Advances in Urinary Diversion: From Cutaneous Ureterostomy to Orthotopic Neobladder Reconstruction—A Comprehensive Review
Abstract
1. Introduction
2. Non-Continent Urinary Diversion
2.1. Cutaneous Ureterostomy
2.2. Ileal Conduit
2.3. Jejunal, Gastric and Colonic Conduit
3. Continent Urinary Diversions
3.1. Ureterosigmoidostomy
3.2. Kock Pouch
3.3. Mainz Pouch
3.4. Cologne Pouch
3.5. Penn Pouch
3.6. Indiana Pouch
3.7. Florida Pouch
3.8. Miami Pouch
3.9. Abol-Enein Pouch
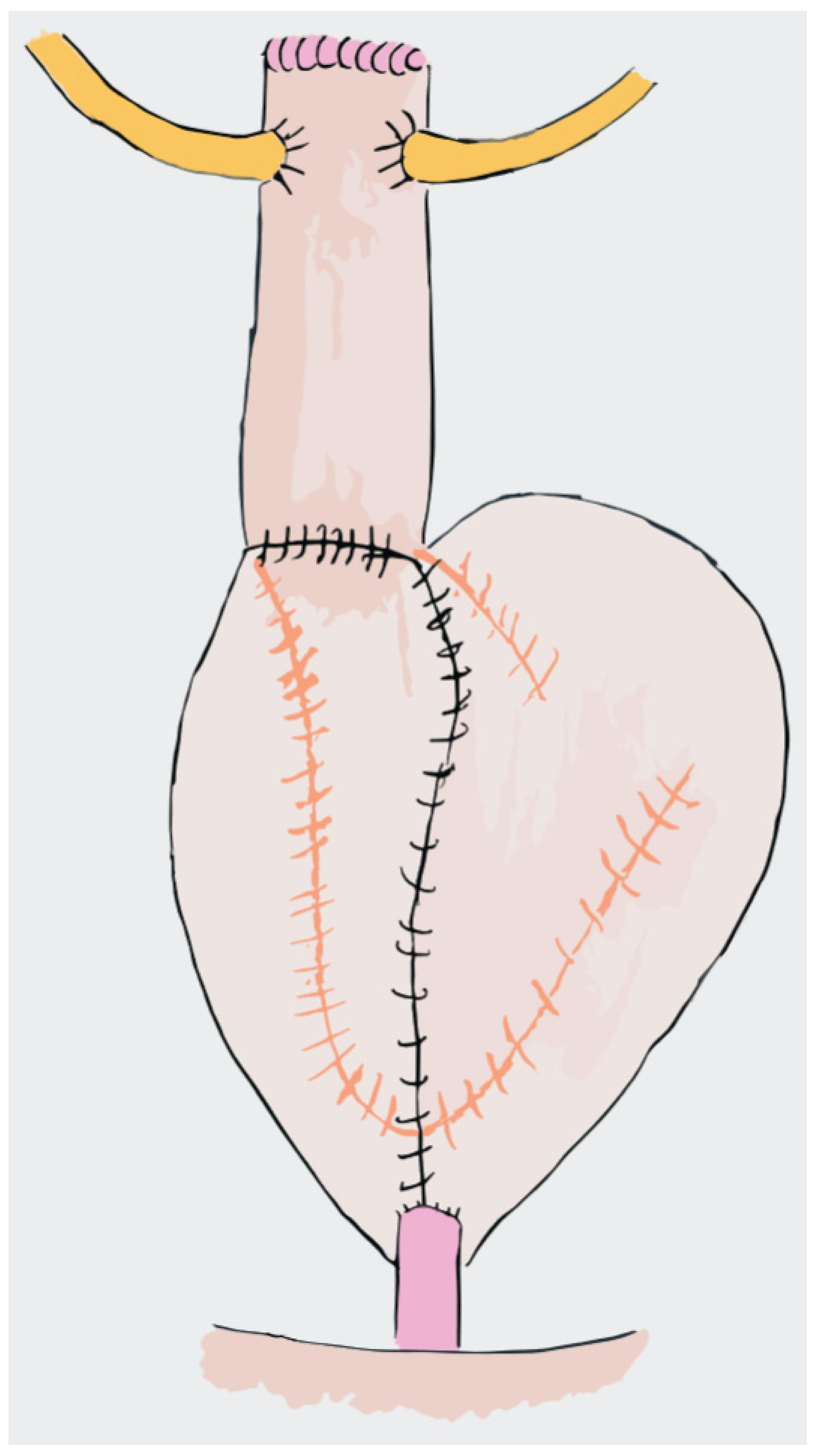
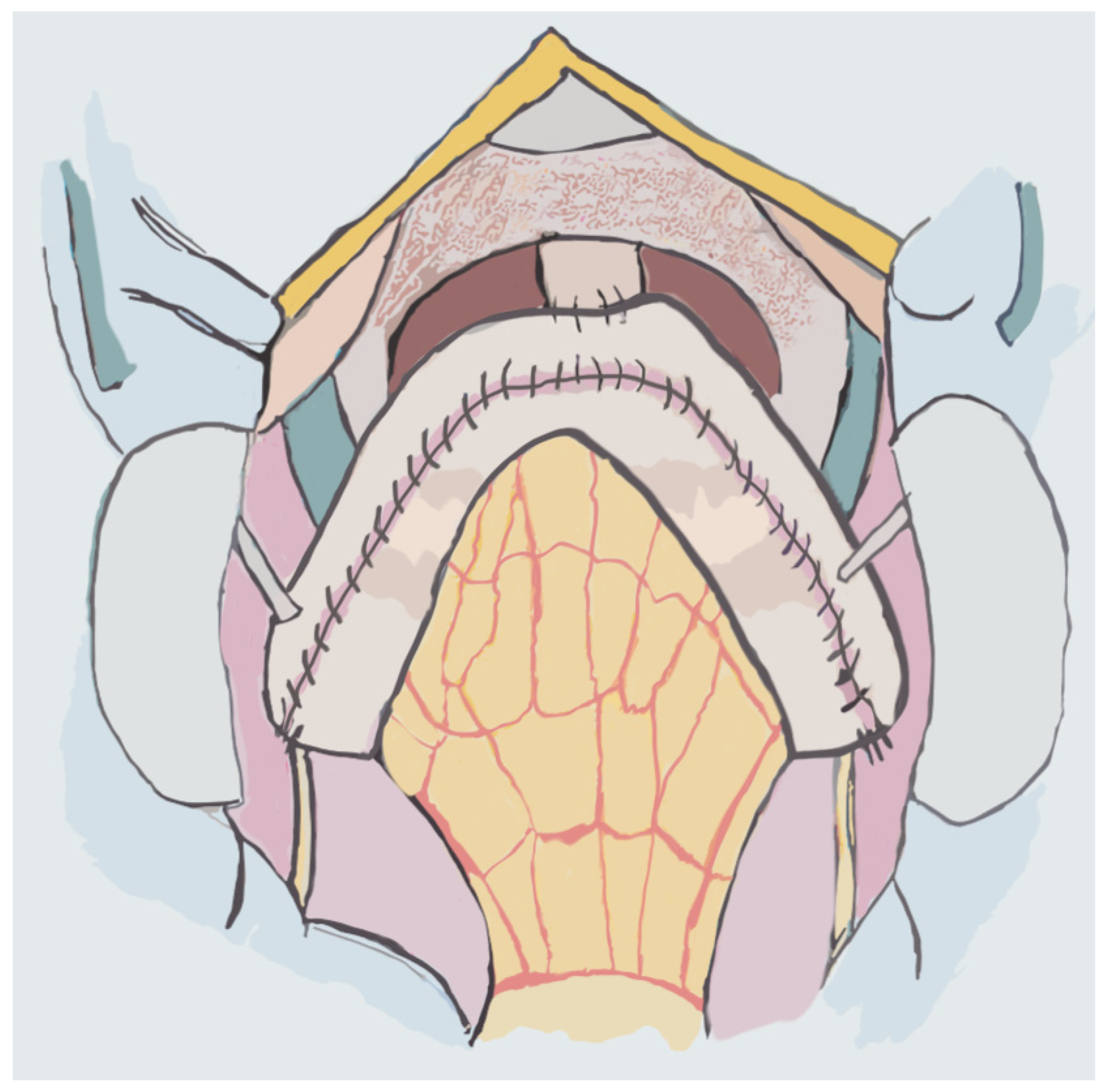
4. Neobladders
4.1. Studer Neobladder
4.2. Hautmann Neobladder
4.3. Kock Ileal Neobladder
4.4. Padua Ileal Neobladder
4.5. Y-Shaped Neobladder
4.6. Camey I-II Neobladder
4.7. U-Shaped Neobladder
4.8. Z-Shaped Neobladder
4.9. Anatolian Ileal Neobladder
4.10. Shell Neobladder
4.11. Florin Neobladder
4.12. Vesuvian Orthotopic Neobladder
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Jubber, I.; Ong, S.; Bukavina, L.; Black, P.C.; Compérat, E.; Kamat, A.M.; Kiemeney, L.; Lawrentschuk, N.; Lerner, S.P.; Meeks, J.J.; et al. Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Eur. Urol. 2023, 84, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Halaseh, S.A.; Halaseh, S.; Alali, Y.; Ashour, M.E.; Alharayzah, M.J. A Review of the Etiology and Epidemiology of Bladder Cancer: All You Need To Know. Cureus 2022, 14, e27330. [Google Scholar] [CrossRef] [PubMed]
- Caballero, J.M.; Gili, J.M.; Pereira, J.C.; Gomáriz, A.; Castillo, C.; Martín-Baranera, M. Risk Factors Involved in the High Incidence of Bladder Cancer in an Industrialized Area in North-Eastern Spain: A Case–Control Study. J. Clin. Med. 2023, 12, 728. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Pandolfo, S.D.; Aveta, A.; Martino, R.; Trama, F.; Caputo, V.F.; Barone, B.; Abate, M.; Sicignano, E.; Cilio, S.; et al. A Comparative Study of the Triglycerides/HDL Ratio and Pseudocholinesterase Levels in Patients with Bladder Cancer. Diagnostics 2022, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Finati, M.; Cinelli, F.; Fanelli, A.; Del Giudice, F.; De Berardinis, E.; Sciarra, A.; Russo, G.; Mancini, V.; D’Altilia, N.; et al. Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J. Pers. Med. 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Aveta, A.; Cacciapuoti, C.; Barone, B.; Di Zazzo, E.; Del Giudice, F.; Maggi, M.; Ferro, M.; Terracciano, D.; Busetto, G.M.; Lucarelli, G.; et al. The Impact of Meat Intake on Bladder Cancer Incidence: Is It Really a Relevant Risk? Cancers 2022, 14, 4775. [Google Scholar] [CrossRef]
- Ferro, M.; Chiujdea, S.; Musi, G.; Lucarelli, G.; Del Giudice, F.; Hurle, R.; Damiano, R.; Cantiello, F.; Mari, A.; Minervini, A.; et al. Impact of Age on Outcomes of Patients With Pure Carcinoma In Situ of the Bladder: Multi-Institutional Cohort Analysis. Clin. Genitourin. Cancer 2022, 20, e166–e172. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Kwon, C.S.; Forsythe, A.; Mamolo, C.M.; Masters, E.T.; Jacobs, I.A. Humanistic and Economic Burden of Non-Muscle Invasive Bladder Cancer: Results of Two Systematic Literature Reviews. Clin. Outcomes Res. 2020, 12, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.D.; Lee, D.J.; Aggarwal, S. Urinary Diversion: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 78, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Bricker, E.M. Bladder Substitution after Pelvic Evisceration. Surg. Clin. N. Am. 1950, 30, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Stangl, F.P.; Thalmann, G.N. Continent Diversion: Five Decades of Developments and Evolution. BJU Int. 2020, 126, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Pyrgidis, N.; Sokolakis, I.; Haltmair, G.; Hatzichristodoulou, G. The Effect of Urinary Diversion on Renal Function after Cystectomy for Bladder Cancer: Comparison between Ileal Conduit, Orthotopic Ileal Neobladder, and Heterotopic Ileocecal Pouch. World J. Urol. 2022, 40, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Check, D.K.; Leo, M.C.; Banegas, M.P.; Bulkley, J.E.; Danforth, K.N.; Gilbert, S.M.; Kwan, M.L.; Rosetti, M.O.; McMullen, C.K. Decision Regret Related to Urinary Diversion Choice among Patients Treated with Cystectomy. J. Urol. 2020, 203, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.L.; Yu, H.-Y.; Setodji, C.; Hanley, J.M.; Litwin, M.S.; Saigal, C.S. Urologic Diseases in America Project Urinary Diversion and Morbidity after Radical Cystectomy for Bladder Cancer. Cancer 2010, 116, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Ellinger, J.; Koeditz, B.; Heidenreich, A.; Hoffmann, M.A. Predictors for Outcome and Complications Related to Urinary Diversion. Anticancer Res. 2021, 41, 5585–5591. [Google Scholar] [CrossRef] [PubMed]
- Presicce, F.; Leonardo, C.; Tuderti, G.; Brassetti, A.; Mastroianni, R.; Bove, A.; Misuraca, L.; Anceschi, U.; Ferriero, M.; Gallucci, M.; et al. Late Complications of Robot-Assisted Radical Cystectomy with Totally Intracorporeal Urinary Diversion. World J. Urol. 2021, 39, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Hatakeyama, S.; Momota, M.; Soma, O.; Hamano, I.; Tanaka, T.; Iwamura, H.; Fujita, N.; Okamoto, T.; Yamamoto, H.; et al. Frailty Is Significantly Associated with the Type of Urinary Diversion in Patients with Muscle-Invasive Bladder Cancer. Int. J. Urol. 2020, 27, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Tatenuma, T.; Tsutsumi, S.; Yasui, M.; Noguchi, G.; Umemoto, S.; Kishida, T. Outcome of Palliative Urinary Diversion and Observation for Malignant Extrinsic Ureteral Obstruction. J. Palliat. Med. 2020, 23, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, C.L.; Lima, E. Urinary Diversions for Radical Cystectomy: A Review of Complications and Their Management. Mini-Invasive Surg. 2021, 5, 28. [Google Scholar] [CrossRef]
- Borzelli, A.; Giurazza, F.; Corvino, F.; De Magistris, G.; Cavaglià, E.; Pane, F.; Coppola, M.; Amodio, F.; Silvestre, M.; Niola, R. Successful Endovascular Treatment of Massive Hematuria Due to Ureteroarterial Fistula Secondary to Ureterocutaneostomy. J. Radiol. Rev. 2021, 8, 51–54. [Google Scholar] [CrossRef]
- Pastore, A.L.; Fuschi, A.; Martoccia, A.; Capone, L.; Velotti, G.; Suraci, P.P.; Silvio, S.; Maggi, M.; Al, S.Y.; Carbone, A. Mp53-06 Quality of Life in Elderly Patients after Minimally Invasive Radical Cystectomy: Comparison Study between Single Stoma Uretero-Cutaneostomy and Intracorporeal Ileal Conduit Urinary Diversion. J. Urol. 2021, 206, e940–e941. [Google Scholar] [CrossRef]
- Asimakidou, M.; De Win, G.; Cherian, A. Laparoscopy-Assisted Ureterostomy— Technique. J. Pediatr. Urol. 2019, 15, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Lowrance, W.T.; Rumohr, J.A.; Clark, P.E.; Chang, S.S.; Smith, J.A.; Cookson, M.S. Urinary Diversion Trends at a High Volume, Single American Tertiary Care Center. J. Urol. 2009, 182, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Tyson, M.D.; Chang, S.S. Conduit Urinary Diversion. Urol. Clin. N. Am. 2018, 45, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, W.F.; Clarke, B.G. Five Years’ Experience with Uretero-Enterostomy by the Combined Technique. J. Urol. 1955, 73, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Villagrá, I.; Gauroy, E.; Ricotta, G.; Navarro, A.S.; Ferron, G.; Martinez, A. 841 How to Improve Bricker Ileal Conduit Functionality. Int. J. Gynecol. Cancer 2024, 34. [Google Scholar] [CrossRef]
- Demaegd, L.; Albersen, M.; Muilwijk, T.; Milenkovic, U.; Moris, L.; Everaerts, W.; Van Poppel, H.; Van der Aa, F.; Joniau, S.; Akand, M. Comparison of Postoperative Complications of Ileal Conduits versus Orthotopic Neobladders. Transl. Androl. Urol. 2020, 9, 2541–2554. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.; Lawrentschuk, N.; Jack, G.S.; Davis, N.F. A Systematic Review and Meta-Analysis of the Long-Term Outcomes of Ileal Conduit and Orthotopic Neobladder Urinary Diversion. Can. Urol. Assoc. J. 2021, 15, E48–E57. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hong, B.; Park, J.-Y.; Lee, Y.; Hwang, J.-H.; Kong, Y.-G.; Kim, Y.-K. Comparison of a Significant Decline in the Glomerular Filtration Rate between Ileal Conduit and Ileal Neobladder Urinary Diversions after Radical Cystectomy: A Propensity Score-Matched Analysis. J. Clin. Med. 2020, 9, 2236. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Inoue, T.; Kawakita, M.; Ito, K.; Okumura, K.; Yamada, H.; Kubota, M.; Fujii, M.; Shimizu, Y.; Yatsuda, J.; et al. Perioperative and Oncological Outcomes of Laparoscopic Radical Cystectomy with Intracorporeal versus Extracorporeal Ileal Conduit: A Matched-Pair Comparison in a Multicenter Cohort in Japan. Int. J. Urol. 2020, 27, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.N.; Medina, L.G.; Aron, M. Robotic-Assisted Ileal Conduit Urinary Diversion. In Techniques of Robotic Urinary Tract Reconstruction: A Complete Approach; Stifelman, M.D., Zhao, L.C., Eun, D.D., Koh, C.J., Eds.; Springer International Publishing: Cham, Switerlands, 2022; pp. 295–310. ISBN 978-3-030-50196-9. [Google Scholar]
- Boehm, D.; Rosenfeld, J.; Ji, E.; Lee, Z. A Review of Bowel-Based Urinary Diversions for the Colorectal Surgeon. Semin. Colon Rectal Surg. 2023, 34, 100960. [Google Scholar] [CrossRef]
- Aoki, T.; Furuya, R.; Fukasawa, M.; Nozawa, M.; Takihana, Y.; Sudoh, M.; Nakagomi, H. Bladder Cancer with Urinary Diversion by a Sigmoid Colon Conduit after Transverse Colon Stoma. IJU Case Rep. 2024, 7, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Hebert, K.; Matta, R.; Fendereski, K.; McCormick, B.; Myers, J. V01-01 Colon Conduit Urinary Diversion: Considerations for the Reconstructive Urologist. J. Urol. 2022, 207, e52. [Google Scholar] [CrossRef]
- Klein, E.A.; Montie, J.E.; Montague, D.K.; Kay, R.; Straffon, R.A. Jejunal Conduit Urinary Diversion. J. Urol. 1986, 135, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kanematsu, A.; Tomono, M.; Kataoka, K.; Beppu, N.; Uchino, M.; Shinohara, H.; Ikeuchi, H.; Yamamoto, S.; Ikeda, M. Urinary Tract Diversion with Gastric Conduit after Total Pelvic Exenteration for Crohn’s Disease-Related Anorectal Cancer: A Case Report. Surg. Case Rep. 2022, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M.; Del Prete, L.; D’amico, G.; Eltemamy, M.; Akshelyan, S.; Maklad, M.; Osman, M.; Wee, A.; Viadya, A.; Abu-Elmagd, K. P-70: Native Jejunal Conduit for Urinary Diversion in Enbloc Liver-Intestine-Kidney Transplantation for a Patient with Irradiation Enteritis and Cystitis. Transplantation 2021, 105, S85. [Google Scholar] [CrossRef]
- Miller, D.T.; Maganty, A.; Theisen, K.M.; Hrebinko, R. Novel Creation of a Noneverted Stoma During Ileal Conduit Urinary Diversion: Technique and Short-Term Outcomes. Urology 2020, 146, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Keady, C.; Hechtl, D.; Joyce, M. When the Bowel Meets the Bladder: Optimal Management of Colorectal Pathology with Urological Involvement. World J. Gastrointest. Surg. 2020, 12, 208–225. [Google Scholar] [CrossRef]
- Rezaee, M.E.; Atwater, B.L.; Bihrle, W.; Schroeck, F.R.; Seigne, J.D. Ileal Conduit Versus Continent Urinary Diversion in Radical Cystectomy: A Retrospective Cohort Study of 30-Day Complications, Readmissions, and Mortality. Urology 2022, 170, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F. Cystectomy and Urinary Diversion–Surgical Considerations.|Urologic Nursing|EBSCOhost. Available online: https://openurl.ebsco.com/contentitem/doi:10.7257%2F1053-816X.2021.41.5.272?sid=ebsco:plink:crawler&id=ebsco:doi:10.7257%2F1053-816X.2021.41.5.272 (accessed on 29 March 2024).
- von Knobloch, R. Radical Cystectomy and Urinary Diversion in Women. Transl. Androl. Urol. 2023, 12, 155–157. [Google Scholar] [CrossRef]
- Przydacz, M.; Corcos, J. Revisiting Ureterosigmoidostomy, a Useful Technique of Urinary Diversion in Functional Urology. Urology 2018, 115, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, A.; Jedidi, L.; Saadi, A.; Essid, N.; Ben Lahouel, S.; Karoui, Y.; Ben Moussa, M. Sigmoid Adenocarcinoma Following Ureterosigmoidostomy: A Known Complication of a No Longer Known Procedure. Int. Urol. Nephrol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.; Fernstrum, A.; Sharma, T.; Mishra, K.; Bukavina, L.; Prunty, M.; Bodner, D. Cutaneous Paraneoplastic Presentation of Colon Cancer in a Patient with History of Ureterosigmoidostomy. Curr. Urol. 2022. [Google Scholar] [CrossRef]
- Niwa, H.; Fukasawa, H.; Kaneko, M.; Matsuyama, T.; Yasuda, H.; Furuya, R. Hypokalemia-Associated Paralysis and Metabolic Acidosis in a Patient with Bilateral Ureterosigmoidostomy. CEN Case Rep. 2015, 5, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Beran, A.; Burlen, J.; Ghazaleh, S.; Aburayyan, K.; Patel, D.; Nehme, C.; Sharma, S.; Ahmed, Z.; Srour, O.; Kobeissy, A. S1689 A Late-Delayed Periodic Hyperammonemic Encephalopathy and Normal Anion Gap Metabolic Acidosis Secondary to Ureterosigmoidostomy. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, S869. [Google Scholar] [CrossRef]
- Bindi, E.; Ilari, M.; Torino, G.; Mariscoli, F.; Nino, F.; Cobellis, G.; Martino, A. Detubularized Ureterosigmoidostomy for the Creation of Continent Neobladder in Children: Cases Report and Review of the Literature. Children 2021, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Soundharya, S.; Sanghvi, B.V.; Mudkhedkar, K.P.; Shah, R.S.; Makhija, D.P.; Mhaskar, S.S.; Khidtta, K.K.; Parelkar, S.V. Detubularized Isolated Ureterosigmoidostomy in a Complicated Common Cloaca: A Case Report. Pediatr. Urol. Case Rep. 2020, 7, 71–76. [Google Scholar] [CrossRef]
- Khan, M.N.; Naqvi, A.H.; Lee, R.E. Carcinoma of Sigmoid Colon Following Urinary Diversion: A Case Report and Review of Literature. World J. Surg. Oncol. 2004, 2, 20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shuford, R.; Ashburn, J.H. Don’t Forget about the K-Pouch! Clin. Colon Rectal Surg. 2022, 35, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Crawshaw, A.; Williams, J.; Woodhouse, F. The Kock Pouch Reconsidered: An Alternative Surgical Technique. Br. J. Nurs. 2014, 23, S26–S29. [Google Scholar] [CrossRef] [PubMed]
- Bröder, S.; Jäger, W.; Thüroff, J.W.; Stein, R. Orthotopic MAINZ Pouch Bladder Substitution—Long-Term Follow-Up. Cent. Eur. J. Urol. 2021, 74, 235–240. [Google Scholar] [CrossRef]
- Jing, S.; Yang, E.; Luo, Z.; Zhang, Y.; Ding, H.; Yang, L.; Dong, Z.; Shang, P.; Yue, Z.; Wu, G.; et al. Perioperative Outcomes and Continence Following Robotic-Assisted Radical Cystectomy with Mainz Pouch II Urinary Diversion in Patients with Bladder Cancer. BMC Cancer 2024, 24, 127. [Google Scholar] [CrossRef] [PubMed]
- Stakhovsky, O.E.; Tymoshenko, A.V.; Voilenko, O.A.; Vitruk, Y.V.; Kononenko, O.A.; Pikul, M.V.; Stakhovsky, E.O. Results of Mainz-Pouch II Transrectal Urinary Diversion from a Single Center. Urologiya 2020, 24. [Google Scholar] [CrossRef]
- Wang, P.; Xiao, S.; Fu, W.; Song, Y.; Sun, S.; Zhang, F.; Shen, D.; Zhu, J.; Wang, Z.; Chen, J.; et al. Robot-assisted Radical Cystectomy with Intracorporeal Mainz Ⅱ Rectosigmoid Pouch for Muscle-invasive Bladder Cancer. Int. J. Med. Robot. 2021, 17, e2284. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liu, J.; Wu, G.; Yang, S.; Luo, C.; Du, T.; Luo, Y.; Bao, J.; Tian, J.; Wang, Z.; et al. Comparison of Open and Intracorporeal Modified Ureterosigmoidostomy (Mainz II) after Laparoscopic Radical Cystectomy with Bladder Cancer. World J. Surg. Oncol. 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Winkler, A.; Vahdad, R.M.; Ekamp, A.; Boemers, T.M. The Cologne Pouch Procedure for Continent Anal Urinary Diversion in Children with Bladder Exstrophy–Epispadias Complex. J. Pediatr. Urol. 2018, 14, 431.e1–431.e6. [Google Scholar] [CrossRef] [PubMed]
- Kaefer, D.; Kim, J.-H. Bladder Reconstruction and Urinary Diversion. In Textbook of Female Urology and Urogynecology; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-314424-3. [Google Scholar]
- Ali-El-Dein, B. The Ureter and Urinary Diversion. In The Ureter: A Comprehensive Review; Abdel-Gawad, M., Ali-El-Dein, B., Barry, J., Stenzl, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 517–559. ISBN 978-3-031-36212-5. [Google Scholar]
- Delshad, S.; Rastad, H.; Mardi, P. Congenital Bladder and Urethral Agenesis: Two Case Reports and Management. Adv. Urol. 2020, 2020, e2782783. [Google Scholar] [CrossRef] [PubMed]
- Mukewar, S.; Hall, P.; Lashner, B.A.; Lopez, R.; Kiran, R.P.; Shen, B. Risk Factors for Nephrolithiasis in Patients with Ileal Pouches. J. Crohns Colitis 2013, 7, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, I.; Basic, D. Modified Mainz Pouch II (Sigma Rectum Pouch) Urinary Diversion: 12 Years Experience. Acta Chir. Iugosl. 2007, 54, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Rg, R. Present Experience with the Indiana Pouch. World J. Urol. 1996, 14, 92–98. [Google Scholar] [CrossRef]
- Llueca, A.; Maazouzi, Y.; Ponce, P.; Serra, A.; Garau, C.; Rodrigo, M. Step by Step Indiana Pouch Construction in a Previously Irradiated Patient with a Cervical Cancer Relapse. Int. J. Surg. Case Rep. 2019, 66, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Monn, M.F.; Kaimakliotis, H.Z.; Cary, K.C.; Pedrosa, J.A.; Flack, C.K.; Koch, M.O.; Bihrle, R. Short-Term Morbidity and Mortality of Indiana Pouch, Ileal Conduit, and Neobladder Urinary Diversion Following Radical Cystectomy. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.G.; Thrasher, J.B.; Park, G.Y.; Kueker, D.C.; Weigel, J.W. Long-Term Complications Related to the Modified Indiana Pouch. Urology 2002, 60, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Polm, P.D.; Wyndaele, M.I.A.; de Kort, L.M.O. Very Long-Term Follow-up of Indiana Pouches Proves Durability. Neurourol. Urodyn. 2023; early view. [Google Scholar] [CrossRef]
- Fu, H.; Davis, L.; Ramakrishnan, V.; Barefoot, T.; Sholtes, C.; Liang, L.; Said, M.; Messer, J. Identify Risk Factors for Perioperative Outcomes in Intracorporeal Urinary Diversion and Extracorporeal Urinary Diversion with Robotic Cystectomy. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2024, 50, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; Ruel, N.H.; Yamzon, J.; Zhumkhawala, A.-A.; Lau, C.S.; Yuh, B.E.; Chan, K.G. Indiana Pouch Continent Cutaneous Urinary Diversion After Robotic-Assisted Radical Cystectomy: A 16-Year Experience. Urology 2024, 183, e325–e327. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, J.L. Remodeled Right Colon: An Alternative Urinary Reservoir. J. Urol. 1987, 138, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Igel, D.A.; Chestnut, C.J.; Lee, E.K. Urinary Diversion and Reconstruction Following Radical Cystectomy for Bladder Cancer: A Narrative Review. AME Med. J. 2021, 6. [Google Scholar] [CrossRef]
- Baboudjian, M.; Gondran-Tellier, B.; Michel, F.; Abdallah, R.; Rouy, M.; Gaillet, S.; Sichez, P.C.; Boissier, R.; Bladou, F.; Lechevallier, E.; et al. Miami Pouch: A Simple Technique for Efficient Continent Cutaneous Urinary Diversion. Urology 2021, 152, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Pattou, M.; Baboudjian, M.; Pinar, U.; Parra, J.; Rouprêt, M.; Karsenty, G.; Phe, V. Continent Cutaneous Urinary Diversion with an Ileal Pouch with the Mitrofanoff Principle versus a Miami Pouch in Patients Undergoing Cystectomy for Bladder Cancer: Results of a Comparative Study. World J. Urol. 2022, 40, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; Angeles, M.A.; Martinez, A.; Ferron, G. Creation of a Miami Pouch in 10 Logical Steps. Gynecol. Oncol. 2018, 151, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Angeles, M.A.; Martínez-Gómez, C.; Martinez, A.; Ferron, G. Laparoscopic Hand-Assisted Miami Pouch after Pelvic Exenteration in 10 Steps. Gynecol. Oncol. 2018, 150, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Salom, E.M.; Mendez, L.E.; Schey, D.; Lambrou, N.; Kassira, N.; Gómez-Marín, O.; Averette, H.; Peñalver, M. Continent Ileocolonic Urinary Reservoir (Miami Pouch): The University of Miami Experience over 15 Years. Am. J. Obstet. Gynecol. 2004, 190, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Alliot, H.; Tapsoba, T.; Paye-Jaouen, A.; Ashkanani, Y.; Josset-Raffet, E.; Natio, L.; Peycelon, M.; El-Ghoneimi, A. A Catheterizable Serous-Lined Urinary Outlet Associated with the Ileal Bladder Augmentation Abol-Enein and Ghoneim Procedure: A Safe and Reliable Procedure in Children. Front. Pediatr. 2024, 12, 1273505. [Google Scholar] [CrossRef] [PubMed]
- Abol-Enein, H.; Salem, M.; Mesbah, A.; Abdel-Latif, M.; Kamal, M.; Shabaan, A.; Ghoneim, M. Continent Cutaneous Ileal Pouch Using the Serous Lined Extramural Valves. The Mansoura Experience in More than 100 Patients. J. Urol. 2004, 172, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Cicione, A.; De Nunzio, C.; Lombardo, R.; Trucchi, A.; Manno, S.; Lima, E.; Tubaro, A. Complications and Quality of Life of Ileal Conduit, Orthotopic Neobladder and Ureterocutaneostomy: Systematic Review of Reports Using the Clavien-Dindo Classification. Minerva Urol. Nefrol. 2020, 72, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Pronk, C.E.; Albers, L.F.; Kuijper, L.D.J.; Hendricksen, K.; Nicolai, M.P.J. Sexual Function after Radical Cystectomy in Males with Bladder Carcinoma: A Six-Year Longitudinal Single-Centre Study. Front. Urol. 2023, 3, 1100516. [Google Scholar] [CrossRef]
- Qu, L.G.; Lawrentschuk, N. Orthotopic Neobladder Reconstruction: Patient Selection And Perspectives. Res. Rep. Urol. 2019, 11, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Gakis, G.; Stenzl, A. Ileal Neobladder and Its Variants. Eur. Urol. Suppl. 2010, 9, 745–753. [Google Scholar] [CrossRef]
- Basford, J.R. The Law of Laplace and Its Relevance to Contemporary Medicine and Rehabilitation. Arch. Phys. Med. Rehabil. 2002, 83, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Colding-Jørgensen, M.; Poulsen, A.L.; Steven, K. Mechanical Characteristics of Tubular and Detubularised Bowel for Bladder Substitution: Theory, Urodynamics and Clinical Results. Br. J. Urol. 1993, 72, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Sidi, A.A.; Reinberg, Y.; Gonzalez, R. Influence of Intestinal Segment and Configuration on the Outcome of Augmentation Enterocystoplasty. J. Urol. 1986, 136, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, N.; Moon, A.; Thorpe, A.C. Metabolic Complications of Urinary Intestinal Diversion. Indian J. Urol. IJU J. Urol. Soc. India 2013, 29, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Urologic Robotic Surgery–Surgical Clinics. Available online: https://www.surgical.theclinics.com/article/S0039-6109(19)30161-6/abstract (accessed on 30 March 2024).
- Bansal, D.; Chaturvedi, S.; Maheshwari, R.; Kumar, A. Role of Laparoscopy in the Era of Robotic Surgery in Urology in Developing Countries. Indian J. Urol. 2021, 37, 32. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, R.; Tuderti, G.; Ferriero, M.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Misuraca, L.; Zampa, A.; Torregiani, G.; Covotta, M.; et al. Open vs Robotic Intracorporeal Padua Ileal Bladder: Functional Outcomes of a Single-Centre RCT. World J. Urol. 2023, 41, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Brassetti, A.; Cacciamani, G.; Anceschi, U.; Ferriero, M.; Tuderti, G.; Miranda, G.; Mastroianni, R.; Desai, M.; Aron, M.; Gill, I.; et al. Long-Term Oncologic Outcomes of Robot-Assisted Radical Cystectomy (RARC) with Totally Intracorporeal Urinary Diversion (ICUD): A Multi-Center Study. World J. Urol. 2020, 38, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Studer, U.E.; Ackermann, D.; Casanova, G.A.; Zingg, E.J. Three Years’ Experience with an Ileal Low Pressure Bladder Substitute. Br. J. Urol. 1989, 63, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.N.; Adding, L.C.; Hosseini, A.; Schumacher, M.C.; Volz, D.; Nilsson, A.; Carlsson, S.; Wiklund, N.P. Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion in Patients with Transitional Cell Carcinoma of the Bladder. Eur. Urol. 2011, 60, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Lavallée, E.; Wiklund, P. The Studer Neobladder: An Established and Reproducible Technique for Intracorporeal Urinary Diversion. Eur. Urol. Open Sci. 2021, 35, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, R.E. Surgery Illustrated–Surgical Atlas. BJU Int. 2010, 105, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Salihagic, I.K.; Hrkac, A.; Ovcaricek, S.; Bokarica, P.; Gilja, I. Outcome of Small versus Big Capacity Hautmann Neobladder Reconstruction: A Prospective Randomized Study—A 5-Year Follow Up. Technol. Health Care 2024, 32, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, J.; He, P.; Zhang, J.; Wang, C.; Zheng, J.; Li, X.; Lang, L.; Zhou, Z.; Chen, Z. Refinement Surgical Technique, and Perioperative and Functional Outcomes in Patients With Robotic Intracorporeal Hautmann Orthotopic Neobladder. Urology 2020, 138, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, R.E.; Volkmer, B.; Egghart, G.; Frohneberg, D.; Gottfried, H.W.; Gschwend, J.; Hefty, R.; Kleinschmidt, K.; Küfer, R.; Miller, K.; et al. Functional Outcome and Complications Following Ileal Neobladder Reconstruction in Male Patients without Tumor Recurrence. More than 35 Years of Experience from a Single Center. J. Urol. 2021, 205, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Nouhaud, F.X.; Williams, M.; Yaxley, W.; Cho, J.; Perera, M.; Thangasamy, I.; Esler, R.; Coughlin, G. Robot-Assisted Orthotopic “W” Ileal Neobladder in Male Patients: Step-by-Step Video-Illustrated Technique and Preliminary Outcomes. J. Robot. Surg. 2020, 14, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Pardalidis, P.; Andriopoulos, N.; Pardalidis, N.; Pardalidis, P.; Andriopoulos, N.; Pardalidis, N. Robotic Orthotopic Neobladder: The Two Chimney Technique. In Modern Approach to Diagnosis and Treatment of Bladder Cancer; IntechOpen: London, UK, 2021; ISBN 978-1-83969-191-1. [Google Scholar]
- Locke, J.A.; Neu, S.; Herschorn, S. Diagnosis and Management of Kock Afferent Nipple Valve Obstruction. Urology 2021, 152, 173–177. [Google Scholar] [CrossRef]
- Pagano, F.; Artibani, W.; Ligato, P.; Piazza, R.; Garbeglio, A.; Passerini, G. Vescica Ileale Padovana:A Technique for Total Bladder Replacement. Eur. Urol. 1990, 17, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Papalia, R.; Misuraca, L.; Tuderti, G.; Minisola, F.; Ferriero, M.; Vallati, G.; Guaglianone, S.; Gallucci, M. Robotic Intracorporeal Padua Ileal Bladder: Surgical Technique, Perioperative, Oncologic and Functional Outcomes. Eur. Urol. 2018, 73, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; De Marco, V.; Sebben, M.; Rizzetto, R.; Cerruto, M.A.; Porcaro, A.B.; Gill, I.S.; Artibani, W. Robot-Assisted Vescica Ileale Padovana: A New Technique for Intracorporeal Bladder Replacement Reproducing Open Surgical Principles. Eur. Urol. 2019, 76, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Anceschi, U.; DI Maida, F.; Flammia, R.S.; Bigazzi, B.; Grosso, A.A.; Fede Spicchiale, C.; Mari, A.; Brassetti, A.; Tuderti, G.; Ferriero, M.C.; et al. Robotic Intracorporeal Padua Ileal Neobladder vs. Florin Pouch: Comparison of Mid-Term Urodynamic and Functional Profiles. Minerva Urol. Nephrol. 2022, 74, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; Angeles, M.A.; Migliorelli, F.; Martinez, A.; Bernard, M.; Ferron, G. Creation of a Y-Shaped Ileal Orthotopic Neobladder after an Anterior Pelvic Exenteration in 10 Logical Steps. Int. J. Gynecol. Cancer 2020, 30, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.; Bellina, M.; Fasolis, G.; Frea, B.; Scarpa, R.M.; Mari, M.; Rolle, L.; Destefanis, P. Y-Neobladder: An Easy, Fast, and Reliable Procedure. Urology 2004, 63, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Chen, S.; Zhang, L.; Li, T.; Sun, S.; Xu, B.; Zhu, W.; Zhang, G.; Zhang, L.; Wu, J.; et al. Robot-Assisted Laparoscopic Radical Cystectomy and Modified Y-Shaped Ileal Orthotopic Neobladder Reconstruction. Front. Surg. 2022, 9, 889536. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Elbendary, M.; Radwan, M.; Abo-Farha, M.O.; Gamil, T.; Azab, S.S.; Elmateet, M.S. Long-Term Evaluation of Modified Orthotopic Y-Shaped Ileal Neobladder (Tanta Pouch) with Left Retro-Colic Chimney. Int. Urol. Nephrol. 2020, 52, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.C.; Fonseca, G.N.; Cerqueira, J.B.G.; Nóbrega, M.S.; Costa, M.R.; Machado, P.C. Laparoscopic Radical Cystectomy with Intracorporeally Constructed Y-Shaped Orthotopic Ileal Neobladder Using Nonabsorbable Titanium Staples Exclusively. Urology 2005, 66, 657. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, J.; Farber, R.D.; Parra, R.O. Camey Procedure. A Continent Urinary Diversion Technique. AORN J. 1991, 54, 34–41, 44. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, G.; Gautier, J.-R.; Quintens, H.; Lasserre, E.; Caissel, J.; Leandri, P. Ileal Low-Pressure Bladder Replacement: Camey Type II. Eur. Urol. 1990, 18, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lilien, O.M.; Camey, M. 25-Year Experience with Replacement of the Human Bladder (Camey Procedure). J. Urol. 2017, 197, S173–S179. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.C.; Araújo, M.B.; Silveira, R.A.; Regadas, R.P.; Pinheiro, D.G.; Messias, F.I.; Argollo, R.S.; Guedes, G.A.; Gadelha, J.B.C.; Fonseca, G.N. Laparoscopic-Assisted Radical Cystectomy with U-Shaped Orthotopic Ileal Neobladder Constructed Using Nonabsorbable Titanium Staples. Urology 2006, 68, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-W.; Wu, C.-C.; Chen, K.-C.; Ho, C.-H. Modified U-Shaped Ileal Neobladder Designed for Facilitating Neobladder-Urethral Anastomosis in Extracorporeal Reconstruction after Robotic-Assisted Radical Cystectomy. J. Cancer Res. Ther. 2019, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, Y.; Yonneau, L.; Lebret, T.; Herve, J.-M.; Butreau, M.; Botto, H. The Z-Shaped Ileal Neobladder after Radical Cystectomy: An 18 Years Experience with 329 Patients: RESULTS OF Z-SHAPED NEOBLADDER. BJU Int. 2011, 108, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qi, H.; Zhou, R.; Jin, X. Early and Late Urodynamic Assessment of the Orthotopic N-shaped Neobladder. Oncol. Lett. 2013, 6, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Talat, Z.; Onal, B.; Cetinel, B.; Demirdag, C.; Citgez, S.; Dogan, C. The Surgical Technique and Initial Outcomes of Anatolian Neobladder: A Novel Technique of Ileal Neobladder after Radical Cystectomy. BMC Urol. 2018, 18, 94. [Google Scholar] [CrossRef]
- Bianchi, R.; Mistretta, F.A.; Musi, G.; Luzzago, S.; Morelli, M.; Lorusso, V.; Catellani, M.; Di Trapani, E.; Cozzi, G.; Ferro, M.; et al. Robot-Assisted Intracorporeal Orthotopic Ileal Neobladder: Description of the “Shell” Technique. J. Clin. Med. 2021, 10, 3601. [Google Scholar] [CrossRef] [PubMed]
- Minervini, A.; Vanacore, D.; Vittori, G.; Milanesi, M.; Tuccio, A.; Siena, G.; Campi, R.; Mari, A.; Gavazzi, A.; Carini, M. Florence Robotic Intracorporeal Neobladder (FloRIN): A New Reconfiguration Strategy Developed Following the IDEAL Guidelines. BJU Int. 2018, 121, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Peteinaris, A.; Gkeka, K.; Katsakiori, P.; Tatanis, V.; Anaplioti, E.; Faitatziadis, S.; Spinos, T.; Obaidat, M.; Vagionis, A.; Polyzonis, S.; et al. Replicating Florence Intracorporeal Neobladder Technique in Laparoscopic Radical Cystectomy: A Retrospective Study. Urology 2024, 183, 106–110. [Google Scholar] [CrossRef]
- Del Biondo, D.; Napodano, G.; Di Giacomo, F.; Di Domenico, D.D.; Feleppa, B.; Yazici, S.; Zito, A.R. A New Technique for Continent Urinary Diversion: Initial Experience and Description of the Technique. Urol. J. 2022, 19, 300–306. [Google Scholar] [CrossRef]
- Del Biondo, D.; Napodano, G.; Barone, B.; Iacone, M.; Grillo, M.; Ottaviano, N.; Piccoli, B.; Di Giacomo, F.; Di Domenico, D.; Yazici, S. The Robotic Intracorporeal Vesuvian Orthotopic Neobladder (VON)—A New Technique for Continent Urinary Diversion: Initial Experience and Description of the Technique. Appl. Sci. 2022, 12, 11616. [Google Scholar] [CrossRef]
- Basic, D.T.; Hadzi-Djokic, J.; Ignjatovic, I. The History of Urinary Diversion. Acta Chir. Iugosl. 2007, 54, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur. Urol. 2009, 55, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijzen, J.A.; de Vries, R.R.; Bex, A.; van der Poel, H.G.; Meinhardt, W.; Antonini, N.; Horenblas, S. Urinary Diversions after Cystectomy: The Association of Clinical Factors, Complications and Functional Results of Four Different Diversions. Eur. Urol. 2008, 53, 834–842; discussion 842–844. [Google Scholar] [CrossRef] [PubMed]
- Donegan, B.; Kingston, L. Quality of Life Following Formation of an Ileal Conduit Due to Urinary Bladder Neoplasm: A Systematic Review. Int. J. Nurs. Pract. 2022, 28, e12988. [Google Scholar] [CrossRef] [PubMed]
- Movassaghi, K.; Shah, S.H.; Cai, J.; Miranda, G.; Fernandez, J.; Duddalwar, V.; Daneshmand, S.; Djaladat, H. Incisional and Parastomal Hernia Following Radical Cystectomy and Urinary Diversion: The University of Southern California Experience. J. Urol. 2016, 196, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Donahue, T.F.; Bochner, B.H.; Sfakianos, J.P.; Kent, M.; Bernstein, M.; Hilton, W.M.; Cha, E.K.; Yee, A.M.; Dalbagni, G.; Vargas, H.A. Risk Factors for the Development of Parastomal Hernia after Radical Cystectomy. J. Urol. 2014, 191, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Korkes, F.; Fernandes, E.; Gushiken, F.A.; Glina, F.P.A.; Baccaglini, W.; Timóteo, F.; Glina, S. Bricker Ileal Conduit vs. Cutaneous Ureterostomy after Radical Cystectomy for Bladder Cancer: A Systematic Review. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2021, 48, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Mally, D.; John, P.; Pfister, D.; Heidenreich, A.; Albers, P.; Niegisch, G. Comparative Analysis of Elderly Patients Undergoing Radical Cystectomy With Ureterocutaneostomy or Ileal Conduit With a Special Focus on Bowl Complications Requiring Surgical Revision. Front. Surg. 2022, 9, 803926. [Google Scholar] [CrossRef] [PubMed]
- Kozacıoğlu, Z.; Değirmenci, T.; Günlüsoy, B.; Ceylan, Y.; Minareci, S. Ureterocutaneostomy: For Whom and When? Turk. J. Urol. 2013, 39, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Goonewardene, S.S.; Ventii, K.; Bahl, A.; Persad, R.; Motiwala, H.; Albala, D. Body Image in Bladder Cancer. In Management of Muscle Invasive Bladder Cancer; Goonewardene, S.S., Ventii, K., Bahl, A., Persad, R., Motiwala, H., Albala, D., Eds.; Springer International Publishing: Cham, Swtzerland, 2021; pp. 555–558. ISBN 978-3-030-57915-9. [Google Scholar]
- Sato, M.; Osawa, T.; Abe, T.; Honda, M.; Higuchi, M.; Yamada, S.; Furumido, J.; Kikuchi, H.; Matsumoto, R.; Sato, Y.; et al. Validation of the Japanese Version of the Body Image Scale for Bladder Cancer Patients. Sci. Rep. 2022, 12, 21544. [Google Scholar] [CrossRef]
- Klein, G.T.; Ajay, D.; Volk, R.J.; Leal, V.; Westney, O.L. Living with Urinary Diversions: Patient Insights to Improve the Perioperative Experience. Urology 2021, 152, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Butea-Bocu, M.; Brock, O.; Hanske, J.; Pucheril, D.; Noldus, J.; Otto, U. Association between Development of Metabolic Acidosis and Improvement of Urinary Continence after Ileal Neobladder Creation. J. Urol. 2020, 203, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Lim, B.; Chi, B.H.; Kim, J.H.; Lee, W.; You, D.; Kim, C.-S. Hybrid Ileal Pouch with Concomitant Anti-Refluxing and Refluxing Ureteroileal Anastomosis. BMC Urol. 2021, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Almassi, N.; Bochner, B.H. Ileal Conduit or Orthotopic Neobladder: Selection and Contemporary Patterns of Use. Curr. Opin. Urol. 2020, 30, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.T.S.; Lawrentschuk, N. Orthotopic Neobladder Reconstruction. Urol. Ann. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bachour, K.; Faiena, I.; Salmasi, A.; Lenis, A.T.; Johnson, D.C.; Pooli, A.; Drakaki, A.; Pantuck, A.J.; Chamie, K. Trends in Urinary Diversion after Radical Cystectomy for Urothelial Carcinoma. World J. Urol. 2018, 36, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Groeben, C.; Koch, R.; Baunacke, M.; Schmid, M.; Borkowetz, A.; Wirth, M.P.; Huber, J. Urinary Diversion After Radical Cystectomy for Bladder Cancer: Comparing Trends in the US and Germany from 2006 to 2014. Ann. Surg. Oncol. 2018, 25, 3502–3509. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wu, K.; Wang, S.; Su, W.; Li, C.; Li, B.; Mao, X. The Impact of Orthotopic Neobladder vs Ileal Conduit Urinary Diversion after Cystectomy on the Survival Outcomes in Patients with Bladder Cancer: A Propensity Score Matched Analysis. Cancer Med. 2020, 9, 7590–7600. [Google Scholar] [CrossRef] [PubMed]
- Thakare, N.; Lamb, B.W.; Biers, S. Orthotopic Bladder Substitution: Surgical Aspects and Optimization of Outcomes. BJUI Compass 2021, 2, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Fasanella, D.; Marchioni, M.; Domanico, L.; Franzini, C.; Inferrera, A.; Schips, L.; Greco, F. Neobladder “Function”: Tips and Tricks for Surgery and Postoperative Management. Life 2022, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Ghodoussipour, S.; Ladi Seyedian, S.S.; Jiang, D.; Lifton, J.; Ahmadi, H.; Wayne, K.; Miranda, G.; Cai, J.; Djaladat, H.; Schuckman, A.; et al. Predictors of Need for Catheterisation and Urinary Retention after Radical Cystectomy and Orthotopic Neobladder in Male Patients. BJU Int. 2021, 128, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Ali-el-Dein, B.; Shaaban, A.A.; Abu-Eideh, R.H.; el-Azab, M.; Ashamallah, A.; Ghoneim, M.A. Surgical Complications Following Radical Cystectomy and Orthotopic Neobladders in Women. J. Urol. 2008, 180, 206–210; discussion 210. [Google Scholar] [CrossRef]
- Situmorang, G.R.; Irdam, G.A.; Abdullah, A. Bowel Obstruction Incidence in Urinary Diversion Patients: A Meta-Analysis Study. Urol. Ann. 2022, 14, 359. [Google Scholar]
- Moeen, A.M.; Safwat, A.S.; Elderwy, A.A.; Behnsawy, H.M.; Osman, M.M.; Hameed, D.A. Management of Neobladder Complications: Endoscopy Comes First. Scand. J. Urol. 2017, 51, 146–151. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, V.D.; Melnick, K.; Yim, K.; Ernandez, J.; Onochie, N.; Clinton, T.N.; Steele, G.S.; Preston, M.A.; Kibel, A.S.; Mossanen, M. Evidence-Based Analysis of the Critical Steps of Radical Cystectomy for Bladder Cancer. J. Clin. Med. 2023, 12, 6845. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N. Enhanced Recovery after Surgery. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S3–S4. [Google Scholar] [CrossRef] [PubMed]
- Schiavina, R.; Droghetti, M.; Bianchi, L.; Ercolino, A.; Chessa, F.; Casablanca, C.; Piazza, P.; Mottaran, A.; Recenti, D.; Salvador, M.; et al. The Robotic Approach Improves the Outcomes of ERAS Protocol after Radical Cystectomy: A Prospective Case-Control Analysis. Urol. Oncol. 2021, 39, 833.e1–833.e8. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.; Liu, Z.; Qi, W.; Lv, G.; Zhong, M.; Liu, X.; Zhu, M.; Jiang, Z.; Chen, S.; et al. The Effect of the Enhanced Recovery after Surgery Program on Radical Cystectomy: A Meta-Analysis and Systematic Review. Front. Surg. 2023, 10, 1101098. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Liu, S.; Lu, Y.; Wei, W.; Han, P. Clinical Efficacy and Safety of Enhanced Recovery after Surgery for Patients Treated with Radical Cystectomy and Ileal Urinary Diversion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Transl. Androl. Urol. 2020, 9, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.N.; Pramesh, C.S.; Rathi, S.; Pantvaidya, G.H. Long-Term Results of Orthotopic Neobladder Reconstruction after Radical Cystectomy. BJU Int. 2003, 91, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Mano, R.; Baniel, J.; Goldberg, H.; Stabholz, Y.; Kedar, D.; Yossepowitch, O. Urinary Tract Infections in Patients with Orthotopic Neobladder. Urol. Oncol. 2014, 32, 50.e9–50.e14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-G.; Song, Q.-X.; Song, B.; Zhang, D.-L.; Zhang, W.; Wang, J.-Y. Diagnosis and Treatment of Urinary Incontinence after Orthotopic Ileal Neobladder in China. Chin. Med. J. 2017, 130, 231–235. [Google Scholar]
- Harano, M.; Eto, M.; Nakamura, M.; Hasegawa, Y.; Kano, M.; Yamaguchi, A.; Naito, S. A Pilot Study of the Assessment of the Quality of Life, Functional Results, and Complications in Patients with an Ileal Neobladder for Invasive Bladder Cancer. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2007, 14, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; He, Q.; Zhang, Y.; Wang, L.; Jin, X.; Bao, C.; Wang, W. Management of Urinary Incontinence after Radical Cystectomy and Orthotopic Neobladder: A Scoping Review of International Practices. Nurs. Open 2023, 10, 6618. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, B.; Napolitano, L.; Reccia, P.; Calace, F.P.; De Luca, L.; Olivetta, M.; Stizzo, M.; Rubinacci, A.; Della Rosa, G.; Lecce, A.; et al. Advances in Urinary Diversion: From Cutaneous Ureterostomy to Orthotopic Neobladder Reconstruction—A Comprehensive Review. J. Pers. Med. 2024, 14, 392. https://doi.org/10.3390/jpm14040392
Barone B, Napolitano L, Reccia P, Calace FP, De Luca L, Olivetta M, Stizzo M, Rubinacci A, Della Rosa G, Lecce A, et al. Advances in Urinary Diversion: From Cutaneous Ureterostomy to Orthotopic Neobladder Reconstruction—A Comprehensive Review. Journal of Personalized Medicine. 2024; 14(4):392. https://doi.org/10.3390/jpm14040392
Chicago/Turabian StyleBarone, Biagio, Luigi Napolitano, Pasquale Reccia, Francesco Paolo Calace, Luigi De Luca, Michelangelo Olivetta, Marco Stizzo, Andrea Rubinacci, Giampiero Della Rosa, Arturo Lecce, and et al. 2024. "Advances in Urinary Diversion: From Cutaneous Ureterostomy to Orthotopic Neobladder Reconstruction—A Comprehensive Review" Journal of Personalized Medicine 14, no. 4: 392. https://doi.org/10.3390/jpm14040392
APA StyleBarone, B., Napolitano, L., Reccia, P., Calace, F. P., De Luca, L., Olivetta, M., Stizzo, M., Rubinacci, A., Della Rosa, G., Lecce, A., Romano, L., Sciorio, C., Spirito, L., Mattiello, G., Vastarella, M. G., Papi, S., Calogero, A., Varlese, F., Tataru, O. S., ... Amicuzi, U. (2024). Advances in Urinary Diversion: From Cutaneous Ureterostomy to Orthotopic Neobladder Reconstruction—A Comprehensive Review. Journal of Personalized Medicine, 14(4), 392. https://doi.org/10.3390/jpm14040392








