Challenges in Diagnosis and Therapeutic Strategies in Late-Onset Multiple Sclerosis
Abstract
1. Introduction
2. Methods
3. Clinical and Radiological Characteristics and Disease Course of LOMS
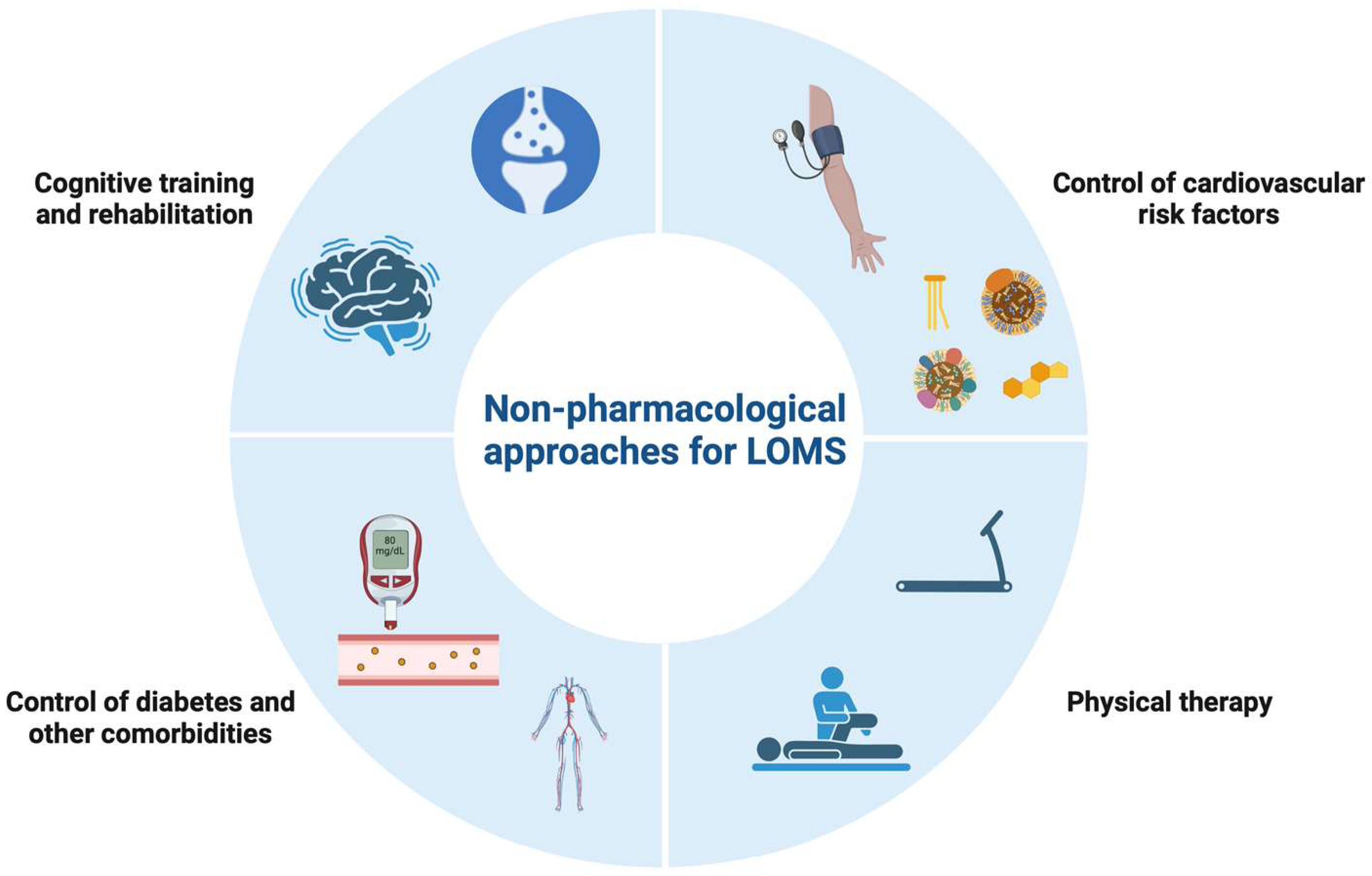
4. Comorbidities
5. Differential Diagnosis
5.1. Vascular Lesions
5.2. Primary and Secondary Central Nervous System Vasculitides
5.3. Acute Disseminated Encephalomyelitis
5.4. Myelopathies
5.5. Optic Neuritis
5.6. Sarcoidosis
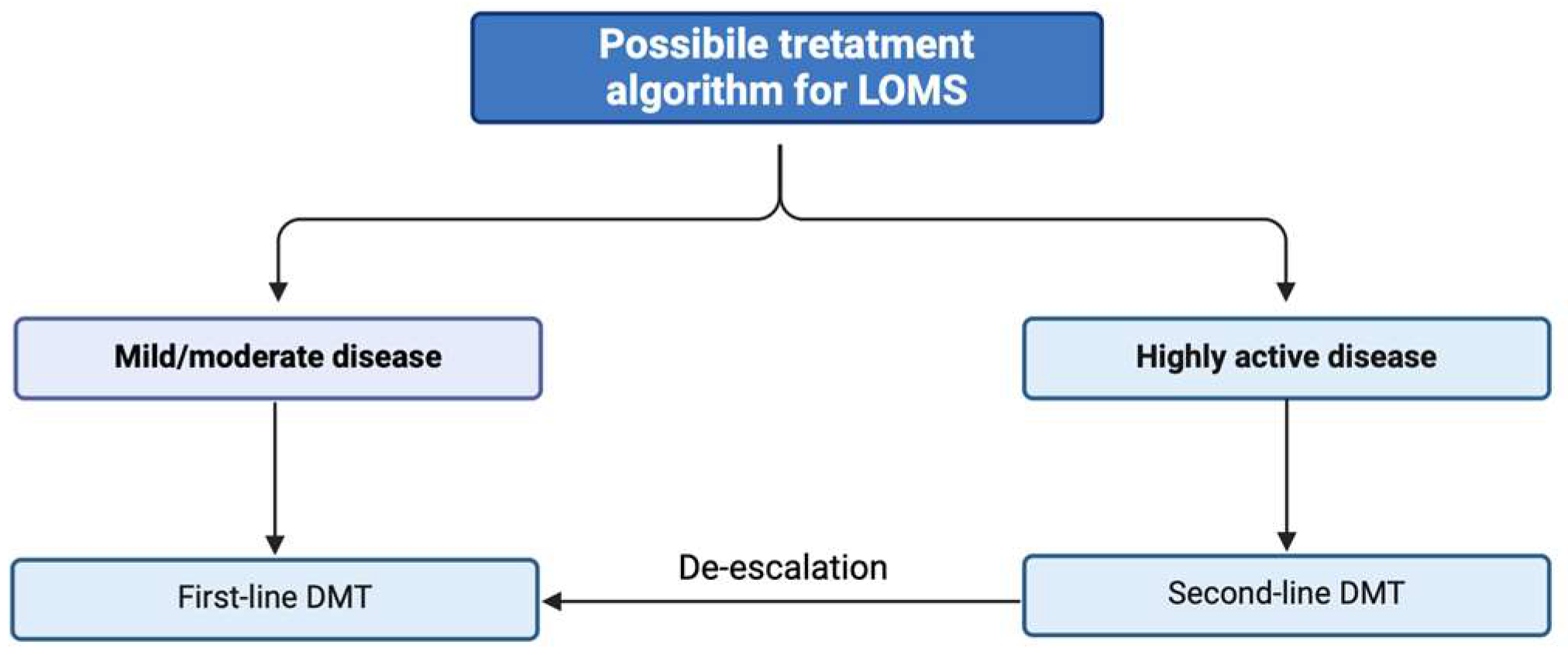
6. Treatment Aspects
6.1. Efficacy of DMTs
6.2. Safety and Tolerability
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- McFarland, H.F.; Martin, R. Multiple sclerosis: A complicated picture of autoimmunity. Nat. Immunol. 2007, 8, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Naegele, M.; Martin, R. The good and the bad of neuroinflammation in multiple sclerosis. Handb. Clin. Neurol. 2014, 122, 59–87. [Google Scholar] [CrossRef]
- Boiko, A.; Vorobeychik, G.; Paty, D.; Devonshire, V.; Sadovnick, D. Early onset multiple sclerosis: A longitudinal study. Neurology 2002, 59, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Polliack, M.L.; Barak, Y.; Achiron, A. Late-onset multiple sclerosis. J. Am. Geriatr. Soc. 2001, 49, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Palathinkara, M.; Razzak, A.N.; Ababneh, O.E.; Cairns, D.; Obeidat, A.Z. Clinical and radiologic differences between early onset, late onset, and very late onset adult multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 80, 105132. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Thygesen, L.C.; Stenager, E.; Laursen, B.; Magyari, M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 2018, 90, e1954–e1963. [Google Scholar] [CrossRef]
- Prosperini, L.; Lucchini, M.; Ruggieri, S.; Tortorella, C.; Haggiag, S.; Mirabella, M.; Pozzilli, C.; Gasperini, C. Shift of multiple sclerosis onset towards older age. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1137–1139. [Google Scholar] [CrossRef]
- Yorio, F.; Marrodan, M.; Farez, M.; Correale, J. Differential Diagnosis in Late Onset Multiple Sclerosis (P1.2-060). Neurology 2019, 92 (Suppl. S15). [Google Scholar] [CrossRef]
- Magyari, M.; Sorensen, P.S. Comorbidity in Multiple Sclerosis. Front. Neurol. 2020, 11, 851. [Google Scholar] [CrossRef]
- Nociti, V.; Romozzi, M. The Importance of Managing Modifiable Comorbidities in People with Multiple Sclerosis: A Narrative Review. J. Pers. Med. 2023, 13, 1524. [Google Scholar] [CrossRef] [PubMed]
- Loma, I.; Heyman, R. Multiple sclerosis: Pathogenesis and treatment. Curr. Neuropharmacol. 2011, 9, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Guillemin, F.; Baumann, C.; Epstein, J.; Kerschen, P.; Garot, T.; Mathey, G.; Debouverie, M. Older Age at Multiple Sclerosis Onset Is an Independent Factor of Poor Prognosis: A Population-Based Cohort Study. Neuroepidemiology 2017, 48, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kalincik, T.; Diouf, I.; Sharmin, S.; Malpas, C.; Spelman, T.; Horakova, D.; Havrdova, E.K.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; et al. Effect of Disease-Modifying Therapy on Disability in Relapsing-Remitting Multiple Sclerosis over 15 Years. Neurology 2021, 96, e783–e797. [Google Scholar] [CrossRef] [PubMed]
- Jakimovski, D.; Eckert, S.P.; Zivadinov, R.; Weinstock-Guttman, B. Considering patient age when treating multiple sclerosis across the adult lifespan. Expert. Rev. Neurother. 2021, 21, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Trojano, M.; Tintore, M.; Montalban, X.; Hillert, J.; Kalincik, T.; Iaffaldano, P.; Spelman, T.; Sormani, M.P.; Butzkueven, H. Treatment decisions in multiple sclerosis—Insights from real-world observational studies. Nat. Rev. Neurol. 2017, 13, 105–118. [Google Scholar] [CrossRef]
- Buscarinu, M.C.; Reniè, R.; Morena, E.; Romano, C.; Bellucci, G.; Marrone, A.; Bigi, R.; Salvetti, M.; Ristori, G. Late-Onset MS: Disease Course and Safety-Efficacy of DMTS. Front. Neurol. 2022, 13, 829331. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.A.; Buron, M.D.; Magyari, M. Late-onset MS is associated with an increased rate of reaching disability milestones. J. Neurol. 2021, 268, 3352–3360. [Google Scholar] [CrossRef]
- Roohani, P.; Emiru, T.; Carpenter, A.; Luzzio, C.; Freeman, J.; Scarberry, S.; Beaver, G.; Davidson, L.; Parry, G. Late onset multiple sclerosis: Is it really late onset? Mult. Scler. Relat. Disord. 2014, 3, 444–449. [Google Scholar] [CrossRef]
- Naseri, A.; Nasiri, E.; Sahraian, M.A.; Daneshvar, S.; Talebi, M. Clinical Features of Late-Onset Multiple Sclerosis: A Systematic Review and Meta-analysis. Mult. Scler. Relat. Disord. 2021, 50, 102816. [Google Scholar] [CrossRef] [PubMed]
- Cazzullo, C.L.; Ghezzi, A.; Marforio, S.; Caputo, D. Clinical picture of multiple sclerosis with late onset. Acta Neurol. Scand. 1978, 58, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, V.; Rodegher, M.; Moiola, L.; Comi, G. Late onset multiple sclerosis: Clinical characteristics, prognostic factors and differential diagnosis. Neurol. Sci. 2004, 25 (Suppl. S4), S350–S355. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.M.; Healy, B.; Augustine, A.; Musallam, A.; Gholipour, T.; Chitnis, T. Effect of gender on late-onset multiple sclerosis. Mult. Scler. 2012, 18, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Rumberg, B.; Berlit, P. Clinical characteristics of patients with late-onset multiple sclerosis. J. Neurol. 2008, 255, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Mirmosayyeb, O.; Brand, S.; Barzegar, M.; Afshari-Safavi, A.; Nehzat, N.; Shaygannejad, V.; Sadeghi Bahmani, D. Clinical Characteristics and Disability Progression of Early- and Late-Onset Multiple Sclerosis Compared to Adult-Onset Multiple Sclerosis. J. Clin. Med. 2020, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Devonshire, V. Is late-onset multiple sclerosis associated with a worse outcome? Neurology 2006, 67, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, J.; Paty, D.; Wonnacott, T.; Feasby, T.; Ebers, G. Multiple sclerosis after age 50. Neurology 1983, 33, 1537–1544. [Google Scholar] [CrossRef]
- Alroughani, R.; Akhtar, S.; Ahmed, S.; Behbehani, R.; Al-Hashel, J. Is Time to Reach EDSS 6.0 Faster in Patients with Late-Onset versus Young-Onset Multiple Sclerosis? PLoS ONE 2016, 11, e0165846. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef]
- Sim, F.J.; Zhao, C.; Penderis, J.; Franklin, R.J. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci. 2002, 22, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Hauer, L.; Perneczky, J.; Sellner, J. A global view of comorbidity in multiple sclerosis: A systematic review with a focus on regional differences, methodology, and clinical implications. J. Neurol. 2021, 268, 4066–4077. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Reingold, S.; Cohen, J.; Stuve, O.; Trojano, M.; Sorensen, P.S.; Cutter, G.; Reider, N. The incidence and prevalence of psychiatric disorders in multiple sclerosis: A systematic review. Mult. Scler. J. 2015, 21, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Nociti, V.; Romozzi, M. Multiple Sclerosis and Autoimmune Comorbidities. J. Pers. Med. 2022, 12, 1828. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, E.; Mohammadi, M.; Moradi, T. High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Mult. Scler. 2013, 19, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, T.B.; Berkowitz, A.L.; Samuels, M.A. Cardiovascular Dysfunction in Multiple Sclerosis. Neurologist 2015, 20, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.M.P.; Taylor, B.V.; Winzenberg, T.; Palmer, A.J.; Blizzard, L.; van der Mei, I. Change and onset-type differences in the prevalence of comorbidities in people with multiple sclerosis. J. Neurol. 2021, 268, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Kowalec, K.; McKay, K.A.; Patten, S.B.; Fisk, J.D.; Evans, C.; Tremlett, H.; Marrie, R.A. Comorbidity increases the risk of relapse in multiple sclerosis: A prospective study. Neurology 2017, 89, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Rudick, R.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010, 74, 1041–1047. [Google Scholar] [CrossRef]
- Marrie, R.A. Comorbidity in multiple sclerosis: Implications for patient care. Nat. Rev. Neurol. 2017, 13, 375–382. [Google Scholar] [CrossRef]
- Marrie, R.A.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 2009, 72, 117–124. [Google Scholar] [CrossRef]
- Berrigan, L.I.; Fisk, J.D.; Patten, S.B.; Tremlett, H.; Wolfson, C.; Warren, S.; Fiest, K.M.; McKay, K.A.; Marrie, R.A. Health-related quality of life in multiple sclerosis: Direct and indirect effects of comorbidity. Neurology 2016, 86, 1417–1424. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef]
- Arias, M.; Dapena, D.; Arias-Rivas, S.; Costa, E.; López, A.; Prieto, J.; Corredera, E. Late onset multiple sclerosis. Neurología 2011, 26, 291–296. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Li, H.; Xiao, L.; Zhou, Z.; Chen, K.; Gui, L.; Hou, X.; Fan, R.; Chen, K.; et al. Clinical, Radiological and Pathological Characteristics between Cerebral Small Vessel Disease and Multiple Sclerosis: A Review. Front. Neurol. 2022, 13, 841521. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Schmidt, H.; Haybaeck, J.; Loitfelder, M.; Weis, S.; Cavalieri, M.; Seiler, S.; Enzinger, C.; Ropele, S.; Erkinjuntti, T.; et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011, 122, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F. Magnetic resonance signal abnormalities in asymptomatic individuals: Their incidence and functional correlates. Eur. Neurol. 1989, 29, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.M. Lacunes: Small, deep cerebral infarcts. Neurology 1965, 15, 774–784. [Google Scholar] [CrossRef]
- Longstreth, W.T., Jr.; Bernick, C.; Manolio, T.A.; Bryan, N.; Jungreis, C.A.; Price, T.R. Lacunar Infarcts Defined by Magnetic Resonance Imaging of 3660 Elderly People: The Cardiovascular Health Study. Arch. Neurol. 1998, 55, 1217–1225. [Google Scholar] [CrossRef]
- Tallantyre, E.C.; Dixon, J.E.; Donaldson, I.; Owens, T.; Morgan, P.S.; Morris, P.G.; Evangelou, N. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011, 76, 534–539. [Google Scholar] [CrossRef]
- Sati, P.; George, I.C.; Shea, C.D.; Gaitán, M.I.; Reich, D.S. FLAIR*: A combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012, 265, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.M.; Scolding, N.J. The diagnosis of primary central nervous system vasculitis. Pract. Neurol. 2020, 20, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Jewells, V.L.; Latchaw, R.E. CNS Vasculitis—An Overview of This Multiple Sclerosis Mimic: Clinical and MRI Implications. Semin. Ultrasound CT MRI 2020, 41, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Maggi, P.; Absinta, M.; Grammatico, M.; Vuolo, L.; Emmi, G.; Carlucci, G.; Spagni, G.; Barilaro, A.; Repice, A.M.; Emmi, L.; et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann. Neurol. 2018, 83, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Marrodan, M.; Fiol, M.P.; Correale, J. Susac syndrome: Challenges in the diagnosis and treatment. Brain 2022, 145, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Susac, J.O.; Murtagh, F.R.; Egan, R.A.; Berger, J.R.; Bakshi, R.; Lincoff, N.; Gean, A.D.; Galetta, S.L.; Fox, R.J.; Costello, F.E.; et al. MRI findings in Susac’s syndrome. Neurology 2003, 61, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.Z.; Yang, H.; Li, D.; Zhao, Z.D.; Sun, Y.J.; Xia, M.R.; Jiang, S.; Zhang, J.W. Clinical comparative analysis of monophasic and multiphasic acute disseminated encephalomyelitis in adults. Arch. Med. Sci. 2023, 19, 687–693. [Google Scholar] [CrossRef]
- Ketelslegers, I.A.; Van Pelt, D.E.; Bryde, S.; Neuteboom, R.F.; Catsman-Berrevoets, C.E.; Hamann, D.; Hintzen, R.Q. Anti-MOG antibodies plead against MS diagnosis in an Acquired Demyelinating Syndromes cohort. Mult. Scler. 2015, 21, 1513–1520. [Google Scholar] [CrossRef]
- Sechi, E.; Cacciaguerra, L.; Chen, J.J.; Mariotto, S.; Fadda, G.; Dinoto, A.; Lopez-Chiriboga, A.S.; Pittock, S.J.; Flanagan, E.P. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD): A Review of Clinical and MRI Features, Diagnosis, and Management. Front. Neurol. 2022, 13, 885218. [Google Scholar] [CrossRef]
- Tavazzi, E.; Ravaglia, S.; Franciotta, D.; Marchioni, E. Differential Diagnosis between Acute Disseminated Encephalomyelitis and Multiple Sclerosis During the First Episode. Arch. Neurol. 2008, 65, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Dale, R.C.; Branson, J.A. Acute disseminated encephalomyelitis or multiple sclerosis: Can the initial presentation help in establishing a correct diagnosis? Arch. Dis. Child. 2005, 90, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, M.; Wen, L.; Wang, Q.; Ding, X.; Wang, J. Clinical Presentation and Outcomes of Acute Disseminated Encephalomyelitis in Adults Worldwide: Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 870867. [Google Scholar] [CrossRef] [PubMed]
- Otallah, S. Acute disseminated encephalomyelitis in children and adults: A focused review emphasizing new developments. Mult. Scler. 2021, 27, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.R.; Sama, A.J.; Schiller, N.C.; Butler, A.J.; Donnally, C.J., 3rd. Cervical Spondylotic Myelopathy: A Guide to Diagnosis and Management. J. Am. Board. Fam. Med. 2020, 33, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Cacciaguerra, L.; Sechi, E.; Rocca, M.A.; Filippi, M.; Pittock, S.J.; Flanagan, E.P. Neuroimaging features in inflammatory myelopathies: A review. Front. Neurol. 2022, 13, 993645. [Google Scholar] [CrossRef] [PubMed]
- Sechi, E.; Flanagan, E.P. Evaluation and Management of Acute Myelopathy. Semin. Neurol. 2021, 41, 511–529. [Google Scholar] [CrossRef]
- Young, W.B. The clinical diagnosis of myelopathy. Semin. Ultrasound CT MRI 1994, 15, 250–254. [Google Scholar] [CrossRef]
- Grill, M.F. Infectious Myelopathies. Continuum 2018, 24, 441–473. [Google Scholar] [CrossRef]
- Kraker, J.A.; Chen, J.J. An update on optic neuritis. J. Neurol. 2023, 270, 5113–5126. [Google Scholar] [CrossRef]
- Bennett, J.L.; Costello, F.; Chen, J.J.; Petzold, A.; Biousse, V.; Newman, N.J.; Galetta, S.L. Optic neuritis and autoimmune optic neuropathies: Advances in diagnosis and treatment. Lancet Neurol. 2023, 22, 89–100. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Pawate, S.; Koth, L.L.; Cho, T.A.; Gelfand, J.M. Neurosarcoidosis: Pathophysiology, Diagnosis, and Treatment. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1084. [Google Scholar] [CrossRef]
- Bayas, A.; Christ, M.; Faissner, S.; Klehmet, J.; Pul, R.; Skripuletz, T.; Meuth, S.G. Disease-modifying therapies for relapsing/active secondary progressive multiple sclerosis—A review of population-specific evidence from randomized clinical trials. Ther. Adv. Neurol. Disord. 2023, 16, 17562864221146836. [Google Scholar] [CrossRef] [PubMed]
- Weideman, A.M.; Tapia-Maltos, M.A.; Johnson, K.; Greenwood, M.; Bielekova, B. Meta-analysis of the Age-Dependent Efficacy of Multiple Sclerosis Treatments. Front. Neurol. 2017, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Gold, R.; Kappos, L.; Arnold, D.L.; Giovannoni, G.; Selmaj, K.; O’Gorman, J.; Stephan, M.; Dawson, K.T. Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: Subgroup analyses of the DEFINE study. J. Neurol. 2013, 260, 2297–2305. [Google Scholar] [CrossRef]
- Newsome, S.D.; Kieseier, B.C.; Arnold, D.L.; Shang, S.; Liu, S.; Hung, S.; Sabatella, G. Subgroup and sensitivity analyses of annualized relapse rate over 2 years in the ADVANCE trial of peginterferon beta-1a in patients with relapsing-remitting multiple sclerosis. J. Neurol. 2016, 263, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.E.; O’Connor, P.; Wolinsky, J.S.; Confavreux, C.; Kappos, L.; Olsson, T.P.; Truffinet, P.; Wang, L.; D’Castro, L.; Comi, G.; et al. Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult. Scler. 2012, 18, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.; Kappos, L.; Calabresi, P.A.; Confavreux, C.; Giovannoni, G.; Galetta, S.L.; Havrdova, E.; Lublin, F.D.; Miller, D.H.; O’Connor, P.W.; et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: Subgroup analyses of AFFIRM and SENTINEL. J. Neurol. 2009, 256, 405–415. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.-W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A Placebo-Controlled Trial of Oral Fingolimod in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2016, 376, 221–234. [Google Scholar] [CrossRef]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2016, 376, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.D.; Arroyo, R.; Boster, A.L.; Boyko, A.N.; Eichau, S.; Ionete, C.; Limmroth, V.; Navas, C.; Pelletier, D.; Pozzilli, C.; et al. Alemtuzumab outcomes by age: Post hoc analysis from the randomized CARE-MS studies over 8 years. Mult. Scler. Relat. Disord. 2021, 49, 102717. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Bar-Or, A.; Cree, B.A.C.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Thakolwiboon, S.; Karukote, A.; Sohn, G.; Jin, D.; Avila, M. Efficacy and Tolerability of Disease Modifying Therapies in Late Onset Multiple Sclerosis (2176). Neurology 2020, 94 (Suppl. S15). [Google Scholar] [CrossRef]
- Zanghì, A.; Avolio, C.; Amato, M.P.; Filippi, M.; Trojano, M.; Patti, F.; D’Amico, E.; Italian MS register. First-line therapies in late-onset multiple sclerosis: An Italian registry study. Eur. J. Neurol. 2021, 28, 4117–4123. [Google Scholar] [CrossRef] [PubMed]
- Shirani, A.; Zhao, Y.; Petkau, J.; Gustafson, P.; Karim, M.E.; Evans, C.; Kingwell, E.; van der Kop, M.L.; Oger, J.; Tremlett, H. Multiple sclerosis in older adults: The clinical profile and impact of interferon Beta treatment. Biomed. Res. Int. 2015, 2015, 451912. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; de Rossi, N.; Scarpazza, C.; Moiola, L.; Cosottini, M.; Gerevini, S.; Capra, R. Natalizumab-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: Findings from an Italian Independent Registry. PLoS ONE 2016, 11, e0168376. [Google Scholar] [CrossRef]
- Prosperini, L.; Scarpazza, C.; Imberti, L.; Cordioli, C.; De Rossi, N.; Capra, R. Age as a risk factor for early onset of natalizumab-related progressive multifocal leukoencephalopathy. J. Neurovirol. 2017, 23, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, F.; Laurent, S.; Fink, G.R.; Barnett, M.H.; Reddel, S.; Hartung, H.P.; Warnke, C. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr. Opin. Neurol. 2019, 32, 305–312. [Google Scholar] [CrossRef]
- Prosperini, L.; Haggiag, S.; Tortorella, C.; Galgani, S.; Gasperini, C. Age-related adverse events of disease-modifying treatments for multiple sclerosis: A meta-regression. Mult. Scler. 2021, 27, 1391–1402. [Google Scholar] [CrossRef]
- Ng, H.S.; Zhu, F.; Zhao, Y.; Yao, S.; Lu, X.; Ekuma, O.; Evans, C.; Fisk, J.D.; Marrie, R.A.; Tremlett, H. Adverse Events Associated With Disease-Modifying Drugs for Multiple Sclerosis. Neurology 2024, 102, e2080062024. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.; Svačina, M.K.; Bobylev, I.; Joshi, A.; Schneider, T.; Lehmann, H.C. Impact of Age and Polytherapy on Fingolimod Induced Bradycardia: A Preclinical Study. J. Neuroimmune Pharmacol. 2017, 12, 204–209. [Google Scholar] [CrossRef] [PubMed]
| LOMS | White Matter Hyperintensities | Lacunar Infarcts | |
|---|---|---|---|
| Age of presentation (years old) | >50 | >55 | >55 |
| Shape | Ovoid | Punctate, focal, and/or confluent | Round or ovoid |
| Size | >3 mm | Usually 3–12 mm | 3–15 mm |
| Sites | Periventricular, cortical or juxtacortical, and infratentorial | Periventricular, adjacent to the lateral ventricular wall, or in the deep white matter | Basal ganglia, thalamus, subcortical white matter, and pons |
| Clinic | Often symptomatic | Silent | Silent or symptomatic |
| MRI findings | |||
| T2/FLAIR | Hyperintense | Hyperintense | Hyperintense |
| Contrast enhancement | Variable | Absent | Variable |
| LOMS | Cervical Spondylotic Myelopathy | |
|---|---|---|
| Age of presentation (years old) | >50 | >50 |
| Level in cervical spine | Upper part | Lower part |
| Size | Larger | Smaller |
| Lesion border definition | Well-defined | Ill-defined |
| Thickness of the cord | Normal | Decreased |
| Edema | Less frequent | More frequent |
| Gadolinium enhancement | In acute phases | Pancake-like or persistent enhancement |
| CSF analysis | ||
| Oligoclonal bands | Common (less than AOMS) | Absent |
| Elevation of proteins | Mild, common | Mild/moderate, common |
| Pleocytosis | Mild, common | Rare |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nociti, V.; Romozzi, M.; Mirabella, M. Challenges in Diagnosis and Therapeutic Strategies in Late-Onset Multiple Sclerosis. J. Pers. Med. 2024, 14, 400. https://doi.org/10.3390/jpm14040400
Nociti V, Romozzi M, Mirabella M. Challenges in Diagnosis and Therapeutic Strategies in Late-Onset Multiple Sclerosis. Journal of Personalized Medicine. 2024; 14(4):400. https://doi.org/10.3390/jpm14040400
Chicago/Turabian StyleNociti, Viviana, Marina Romozzi, and Massimiliano Mirabella. 2024. "Challenges in Diagnosis and Therapeutic Strategies in Late-Onset Multiple Sclerosis" Journal of Personalized Medicine 14, no. 4: 400. https://doi.org/10.3390/jpm14040400
APA StyleNociti, V., Romozzi, M., & Mirabella, M. (2024). Challenges in Diagnosis and Therapeutic Strategies in Late-Onset Multiple Sclerosis. Journal of Personalized Medicine, 14(4), 400. https://doi.org/10.3390/jpm14040400







