Preoperative Glycosylated Haemoglobin Screening to Identify Older Adult Patients with Undiagnosed Diabetes Mellitus—A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Participants
2.3. Outcome Parameters
2.4. Data Sources
2.5. Statistical Methods
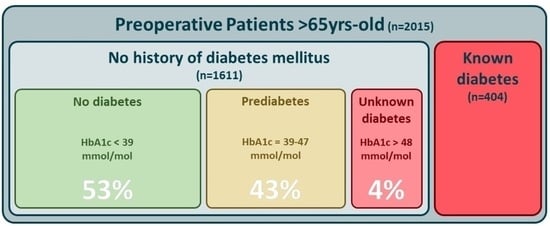
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Vries, F.E.E.; Gans, S.L.; Solomkin, J.S.; Allegranzi, B.; Egger, M.; Dellinger, E.P.; A Boermeester, M. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br. J. Surg. 2017, 104, e95–e105. [Google Scholar] [CrossRef]
- Yong, P.H.; Weinberg, L.; Torkamani, N.; Churilov, L.; Robbins, R.J.; Ma, R.; Bellomo, R.; Lam, Q.T.; Burns, J.D.; Hart, G.K.; et al. The Presence of Diabetes and Higher HbA1c Are Independently Associated with Adverse Outcomes after Surgery. Diabetes Care 2018, 41, 1172–1179. [Google Scholar] [CrossRef]
- Frisch, A.; Chandra, P.; Smiley, D.; Peng, L.; Rizzo, M.; Gatcliffe, C.; Hudson, M.; Mendoza, J.; Johnson, R.; Lin, E.; et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010, 33, 1783–1788. [Google Scholar] [CrossRef]
- Kotagal, M.; Symons, R.G.; Hirsch, I.B.; Umpierrez, G.E.; Dellinger, E.P.; Farrokhi, E.T.; Flum, D.R. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann. Surg. 2015, 261, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.Y.; Nuttall, G.A.; Abel, M.D.; Mullany, C.J.; Schaff, H.V.; Williams, B.A.; Schrader, L.M.; Rizza, R.A.; McMahon, M.M. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin. Proc. 2005, 80, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Curletto, G.; Cipullo, D.; de la Longrais, R.R.; Trento, M.; Passera, P.; Taulaigo, A.V.; Di Miceli, S.; Cenci, A.; Dalmasso, P.; et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care 2014, 37, 1668–1674. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Isaacs, S.D.; Bazargan, N.; You, X.; Thaler, L.M.; Kitabchi, A.E. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Binney, Z.O.; Khakharia, A.; Long, C.A.; Brewster, L.P.; Wilson, P.W.; Jordan, W.D.; Duwayri, Y. High hemoglobin A1c associated with increased adverse limb events in peripheral arterial disease patients undergoing revascularization. J. Vasc. Surg. 2018, 67, 217–228.e1. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.W.; Ti, L.K.; Lean, L.L.; Seet, E.; Paramasivan, A.; Liu, W.; Wang, J.; Chua, V.; Liew, L.Q. The neglected perioperative population of undiagnosed diabetics—A retrospective cohort study. BMC Surg. 2020, 20, 188. [Google Scholar] [CrossRef]
- Carpenter, D.L.; Gregg, S.R.; Xu, K.; Buchman, T.G.; Coopersmith, C.M. Prevalence and Impact of Unknown Diabetes in the ICU. Crit. Care Med. 2015, 43, e541–e550. [Google Scholar] [CrossRef]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. S14), LP-S31.
- WHO. Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. 2011. Available online: https://apps.who.int/iris/handle/10665/70523 (accessed on 20 December 2022).
- Cosentino, F.; Grant, P.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar]
- van den Boom, W.; Schroeder, R.A.; Manning, M.W.; Setji, T.L.; Fiestan, G.-O.; Dunson, D.B. Effect of A1C and Glucose on Postoperative Mortality in Noncardiac and Cardiac Surgeries. Diabetes Care 2018, 41, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Shohat, N.; Muhsen, K.; Gilat, R.; Rondon, A.J.; Chen, A.F.; Parvizi, J. Inadequate Glycemic Control Is Associated with Increased Surgical Site Infection in Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2018, 33, 2312–2321.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, X.; Jin, X.; Lv, M.; Li, X.; Dou, J.; Zeng, J.; An, P.; Chen, Y.; Chen, K.; et al. Effects of Preoperative HbA1c Levels on the Postoperative Outcomes of Coronary Artery Disease Surgical Treatment in Patients with Diabetes Mellitus and Nondiabetic Patients: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2020, 2020, 3547491. [Google Scholar] [CrossRef] [PubMed]
- Underwood, P.; Askari, R.; Hurwitz, S.; Chamarthi, B.; Garg, R. Preoperative A1C and Clinical Outcomes in Patients with Diabetes Undergoing Major Noncardiac Surgical Procedures. Diabetes Care 2014, 37, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Dronge, A.S.; Perkal, M.F.; Kancir, S.; Concato, J.; Aslan, M.; Rosenthal, R.A. Long-term Glycemic Control and Postoperative Infectious Complications. Arch. Surg. 2006, 141, 375–380, discussion 380. [Google Scholar] [CrossRef] [PubMed]
- Joint British Diabetes Societies for Inpatient Care. Management of Adults with Diabetes Undergoing Surgery and Elective Procedures: Improving Standards (JBDS-IP Guideline); Association of British Clinical Diabetologists: Leeds, UK, 2016. [Google Scholar]
- Lazar, H.L.; McDonnell, M.; Chipkin, S.R.; Furnary, A.P.; Engelman, R.M.; Sadhu, A.R.; Bridges, C.R.; Haan, C.K.; Svedjeholm, R.; Taegtmeyer, H.; et al. The Society of Thoracic Surgeons Practice Guideline Series: Blood Glucose Management During Adult Cardiac Surgery. Ann. Thorac. Surg. 2009, 87, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- Williams, R.; Colagiuri, S.; Chan, J.; Almutairi, R.; Montoya, P.A.; Basit, A.; Beran, D.; Besançon, S.; Bommer, C.; Borgnakk, W.; et al. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Myles, P.S.; A Shulman, M.; Heritier, S.; Wallace, S.; McIlroy, D.R.; McCluskey, S.; Sillar, I.; Forbes, A. Validation of days at home as an outcome measure after surgery: A prospective cohort study in Australia. BMJ Open 2017, 7, e015828. [Google Scholar] [CrossRef]
- Bell, M.; Eriksson, L.I.; Svensson, T.; Hallqvist, L.; Granath, F.; Reilly, J.; Myles, P.S. Days at Home after Surgery: An Integrated and Efficient Outcome Measure for Clinical Trials and Quality Assurance. EClinicalMedicine 2019, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Jaeger, M.; Baxter, M.; VanDenKerkhof, E.; van Vlymen, J. Postoperative dysglycemia in elective non-diabetic surgical patients: A prospective observational study. Can. J. Anaesth. 2016, 63, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Koumpan, Y.; VanDenKerkhof, E.; van Vlymen, J. An observational cohort study to assess glycosylated hemoglobin screening for elective surgical patients. Can. J. Anaesth. 2014, 61, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.; Massey, F. Introduction to Statistical Analysis; McGraw-Hill: New York City, NY, USA, 1983. [Google Scholar]
- Clopper, C.J.; Pearson, E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Koenker, R.; Bassett, G. Regression Quantiles. Econometrica 1978, 46, 33–50. [Google Scholar] [CrossRef]
- Bardia, A.; Khabbaz, K.; Mueller, A.; Mathur, P.; Novack, V.; Talmor, D.; Subramaniam, B. The Association between Preoperative Hemoglobin A1C and Postoperative Glycemic Variability on 30-Day Major Adverse Outcomes Following Isolated Cardiac Valvular Surgery. Anesth. Analg. 2017, 124, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Gianchandani, R.Y.; Saberi, S.; Zrull, C.A.; Patil, P.V.; Jha, L.; Kling-Colson, S.C.; Gandia, K.G.; DuBois, E.C.; Plunkett, C.D.; Bodnar, T.W.; et al. Evaluation of Hemoglobin A1c Criteria to Assess Preoperative Diabetes Risk in Cardiac Surgery Patients. Diabetes Technol. Ther. 2011, 13, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- McGinn, J.T.; A Shariff, M.; Bhat, T.M.; Azab, B.; Molloy, W.J.; Quattrocchi, E.; Farid, M.; Eichorn, A.M.; Dlugacz, Y.D.; A Silverman, R. Prevalence of Dysglycemia Among Coronary Artery Bypass Surgery Patients with No Previous Diabetic History. J. Cardiothorac. Surg. 2011, 6, 104. [Google Scholar] [CrossRef]
- Narayan, P.; Kshirsagar, S.N.; Mandal, C.K.; Ghorai, P.A.; Rao, Y.M.; Das, D.; Saha, A.; Chowdhury, S.R.; Rupert, E.; Das, M. Preoperative Glycosylated Hemoglobin: A Risk Factor for Patients Undergoing Coronary Artery Bypass. Ann. Thorac. Surg. 2017, 104, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, J.D.; Lepkowsky, E.R.; Callari, M.M.; Jordan, E.T.; Koenig, J.A.; Sirounian, G.H. The Prevalence of Diabetes Mellitus and Routine Hemoglobin A1c Screening in Elective Total Joint Arthroplasty Patients. J. Arthroplast. 2017, 32, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.I.; Kong, A.; Churilov, L.; Nanayakkara, N.; Chiu, W.L.; Sumithran, P.; Djukiadmodjo, F.; Premaratne, E.; Owen-Jones, E.; Hart, G.K.; et al. Using Automated HbA1c Testing to Detect Diabetes Mellitus in Orthopedic Inpatients and Its Effect on Outcomes. PLoS ONE 2017, 12, e0168471. [Google Scholar] [CrossRef]
- Stenberg, E.; Szabo, E.; Näslund, I. Is glycosylated hemoglobin A1 c associated with increased risk for severe early postoperative complications in nondiabetics after laparoscopic gastric bypass? Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2014, 10, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Walędziak, M.; Hady, H.R.; Czerniawski, M.; Proczko-Stepaniak, M.; Szymański, M.; Dowgiałło-Wnukiewicz, N.; Kozera, P.; Szeliga, J.; Orłowski, M.; et al. Type 2 Diabetes Mellitus and Preoperative HbA1c Level Have no Consequence on Outcomes after Laparoscopic Sleeve Gastrectomy—A Cohort Study. Obes. Surg. 2019, 29, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, C.J.; Liang, M.K.; Nguyen, M.T.; Nguyen, D.H.; Holihan, J.L.; Alawadi, Z.M.; Roth, J.S.; Wray, C.J.; Ko, T.C.; Kao, L.S. Preoperative Glycosylated Hemoglobin and Postoperative Glucose Together Predict Major Complications after Abdominal Surgery. J. Am. Coll. Surg. 2015, 221, 854–861e1. [Google Scholar] [CrossRef] [PubMed]
- Hjellestad, I.D.; Søfteland, E.; Husebye, E.S.; Jonung, T. HbA1c predicts long-term postoperative mortality in patients with unknown glycemic status at admission for vascular surgery: An exploratory study. J. Diabetes 2019, 11, 466–476. [Google Scholar] [CrossRef]
- Carson, A.P.; Reynolds, K.; Fonseca, V.A.; Muntner, P. Comparison of A1C and Fasting Glucose Criteria to Diagnose Diabetes Among U.S. Adults. Diabetes Care 2010, 33, 95–97. [Google Scholar] [CrossRef]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Gregg, E.W.; Ford, E.S.; Geiss, L.S.; Bainbridge, K.E.; Fradkin, J.E. Prevalence of Diabetes and High Risk for Diabetes Using A1C Criteria in the U.S. Population in 1988–2006. Diabetes Care 2010, 33, 562–568. [Google Scholar] [CrossRef]
- Iavazzo, C.; McComiskey, M.; Datta, M.; Ryan, M.; Kiernan, J.; Winter-Roach, B.; Slade, R.; Smith, M. Preoperative HBA1c and risk of postoperative complications in patients with gynaecological cancer. Arch. Gynecol. Obstet. 2016, 294, 161–164. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; A Thorell, A.; Soop, M.; O Ljungqvist, O.; Nygren, J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br. J. Surg. 2009, 96, 1358–1364. [Google Scholar] [CrossRef]
- Hudson, C.C.C.; for members of the Cardiothoracic Anesthesiology Research Endeavors (C.A.R.E.) Group; Welsby, I.J.; Phillips-Bute, B.; Mathew, J.P.; Lutz, A.; Hughes, G.C.; Stafford-Smith, M. Glycosylated hemoglobin levels and outcome in non-diabetic cardiac surgery patients. Can. J. Anaesth. 2010, 57, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, F.; Santarpino, G.; Gatti, G.; Reichart, D.; Onorati, F.; Faggian, G.; Dalén, M.; Khodabandeh, S.; Fischlein, T.; Maselli, D.; et al. Utility of glycated hemoglobin screening in patients undergoing elective coronary artery surgery: Prospective, cohort study from the E-CABG registry. Int. J. Surg. 2018, 53, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kocogulları, C.U.; Kunt, A.T.; Aksoy, R.; Duzyol, C.; Parlar, H.; Saskın, H.; Fındık, O. Hemoglobin A1c Levels Predicts Acute Kidney Injury after Coronary Artery Bypass Surgery in Non-Diabetic Patients. Rev. Bras. Cir. Cardiovasc. 2017, 32, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Perrotti, A.; Reichart, D.; Maschietto, L.; Onorati, F.; Chocron, S.; Dalén, M.; Svenarud, P.; Faggian, G.; Santarpino, G.; et al. Glycated Hemoglobin and Risk of Sternal Wound Infection After Isolated Coronary Surgery. Circ. J. 2016, 81, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Walid, M.S.; Newman, B.F.; Yelverton, J.C.; Nutter, J.P.; Ajjan, M.; Robinson, J.S. Prevalence of previously unknown elevation of glycosylated hemoglobin in spine surgery patients and impact on length of stay and total cost. J. Hosp. Med. 2010, 5, E10–E14. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Type 2 Diabetes: Prevention in People at High Risk (NICE Guideline PH38), (Update 2017); National Institute for Health and Care Excellence: Mancchester, UK, 2017. [Google Scholar]
- World Health Organization (WHO). International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia : Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Kahn, R.; Alperin, P.; Eddy, D.; Borch-Johnsen, K.; Buse, J.; Feigelman, J.; Gregg, E.; Holman, R.R.; Kirkman, M.S.; Stern, M.; et al. Age at initiation and frequency of screening to detect type 2 diabetes: A cost-effectiveness analysis. Lancet 2010, 375, 1365–1374. [Google Scholar] [CrossRef]
- Hulst, A.H.; Polderman, J.A.W.; Kooij, F.O.; Vittali, D.; Lirk, P.; Hollmann, M.W.; DeVries, J.H.; Preckel, B.; Hermanides, J. Comparison of perioperative glucose regulation in patients with type 1 vs type 2 diabetes mellitus: A retrospective cross-sectional study. Acta Anaesthesiol. Scand. 2019, 63, 314–321. [Google Scholar] [CrossRef]

| Characteristic | All Patients (n = 697) | No DM (n = 372) | Prediabetes (n = 299) | Undiagnosed DM (n = 26) |
|---|---|---|---|---|
| Age, years | 73 (6) | 72 (5) | 73 (6) | 76 (7) |
| Sex, female, n | 323 (46.3%) | 169 (45.4%) | 143 (47.8%) | 11 (42.3%) |
| BMI, kg m−2 | 26 (4) | 26 (4) | 26 (4) | 27 (4) |
| ASA score, n | ||||
| 1 or 2 | 473 (67.9%) | 260 (69.9%) | 197 (65.9%) | 16 (61.5%) |
| ≥3 | 224 (32.1%) | 112 (30.1%) | 102 (34.1%) | 10 (38.5%) |
| Maximum MET, n | ||||
| <7 | 240 (39.5%) | 126 (38.8%) | 104 (39.8%) | 10 (47.6%) |
| ≥7 | 367 (60.5%) | 199 (61.2%) | 157 (60.2%) | 11 (52.4%) |
| Cardiovascular history, n | ||||
| HT | 317 (45.5%) | 160 (43.0%) | 141 (47.2%) | 16 (61.5%) |
| IHD | 96 (13.8%) | 40 (10.8%) | 50 (16.7%) | 6 (23.1%) |
| CVA/TIA | 76 (10.9%) | 43 (11.6%) | 29 (9.7%) | 4 (15.4%) |
| PVD | 25 (3.6%) | 11 (3.0%) | 14 (4.7%) | 0 |
| Surgical specialty, n | ||||
| Gynaecology | 49 (7.0%) | 26 (7.0%) | 22 (7.4%) | 1 (3.8%) |
| Gastrointestinal | 95 (13.6%) | 54 (14.5%) | 38 (12.7%) | 3 (11.5%) |
| Orthopaedic | 106 (15.2%) | 62 (16.7%) | 40 (13.4%) | 4 (15.4%) |
| Urology | 62 (8.9%) | 36 (9.7%) | 23 (7.7%) | 3 (11.5%) |
| Neurosurgery | 37 (5.3%) | 19 (5.1%) | 16 (5.4%) | 2 (7.7%) |
| Other | 349 (49.9%) | 175 (47.0%) | 160 (53.5%) | 13 (50.0%) |
| Surgical risk, n | ||||
| Minor | 418 (60.0%) | 224 (60.2%) | 177 (59.2%) | 17 (65.4%) |
| Moderate | 220 (31.6%) | 121 (32.5%) | 92 (30.8%) | 7 (26.9%) |
| Major | 59 (8.5%) | 27 (7.3%) | 30 (10.0%) | 2 (7.7%) |
| Anaesthesia type, n | ||||
| General | 594 (85.3%) | 319 (85.8%) | 253 (84.6%) | 22 (88.0%) |
| Neuraxial | 36 (5.2%) | 20 (5.4%) | 15 (5.0%) | 1 (4.0%) |
| PNB | 24 (3.4%) | 15 (4.0%) | 9 (3.0%) | 0 |
| Sedation | 42 (6.0%) | 18 (4.9%) | 22 (7.4%) | 2 (8.0%) |
| HbA1c (mmol mol−1) | HbA1c (%) | n | Prevalence (%) | 95% Confidence Interval | |
|---|---|---|---|---|---|
| No diabetes | <39 | <5.7% | 372 | 53.4 | 49.6 to 57.1 |
| Prediabetes | ≥39 and <48 | 5.7–6.5% | 299 | 42.9 | 39.2 to 46.7 |
| Diabetes | ≥48 | 6.5% | 26 | 3.7 | 2.5 to 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Wilpe, R.; van Zuylen, M.L.; Hermanides, J.; DeVries, J.H.; Preckel, B.; Hulst, A.H. Preoperative Glycosylated Haemoglobin Screening to Identify Older Adult Patients with Undiagnosed Diabetes Mellitus—A Retrospective Cohort Study. J. Pers. Med. 2024, 14, 219. https://doi.org/10.3390/jpm14020219
van Wilpe R, van Zuylen ML, Hermanides J, DeVries JH, Preckel B, Hulst AH. Preoperative Glycosylated Haemoglobin Screening to Identify Older Adult Patients with Undiagnosed Diabetes Mellitus—A Retrospective Cohort Study. Journal of Personalized Medicine. 2024; 14(2):219. https://doi.org/10.3390/jpm14020219
Chicago/Turabian Stylevan Wilpe, Robert, Mark L. van Zuylen, Jeroen Hermanides, J. Hans DeVries, Benedikt Preckel, and Abraham H. Hulst. 2024. "Preoperative Glycosylated Haemoglobin Screening to Identify Older Adult Patients with Undiagnosed Diabetes Mellitus—A Retrospective Cohort Study" Journal of Personalized Medicine 14, no. 2: 219. https://doi.org/10.3390/jpm14020219