Noninvasive Measures of the Implant Primary Stability Using Synthetic Bone Models. Clin. Implant Dent. Relat. Res. 2011, 15, 251–261. [Google Scholar] [CrossRef]
- Wada, M.; Tsuiki, Y.; Suganami, T.; Ikebe, K.; Sogo, M.; Ikuhisa, O.; Maeda, Y. The Relationship Between the Bone Characters Obtained by CBCT and Primary Stability of the Implants. Int. J. Implant Dent. 2015, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Ko, F.C.; Fullam, S.; Virdi, A.S.; Wimmer, M.A.; Sumner, D.R.; Ross, R.D. The Relative Contribution of Bone Microarchitecture and Matrix Composition to Implant Fixation Strength in Rats. J. Orthop. Res. 2021, 40, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, F.; Tozlu, M.; Çakan, D.G. Quantitative Evaluation of Alveolar Cortical Bone Density in Adults With Different Vertical Facial Types Using Cone-Beam Computed Tomography. Korean J. Orthod. 2014, 44, 36. [Google Scholar] [CrossRef]
- Akça, K.; Çehreli, M.C. Biomechanical Consequences of Progressive Marginal Bone Loss Around Oral Implants: A Finite Element Stress Analysis. Med. Biol. Eng. Comput. 2006, 44, 527–535. [Google Scholar] [CrossRef]
- Sotto-Maior, B.S.; Lima, C.d.A.; Senna, P.M.; Camargos, G.d.V.; Cury, A.A.D.B. Biomechanical Evaluation of Subcrestal Dental Implants With Different Bone Anchorages. Braz. Oral Res. 2014, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C. Evaluation of Mandibular Cortical Bone Thickness for Placement of Temporary Anchorage Devices (TADs). Korean J. Orthod. 2012, 42, 110. [Google Scholar] [CrossRef]
- Assenza, B.; Tripodi, D.; Scarano, A.; Perrotti, V.; Piattelli, A.; Iezzi, G.; D’Ercole, S. Bacterial Leakage in Implants With Different Implant–Abutment Connections: An in Vitro Study. J. Periodontol. 2012, 83, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Assenza, B.; Scarano, A.; Leghissa, G.; Carusi, G.; Thams, U.; Roman, F.S.; Piattelli, A. Screw-vs Cement-Implant–Retained Restorations: An Experimental Study in the Beagle. Part 1. Screw and Abutment Loosening. J. Oral Implantol. 2005, 31, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Radwan, M.M.; Bushra, S. Influence of Thermo-Mechanical Aging on Straight Versus Angled Multi-Unit Abutments Screw Joint Stability. Egypt. Dent. J. 2023, 69, 487–495. [Google Scholar] [CrossRef]
- Chaar, M.S.; Att, W.; Strub, J.R. Prosthetic Outcome of Cement-retained Implant-supported Fixed Dental Restorations: A Systematic Review. J. Oral Rehabil. 2011, 38, 697–711. [Google Scholar] [CrossRef]
- Hamed, M.T.; Mously, H.A.; Alamoudi, S.K.; Hashem, A.B.H.; Naguib, G.H. A Systematic Review of Screw Versus Cement-Retained Fixed Implant Supported Reconstructions. Clin. Cosmet. Investig. Dent. 2020, 12, 9–16. [Google Scholar] [CrossRef]
- DuVall, N.; DeReis, S.P.; Vandewalle, K.S. Fracture Strength of Various titanium-based, CAD-CAM and PFM Implant Crowns. J. Esthet. Restor. Dent. 2020, 33, 522–530. [Google Scholar] [CrossRef]
- Weber, H.P.; Kim, D.M.; Ng, M.W.; Hwang, J.W.; Fiorellini, J.P. Peri-implant Soft-tissue Health Surrounding Cement- and Screw-retained Implant Restorations: A Multi-center, 3-year Prospective Study. Clin. Oral Implant. Res. 2006, 17, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Chatzistavrianou, D.; Shahdad, S. An Alternative Design to Overcome the Problem of Unfavorable Implant Angulations for a Screw-Retained, Implant-Supported Fixed Prosthesis: Two Clinical Reports. J. Prosthodont. 2015, 24, 589–593. [Google Scholar] [CrossRef]







| Material | Young’s Modulus (E MPa) | Poisson’s Ratio (ν) | Shear Modulus (G Mpa) | References |
|---|---|---|---|---|
| Cortical bone | Ex 12,600 Ev 12,600 Ez 19,400 | νxy 0.300 νyz 0.253 νxz 0.253 νyx 0.300 νzy 0.390 νzx 0.390 | Gxy 4,850 Gyz 5.700 Gxz 5.700 | [12] |
| Trabecular bone | Ex 1148 Ev 210 Ez 1148 | νxy 0.055 νyz 0.010 νxz 0.322 νyx 0.010 νzy 0.055 νzx 0.322 | Gxy 68 Gyz 68 Gxz 434 | [12] |
| Mucosa | 2.8 | 0.40 | [13] | |
| Titanium | 110.000 | 0.33 | [14] | |
| Zirconia | 210.000 | 0.30 | [15] | |
| Resin composite | 12.000 | 0.33 | [16] | |
| Resin cement | 5.100 | 0.27 | [16] | |
| Bone graft | 3450 | 0.31 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdoğdu, M.; Demirel, M.G.; Mohammadi, R.; Güntekin, N. Assessment of the Impact of Bone Quality and Abutment Configuration on the Fatigue Performance of Dental Implant Systems Using Finite Element Analysis (FEA). J. Pers. Med. 2024, 14, 1040. https://doi.org/10.3390/jpm14101040
Erdoğdu M, Demirel MG, Mohammadi R, Güntekin N. Assessment of the Impact of Bone Quality and Abutment Configuration on the Fatigue Performance of Dental Implant Systems Using Finite Element Analysis (FEA). Journal of Personalized Medicine. 2024; 14(10):1040. https://doi.org/10.3390/jpm14101040
Chicago/Turabian StyleErdoğdu, Meryem, Mehmet Gökberkkaan Demirel, Reza Mohammadi, and Neslihan Güntekin. 2024. "Assessment of the Impact of Bone Quality and Abutment Configuration on the Fatigue Performance of Dental Implant Systems Using Finite Element Analysis (FEA)" Journal of Personalized Medicine 14, no. 10: 1040. https://doi.org/10.3390/jpm14101040








