Urinary L-FABP Assay in the Detection of Acute Kidney Injury following Haematopoietic Stem Cell Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Haematological Conditions and Conditioning Regimens
2.2.1. Patients with Lymphoma Were Conditioned with Either
- (a)
- Etoposide 200 mg/m2 in 1000 mL of normal saline over 2 h and Cytosine Arabinoside 200 mg/m2 12 hourly over 30 min for 4 days followed by Melphalan 140 mg/m2 IV in 250 mL of normal saline (dose reduction to 120 mg/m2 if SCr > 200 μmol/L) pre-transplant OR
- (b)
- Carmustine 400 mg/m2 over 60 min (single dose) and Thiotepa 5 mg/Kg IV 12 hourly in 250 mL of normal saline for 2 days pre-transplant.
2.2.2. Patients with Myelodysplastic and Myeloproliferative Disorders Were Broadly Classified under Other Haematological Conditions and Were Divided into Two Subgroups
- (a)
- Patients who received reduced-intensity (non-myeloablative) allogenic HSCT with Fludarabine (30 mg/m2 IV over 30 min for 5 days) and Busulphan (3.2 mg/m2 over 30 min for 3 days) and/or Cytosine Arabinoside 2 g/m2 IV over 4 h for 4 days) followed infrequently by Melphalan (140 mg/ m2 in 250 mL of normal saline) or total body irradiation (TBI)-single fraction of 200 cGy/2 Gy.
- (b)
- Patients who received full-intensity (myeloablative) allogenic HSCT with Lomustine (200 mg/m2 single dose), Etoposide (200 mg/m2 over 2 h in 1000 mL of normal saline) and Cytosine Arabinoside (200 mg/m2 12 hourly over 30 min for 4 days followed by Melphalan 140 mg/m2 IV in 250 mL of normal saline and TBI fractions of 330 cGy for 3–4 days).
2.3. AKI Definition
2.4. Study Measurements and Laboratory Analysis
2.5. Statistics
3. Results
3.1. Patient Characteristics
3.2. Haematopoietic Stem Cell Transplantation and Acute Kidney Injury
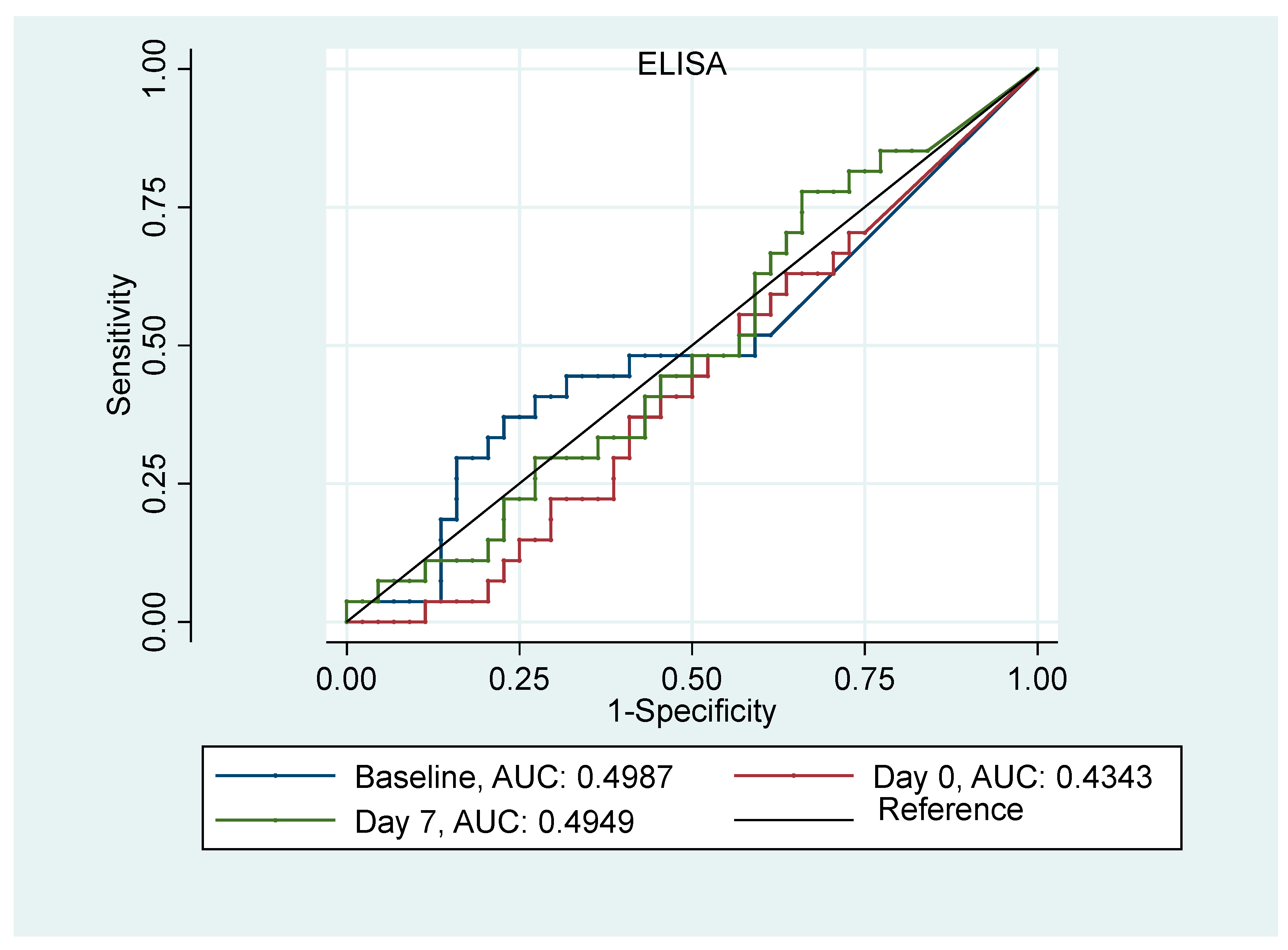
3.3. AKI Prediction Analysis
3.4. Survival Analysis and uL-FABP ELISA
3.5. Association of uL-FABP ELISA with POC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horowitz, M.M. Uses and Growth of Hematopoietic Cell Transplantation. In Thomas’ Hematopoietic Cell Transplantation; Wiley: Hoboken, NJ, USA, 2003; pp. 9–15. [Google Scholar] [CrossRef]
- Renaghan, A.D.; Jaimes, E.A.; Malyszko, J.; Perazella, M.A.; Sprangers, B.; Rosner, M.H. Acute kidney injury and CKD associated with hematopoietic stem cell transplantation. Clin. J. Am. Soc. Nephrol. 2020, 15, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kersting, S.; Koomans, H.A.; Hené, R.J.; Verdonck, L.F. Acute renal failure after allogeneic myeloablative stem cell transplantation: Retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant. 2007, 39, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Gonçalves, S.; Jorge, S.; Raimundo, M.; Resende, L.; Lourenço, F.; Martins, C.; Carmo, J.A.D.; Lacerda, J.M.F.; Prata, M.M. Contemporary analysis of the influence of acute kidney injury after reduced intensity conditioning haematopoietic cell transplantation on long-term survival. Bone Marrow Transplant. 2008, 42, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Jorge, S.; Silva, S.; de Almeida, E.; Abreu, F.; Martins, C.; Carmo, J.A.D.; Lacerda, J.F.; Prata, M.M. Acute renal failure following myeloablative autologous and allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2006, 38, 707. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Guthrie, K.; Batchelder, A.; Schoch, G.; Aboulhosn, N.; Manchion, J.; Mcdonald, G.B. Acute renal failure after myeloablative hematopoietic cell transplant: Incidence and risk factors. Kidney Int. 2005, 67, 272–277. [Google Scholar] [CrossRef]
- Zager, R.A.; O’Quigley, J.; Zager, B.K.; Alpers, C.; Shulman, H.; Gamelin, L.; Stewart, P.; Thomas, E. Acute renal failure following bone marrow transplantation: A retrospective study of 272 patients. Am J. Kidney Dis. 1989, 13, 210–216. [Google Scholar] [CrossRef]
- Parikh, C.R.; Schrier, R.W.; Storer, B.; Diaconescu, R.; Sorror, M.L.; Maris, M.B.; Maloney, D.G.; McSweeney, P.; Storb, R.; Sandmaier, B.M. Comparison of ARF after myeloablative and nonmyeloablative hematopoietic cell transplantation. Am. J. Kidney Dis. 2005, 45, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Abramson, M.H.; Gutgarts, V.; Zheng, J.; Maloy, M.A.; Ruiz, J.D.; Scordo, M.; Jaimes, E.A.; Sathick, I.J. Acute kidney injury in the modern era of allogeneic hematopoietic stem cell transplantation. Clin. J. Am. Soc. Nephrol. 2021, 16, 1318–1327. [Google Scholar] [CrossRef]
- Schrier, R.W.; Parikh, C.R. Comparison of renal injury in myeloablative autologous, myeloablative allogeneic and non-myeloablative allogeneic haematopoietic cell transplantation. Nephrol. Dial. Transplant. 2005, 20, 678–683. [Google Scholar] [CrossRef]
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Nephron. Clin. Pract. 2012, 4, c178–c184. [Google Scholar] [CrossRef] [PubMed]
- Slocum, J.L.; Heung, M.; Pennathur, S. Marking renal injury: Can we move beyond serum creatinine? Transl. Res. 2012, 159, 277–289. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Kashani, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J. Thorac. Dis. 2016, 8, E305–E311. [Google Scholar] [CrossRef]
- Dalton, R.N. Serum creatinine and glomerular filtration rate: Perception and reality. Clin. Chem. 2010, 56, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Samuels, J.; Ng, C.S.; Nates, J.; Price, K.; Finkel, K.; Salahudeen, A.; Shaw, A. Small increases in serum creatinine are associated with prolonged ICU stay and increased hospital mortality in critically ill patients with cancer. Support. Care Cancer 2011, 19, 1527. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.; Vaidya, V.S.; Waikar, S.S.; Collings, F.B.; Sunderland, K.E.; Gioules, C.J.; Bonventre, J.V. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010, 77, 708. [Google Scholar] [CrossRef]
- Kamijo, A.; Kimura, K.; Sugaya, T.; Yamanouchi, M.; Hikawa, A.; Hirano, N.; Hirata, Y.; Goto, A.; Omata, M. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J. Lab. Clin. Med. 2004, 143, 23–30. [Google Scholar] [CrossRef]
- Thi, T.N.D.; Gia, B.N.; Le Thi, H.L.; Thi, T.N.C.; Thanh, H.P. Evaluation of Urinary L-Fabp as an Early Marker for Diabetic Nephropathy in Type 2 Diabetic Patients. J. Med. Biochem. 2020, 39, 224–230. [Google Scholar] [CrossRef]
- González, J.; Jatem, E.; Roig, J.; Valtierra, N.; Ostos, E.; Abó, A.; Santacana, M.; García, A.; Segarra, A. Usefulness of urinary biomarkers to estimate the interstitial fibrosis surface in diabetic nephropathy with normal kidney function. Nephrol. Dial. Transplant. 2022, 37, 2102–2110. [Google Scholar] [CrossRef]
- Chiang, T.H.; Yo, C.H.; Hoong Lee, G.; Mathew, A.; Sugaya, T.; Li, W.Y.; Lee, C.C. Accuracy of Liver-Type Fatty Acid-Binding Protein in Predicting Acute Kidney Injury: A Meta-Analysis. J. Appl. Lab. Med. 2022, 7, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Portilla, D.; Dent, C.; Sugaya, T.; Nagothu, K.; Kundi, I.; Moore, P.; Noiri, E.; Devarajan, P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008, 73, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sugaya, T.; Node, K.; Ueda, Y.; Koide, H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am. J. Kidney Dis. 2006, 47, 439–444. [Google Scholar] [CrossRef]
- Spector, A.A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [Google Scholar] [CrossRef]
- Veerkamp, J.H.; van Kuppevelt, T.H.M.S.M.; Maatman, R.G.H.J.; Prinsen, C.F.M. Structural and functional aspects of cytosolic fatty acid-binding proteins. Prostaglandins Leukot. Essent. Fat. Acids 1993, 49, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, J.H.; Peeters, R.A.; Maatman, R.G.H.J. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim. Biophys. Acta 1991, 1081, 1–24. [Google Scholar] [CrossRef]
- Simon, N.; Hertig, A. Alteration of Fatty Acid Oxidation in Tubular Epithelial Cells: From Acute Kidney Injury to Renal Fibrogenesis. Front. Med. 2015, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Issemann, I.; Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef]
- Kamijo, A.; Kimura, K.; Sugaya, T.; Yamanouchi, M.; Hase, H.; Kaneko, T.; Hirata, Y.; Goto, A.; Fujita, T.; Omata, M. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 2002, 62, 1628–1637. [Google Scholar] [CrossRef]
- Prowle, J.R.; Calzavacca, P.; Licari, E.; Ligabo, E.V.; Echeverri, J.E.; Bagshaw, S.M.; Haase-Fielitz, A.; Haase, M.; Ostland, V.; Noiri, E.; et al. Combination of biomarkers for diagnosis of acute kidney injury after cardiopulmonary bypass. Ren. Fail. 2015, 37, 408–416. [Google Scholar] [CrossRef]
- Zeng, X.F.; Li, J.M.; Tan, Y.; Wang, Z.-F.; He, Y.; Chang, J.; Zhang, H.; Zhao, H.; Bai, X.; Xie, F.; et al. Performance of urinary NGAL and L-FABP in predicting acute kidney injury and subsequent renal recovery: A cohort study based on major surgeries. Clin. Chem. Lab. Med. 2014, 52, 671–678. [Google Scholar] [CrossRef]
- Parr, S.K.; Clark, A.J.; Bian, A.; Shintani, A.K.; Wickersham, N.E.; Ware, L.B.; Ikizler, T.A.; Siew, E.D. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2014, 87, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.D.; Ware, L.B.; Bian, A.; Shintani, A.; Eden, S.K.; Wickersham, N.; Cripps, B.; Ikizler, T.A. Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int. 2013, 84, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa, I.; Montoliu, C.; Urios, A.; Andrés-Costa, M.J.; Giménez-Garzó, C.; Juan, I.; Puchades, M.J.; Blasco, M.L.; Carratalá, A.; Sanjuán, R.; et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessel. 2014, 30, 703–711. [Google Scholar] [CrossRef]
- Liu, S.; Che, M.; Xue, S.; Xie, B.; Zhu, M.; Lu, R.; Zhang, W.; Qian, J.; Yan, Y. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomark. 2013, 18, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zdziechowska, M.; Gluba-Brzózka, A.; Poliwczak, A.R.; Franczyk, B.; Kidawa, M.; Zielinska, M.; Rysz, J. Serum NGAL, KIM-1, IL-18, L-FABP: New biomarkers in the diagnostics of acute kidney injury (AKI) following invasive cardiology procedures. Int. Urol. Nephrol. 2020, 52, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.; Suzuki, K.; Okada, H.; Ishihara, T.; Minamiyama, T.; Kamidani, R.; Kitagawa, Y.; Fukuta, T.; Suzuki, K.; Miyake, T.; et al. Urinary liver-type fatty acid-binding protein levels may be associated with the occurrence of acute kidney injury induced by trauma. Front. Med. 2024, 11, 1346183. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Hyun, M.C.; Choi, B.S.; Chun, S.Y.; Cho, M.H. Acute kidney injury after using contrast during cardiac catheterization in children with heart disease. J. Korean Med. Sci. 2014, 29, 1102–1107. [Google Scholar] [CrossRef]
- Katoh, H.; Nozue, T.; Kimura, Y.; Nakata, S.; Iwaki, T.; Kawano, M.; Kawashiri, M.-A.; Michishita, I.; Yamagishi, M. Elevation of urinary liver-type fatty acid-binding protein as predicting factor for occurrence of contrast-induced acute kidney injury and its reduction by hemodiafiltration with blood suction from right atrium. Heart Vessel. 2013, 29, 191–197. [Google Scholar] [CrossRef]
- Manabe, K.; Kamihata, H.; Motohiro, M.; Senoo, T.; Yoshida, S.; Iwasaka, T. Urinary liver-type fatty acid-binding protein level as a predictive biomarker of contrast-induced acute kidney injury. Eur. J. Clin. Investig. 2012, 42, 557–563. [Google Scholar] [CrossRef]
- Kokot, M.; Biolik, G.; Ziaja, D.; Fojt, T.; Kedzierski, L.; Antoniak, K.; Janowska, M.; Pawlicki, K.; Ziaja, K.; Dulawa, J. Assessment of subclinical acute kidney injury after abdominal aortic aneurysm surgery using novel markers: L-FABP and H-FABP. Nefrologia 2014, 34, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Okuda, H.; Obata, Y.; Kamijo-Ikemori, A.; Inoue, S. Quantitative and qualitative analyses of urinary L-FABP for predicting acute kidney injury after emergency laparotomy. J. Anesth. 2022, 36, 38–45. [Google Scholar] [CrossRef]
- Yanishi, M.; Kinoshita, H. Urinary L-type fatty acid-binding protein is a predictor of cisplatin-induced acute kidney injury. BMC Nephrol. 2022, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Shingai, N.; Morito, T.; Najima, Y.; Igarashi, A.; Kobayashi, T.; Doki, N.; Kakihana, K.; Ohashi, K.; Ando, M. Urinary Liver-Type Fatty Acid-Binding Protein Linked with Increased Risk of Acute Kidney Injury after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 2010–2014. [Google Scholar] [CrossRef] [PubMed]
- Endre, Z.H. Acute kidney injury: Definitions and new paradigms. Adv. Chronic Kidney Dis. 2008, 15, 213–221. [Google Scholar] [CrossRef]
- Mitsides, N.; Mitra, V.; Saha, A.; Harris, S.; Kalra, P.A.; Mitra, S. Urinary Liver-Type Fatty Acid Binding Protein, a Biomarker for Disease Progression, Dialysis and Overall Mortality in Chronic Kidney Disease. J. Pers. Med. 2023, 13, 1481. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Ichibayashi, R.; Yamamoto, S.; Nakamichi, Y.; Watanabe, M.; Honda, M. Clinical significance of urinary L-FABP in the emergency department. Int. J. Emerg. Med. 2019, 12, 24. [Google Scholar] [CrossRef]
- Tabbara, I.A.; Zimmerman, K.; Morgan, C.; Nahleh, Z. Allogeneic hematopoietic stem cell transplantation: Complications and results. Arch. Intern. Med. 2002, 162, 1558–1566. [Google Scholar] [CrossRef]
- NCEPOD. A Review of the Care of Patients Who Died in Hospital with a Primary Diagnosis of Acute Kidney Injury (Acute Renal Failure); NCEPOD Report: Adding Insult to injury; NCEPOD: London, UK, 2009. [Google Scholar]
- Hou, S.H.; Bushinsky, D.A.; Wish, J.B.; Cohen, J.J.; Harrington, J.T. Hospital-acquired renal insufficiency: A prospective study. Am. J. Med. 1983, 74, 243–248. [Google Scholar] [CrossRef]
- Shusterman, N.; Strom, B.L.; Murray, T.G.; Morrison, G.; West, S.L.; Maislin, G. Risk factors and outcome of hospital-acquired acute renal failure: Clinical epidemiologic study. Am. J. Med. 1987, 83, 65–71. [Google Scholar] [CrossRef]
- Liaño, F.; Junco, E.; Pascual, J.; Madero, R.; Verde, E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int. Suppl. 1998, 66, S16–S24. [Google Scholar] [PubMed]
- Cosentino, F.; Chaff, C.; Piedmonte, M. Risk factors influencing survival in ICU acute renal failure. Nephrol. Dial. Transplant. 1994, 9 (Suppl. S4), 179–182. [Google Scholar] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]

| n = 85 | Allogenic HSCT n = 35 (41.2%) | Autologous HSCT n = 50 (58.8%) | p-Value |
|---|---|---|---|
| Age median (IQR) [range] 62 (56, 68) [24–75] | 59 (48, 67) [24–70] | 63 (59, 69) [32–75] | <0.001 |
| Gender n (%) | |||
| Female, 30 (35%) | 8 (23%) | 22 (44%) | 0.076 |
| Male, 55 (65%) | 27 (77%) | 28 (56%) | |
| Ethnicity n (%) | |||
| White, 78 (95%) | 33 (94%) | 45 (90%) | 0.350 |
| Asian, 4 (5%) | 1 (3%) | 3 (6%) | |
| Black, 2 (2%) | - | 2 (4%) | |
| Other, 1 (1%) | 1 (3%) | - | |
| Primary Haematological Condition n (%) | |||
| Lymphoma, 6 (7%) | 2 (6%) | 4 (8%) | <0.001 |
| Multiple Myeloma, 46 (54%) | 2 (6%) | 44 (88%) | |
| Other, 33 (39%) | 31 (88%) | 2 (4%) | |
| Medical History n (%) | |||
| Diabetes, 12 (14%) | 3 (9%) | 9 (18%) | 0.360 |
| Hypertension, 32 (38%) | 10 (29%) | 22 (44%) | 0.220 |
| CKD, 12 (14%) | 2 (6%) | 10 (20%) | 0.120 |
| ACE inhibitor | |||
| Ramipril, 10 (12%) | 3 (9%) | 7 (14%) | |
| Lisinopril, 5 (6%) | 2 (6%) | 3 (6%) | |
| Perindopril, 1 (1%) | 1 (3%) | - | |
| Kidney Function Status pre-HSCT n (%) | |||
| Creatinine Clearance > 30 mL/min 75 (88%) Creatinine clearance < 30 mL/min 10 (12%) | 28 (80%) 7 (20%) | 47 (94%) 3 (6%) | 0.130 |
| Creatinine μmol/L median (IQR) [range] | |||
| Baseline | 87 (75, 101) [58–263] | 80 (66, 103) [44–258] | 0.390 |
| Day of transplant | 71 (59, 100) [8–258] | 74 (63, 94) [40–273] | 0.460 |
| Day 7 | 75 (63, 4) [36–230] | 77 (2, 104) [38–329] | 0.530 |
| Day 30 | 96 (78, 129) [50–210] | 89 (70, 110) [44–288] | 0.160 |
| Highest Serum Creatinine μmol/L median (IQR) [range] | 121 (98, 159) [57–310] | 97 (85, 131) [52–449] | 0.033 |
| Uncorrected uL-FABP ELISA mcg median (IQR) [range] | |||
| Baseline 0.5 (0, 2.8) [0, 126.2] | 0.8 (0, 3.1) [0–62.1] | 0.2 (0, 2.0) [0–126.2] | 0.310 |
| Day 0 2.1 (0.1, 7.3) [0, 262.4] | 2.2 (0.7, 7.2) [0–173.1] | 2.1 (0, 8.5) [0–262.4] | 0.300 |
| Day 7 6.4 (1.8, 15.5) [0, 79.5] | 6.9 (2.4, 18.5) [0–79.5] | 5.5 (1.3, 10.2) [0–68.8] | 0.180 |
| Corrected uL-FABP ELISA mcg/gCr median (IQR) [range] | |||
| Baseline 0.6 (0, 5.4) [0, 96.3] | 1.6 (0.1, 3.0) [0–96.3] | 0.6 (0, 6.4) [0–70.3] | 0.480 |
| Day 0 5.1 (0.7, 17.1) [0, 662.8] | 5.4 (2.0, 10.1) [0–49.0] | 4.4 (0, 18.0) [0–662.9] | 0.980 |
| Day 7 5.9 (2.0, 16.5) [0, 125.5] | 10.3 (3.2, 23.0) [0–125.5] | 5.1 (1.1, 11.8) [0–47.1] | 0.041 |
| AKI in the first 30 days, n (%) | 20(57%) | 13(26%) | 0.008 |
| No AKI AKI score = 1 AKI score = 2 AKI score = 3 | 15 14 5 1 | 37 12 0 1 | |
| Mortality 18 months post-HSCT, n (%) | 10 (26%) | 2 (4%) | 0.004 |
| Allogenic HSCT | Autologous HSCT | |||||
|---|---|---|---|---|---|---|
| No AKI | AKI | p-Value | No AKI | AKI | p-Value | |
| uL-FABP ELISA mcg/gCr, Median (IQR) [range], | ||||||
| Baseline | 1.6 (0.3, 2.2) [0–30.5] | 0.8 (0, 3.0) [0–96.3] | 0.470 | 0.2 (0, 2.6) [0–70.3] | 2.8 (0, 7.7) [0–12.2] | 0.760 |
| Day of Transplant | 5.5 (1.7, 16.1) [0–49.0] | 4.1 (1.6, 8.4) [0–34.7] | 0.690 | 4.0 (0, 17.1) [0–101.8] | 4.4 (0, 14.9) [0–77.2] | 0.720 |
| Day 7th post-transplant | 14.6 (4.8, 38.2) [0–74.1] | 5.1 (3.0, 20.2) [0–125.5] | 0.370 | 3.5 (0, 9.8) [0–47.1] | 6.4 (2.2, 9.2) [0–19.7] | 0.720 |
| Serum Creatinine μmol/L Median (IQR) [range] | ||||||
| Baseline | 94 (80, 101) [59–132] | 81 (73, 104) [58–263] | 0.590 | 79 (67, 100) [44–225] | 93 (65, 140) [58–258] | 0.340 |
| Day of Transplant | 68 (58, 88) [49–102] | 78 (63, 105) [41–258] | 0.220 | 73 (63, 92) [40–203] | 94 (70, 143) [57–273] | 0.090 |
| Day 7th post-transplant | 72 (63, 77) [36–94] | 86 (61, 100) [44–230] | 0.130 | 76 (55, 92) [38–228] | 99 (68, 130) [60–329] | 0.073 |
| Variable | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| Log (Baseline uL-FABP ELISA) | 1.01 (0.85,1.19) | 0.90 |
| Log (uL-FABP ELISA Day 0) | 1.004 (0.85,1.29) | 0.97 |
| Log (uL-FABP ELISA Day 7 *) | 1.06 (0.87,1.31) | 0.62 |
| Baseline SCr | 1.01 (1, 1.019) | 0.21 |
| Day 0 SCR | 1.01 (1, 1.024) | 0.07 |
| Day 7 SCR | 1.02 (1, 1.033) | 0.03 |
| Graft type | ||
| Allogenic | 1 | 0.004 |
| Autologous | 0.26 (0.1, 0.651) | |
| Condition | ||
| Multiple Myeloma | 1 | 0.09 |
| Other | 2.17 (0.9, 5.364) | |
| Sex | ||
| Female | 1 | 0.09 |
| Male | 2.29 (0.89, 6.304) | |
| CKD | ||
| No | 1 | 0.14 |
| Yes | 2.53 (0.74, 9.319) | |
| Diabetes | ||
| No | 1 | 0.83 |
| Yes | 1.15 (0.31, 3.949) | |
| Hypertension | ||
| No | 1 | 0.79 |
| Yes | 1.13 (0.46, 2.771) | |
| Creatinine clearance at baseline <30 mL/min or SCr > 200 mmol/L | ||
| Yes | 1 | 0.012 |
| No | 0.13 (0.018, 0.54) |
| Variables in the Model | Odds Ratio (95% CI), p-Value for uL-FABP | Odds Ratio (95% CI), p-Value for Graft Type | Odds Ratio (95% CI), p-Value for SCr |
|---|---|---|---|
| Baseline uL-FABP, Graft type, Baseline Sc | 0.94 (0.76, 1.14) 0.53 | 0.26 (0.095, 0.67) 0.006 | 1.009 (0.99, 1.02) 0.15 |
| Day 0 uL-FABP, Graft type, SCr Day 0 | 0.86 (0.69, 1.06) 0.17 | 0.19 (0.06, 0.53) 0.002 | 1.02 (1.01, 1.04) 0.011 |
| Day 7 * uL-FABP, Graft type, SCr Day 7 | 0.98 (0.78, 1.25) 0.87 | 0.23 (0.07, 0.72) 0.015 | 1.02 (1.01, 1.05) 0.019 |
| ELISA <12.5 | ELISA ≥12.5 | Sensitivity, 95% CI | 0.99 (0.97, 1) | |
| POC = 1 | 227 | 17 | Specificity, 95% CI | 0.65 (0.49, 0.78) |
| POC ≠ 1 | 2 | 31 | Positive Predictive Value, 95% CI | 0.93 (0.89, 0.96) |
| Negative Predictive Value, 95% CI | 0.94 (0.8, 0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, R.; Tholouli, E.; Rajai, A.; Saha, A.; Mitra, S.; Mitsides, N. Urinary L-FABP Assay in the Detection of Acute Kidney Injury following Haematopoietic Stem Cell Transplantation. J. Pers. Med. 2024, 14, 1046. https://doi.org/10.3390/jpm14101046
Mitra R, Tholouli E, Rajai A, Saha A, Mitra S, Mitsides N. Urinary L-FABP Assay in the Detection of Acute Kidney Injury following Haematopoietic Stem Cell Transplantation. Journal of Personalized Medicine. 2024; 14(10):1046. https://doi.org/10.3390/jpm14101046
Chicago/Turabian StyleMitra, Roshni, Eleni Tholouli, Azita Rajai, Ananya Saha, Sandip Mitra, and Nicos Mitsides. 2024. "Urinary L-FABP Assay in the Detection of Acute Kidney Injury following Haematopoietic Stem Cell Transplantation" Journal of Personalized Medicine 14, no. 10: 1046. https://doi.org/10.3390/jpm14101046






