Prognostic Role of Initial Thromboelastography in Emergency Department Patients with Primary Postpartum Hemorrhage: Association with Massive Transfusion
Abstract
1. Introduction
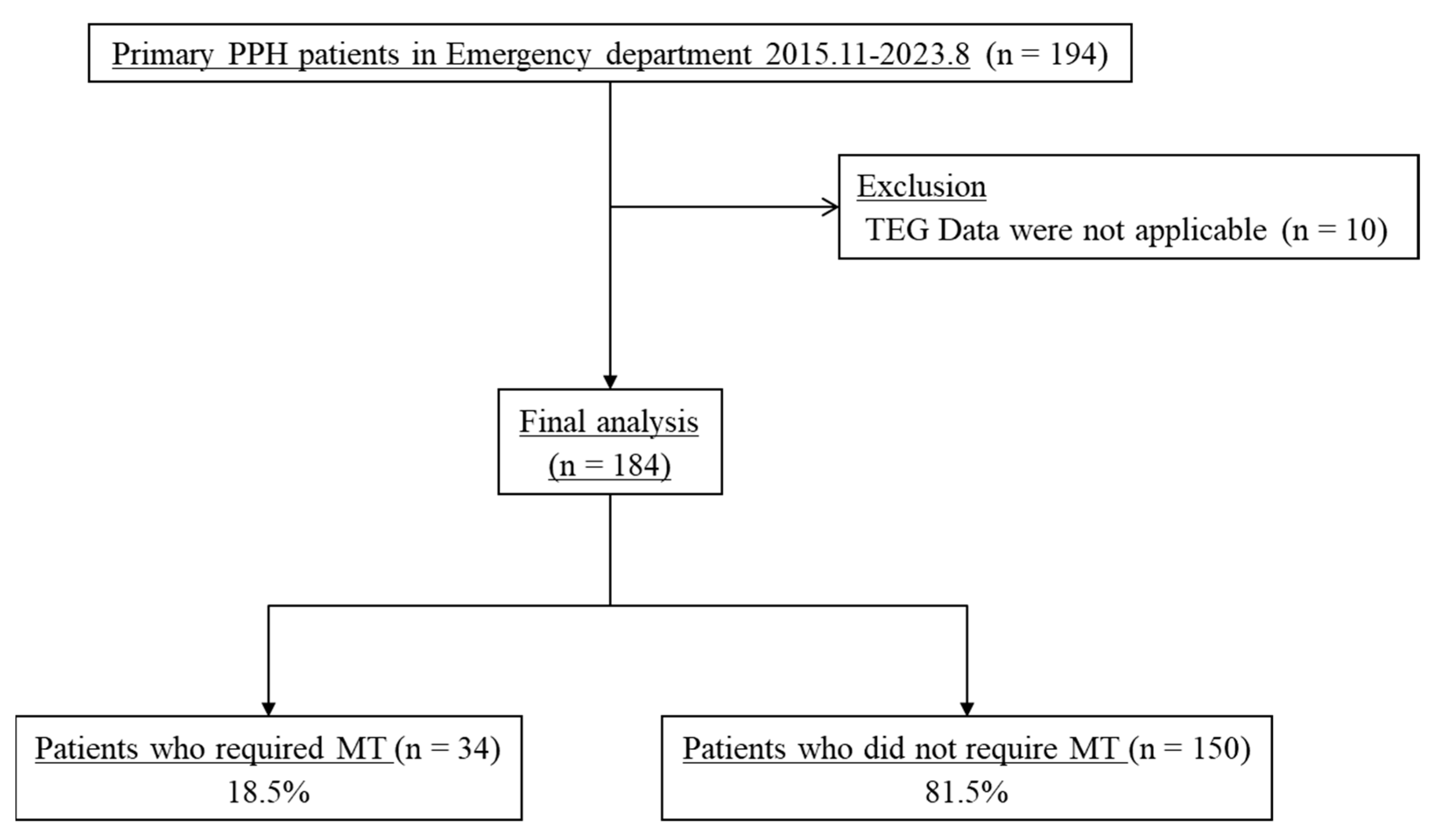
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Collection
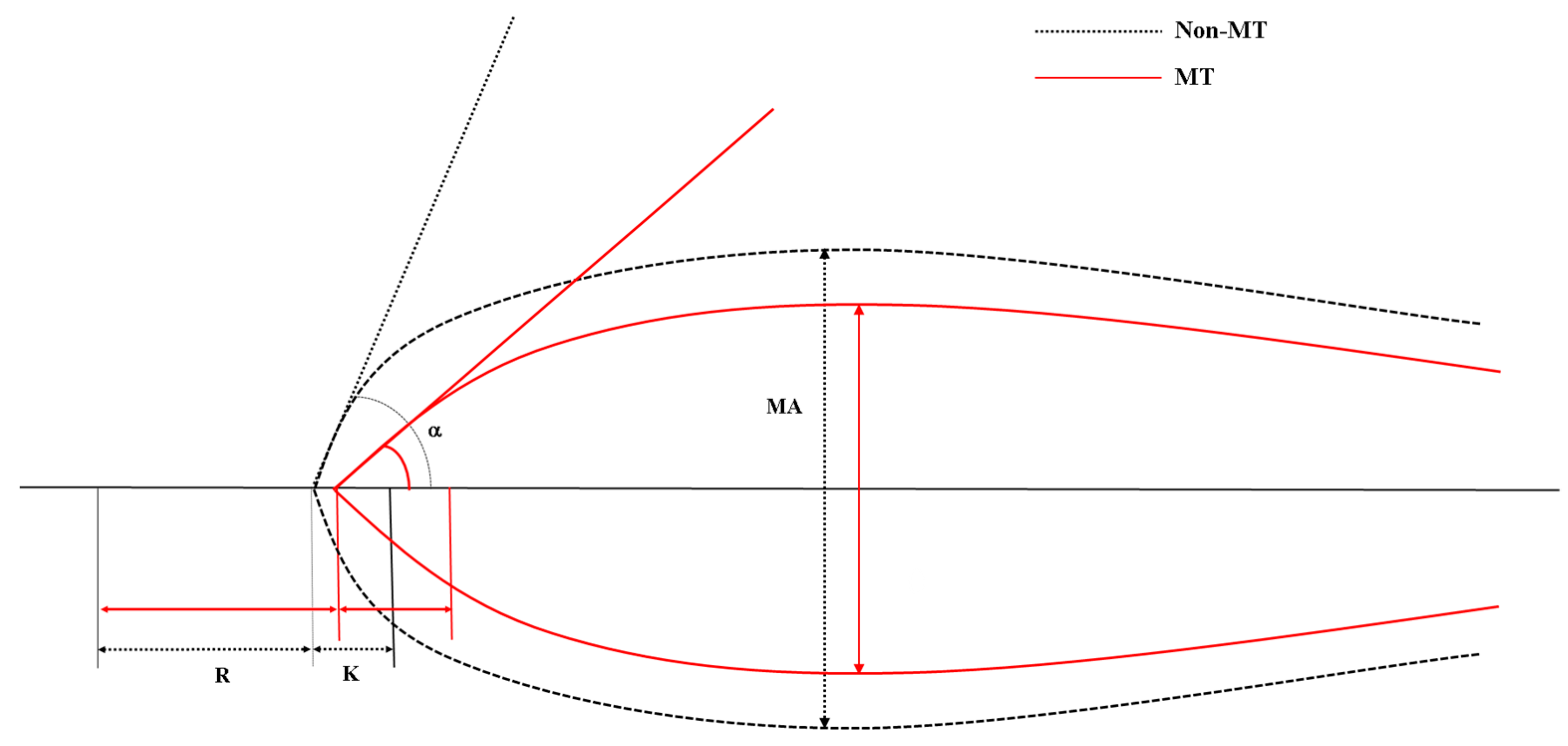
2.3. Thromboelastography
2.4. Statistical Analysis
3. Results
3.1. Baseline and Clinical Characteristics
3.2. Thromboelastographic Analysis
3.3. Predicting Factors Associated with the Need for Massive Transfusion
3.4. Performance Parameters for TEG values and Shock Index to Predict the Need for Massive Transfusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [PubMed]
- Evensen, A.; Anderson, J.M.; Fontaine, P. Postpartum Hemorrhage: Prevention and Treatment. Am. Fam. Physician 2017, 95, 442–449. [Google Scholar]
- Magann, E.F.; Evans, S.; Hutchinson, M.; Collins, R.; Lanneau, G.; Morrison, J.C. Postpartum hemorrhage after cesarean delivery: An analysis of risk factors. South Med. J. 2005, 98, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Girault, A.; Deneux-Tharaux, C.; Sentilhes, L.; Maillard, F.; Goffinet, F. Undiagnosed abnormal postpartum blood loss: Incidence and risk factors. PLoS ONE 2018, 13, e0190845. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Ikeda, T.; Sekizawa, A.; Tanaka, H.; Nakamura, M.; Katsuragi, S.; Osato, K.; Tanaka, K.; Murakoshi, T.; Nakata, M.; et al. Recommendations for saving mothers’ lives in Japan: Report from the Maternal Death Exploratory Committee (2010–2014). J. Obstet. Gynaecol. Res. 2016, 42, 1637–1643. [Google Scholar] [CrossRef]
- Rath, W.H. Postpartum hemorrhage--update on problems of definitions and diagnosis. Acta Obstet. Gynecol. Scand. 2011, 90, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Sohn, C.H.; Kim, W.Y.; Kim, S.R.; Seo, D.W.; Ryoo, S.M.; Lee, Y.S.; Lee, J.H.; Oh, B.J.; Won, H.S.; Shim, J.Y.; et al. An increase in initial shock index is associated with the requirement for massive transfusion in emergency department patients with primary postpartum hemorrhage. Shock 2013, 40, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.L.; Cottam, K.; Hezelgrave, N.L.; Seed, P.T.; Briley, A.; Bewley, S.; Chappell, L.C.; Shennan, A.H. Determination of Normal Ranges of Shock Index and Other Haemodynamic Variables in the Immediate Postpartum Period: A Cohort Study. PLoS ONE 2016, 11, e0168535. [Google Scholar] [CrossRef]
- Muñoz, M.; Stensballe, J.; Ducloy-Bouthors, A.S.; Bonnet, M.P.; De Robertis, E.; Fornet, I.; Goffinet, F.; Hofer, S.; Holzgreve, W.; Manrique, S.; et al. Patient blood management in obstetrics: Prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood Transfus. 2019, 17, 112–136. [Google Scholar] [CrossRef]
- Reikvam, H.; Steien, E.; Hauge, B.; Liseth, K.; Hagen, K.G.; Størkson, R.; Hervig, T. Thrombelastography. Transfus. Apher. Sci. 2009, 40, 119–123. [Google Scholar] [CrossRef]
- Mohamed, M.; Majeske, K.; Sachwani, G.R.; Kennedy, K.; Salib, M.; McCann, M. The impact of early thromboelastography directed therapy in trauma resuscitation. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Trautman, C.L.; Palmer, W.C.; Taner, C.B.; Canabal, J.M.; Getz, T.; Goldman, A.; Heckman, M.G.; Diehl, N.N.; Lee, D.D.; Stancampiano, F.F. Thromboelastography as a Predictor of Outcomes Following Liver Transplantation. Transplant. Proc. 2017, 49, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Welsby, I.J.; Jiao, K.; Ortel, T.L.; Brudney, C.S.; Roche, A.M.; Bennett-Guerrero, E.; Gan, T.J. The kaolin-activated Thrombelastograph predicts bleeding after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2006, 20, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Kim, Y.J.; Jeon, S.B.; Kim, W.Y. Thromboelastography for prediction of hemorrhagic transformation in patients with acute ischemic stroke. Am. J. Emerg. Med. 2020, 38, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Koami, H.; Sakamoto, Y.; Ohta, M.; Goto, A.; Narumi, S.; Imahase, H.; Yahata, M.; Miike, T.; Iwamura, T.; Yamada, K.C.; et al. Can rotational thromboelastometry predict septic disseminated intravascular coagulation? Blood Coagul. Fibrinolysis 2015, 26, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.; Abdul-Kadir, R.; Thachil, J. Management of coagulopathy associated with postpartum hemorrhage: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.W.; Bell, S.F.; de Lloyd, L.; Collis, R.E. Management of postpartum haemorrhage: From research into practice, a narrative review of the literature and the Cardiff experience. Int. J. Obstet. Anesth. 2019, 37, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Sohn, C.H.; Kim, Y.J.; Seo, D.W.; Won, H.S.; Shim, J.Y.; Lim, K.S.; Kim, W.Y. Blood lactate concentration and shock index associated with massive transfusion in emergency department patients with primary postpartum haemorrhage. Br. J. Anaesth. 2018, 121, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Erez, O.; Novack, L.; Beer-Weisel, R.; Dukler, D.; Press, F.; Zlotnik, A.; Than, N.G.; Tomer, A.; Mazor, M. DIC score in pregnant women--a population based modification of the International Society on Thrombosis and Hemostasis score. PLoS ONE 2014, 9, e93240. [Google Scholar] [CrossRef]
- Ramler, P.I.; van den Akker, T.; Henriquez, D.; Zwart, J.J.; van Roosmalen, J.; van Lith, J.M.M.; van der Bom, J.G. Women receiving massive transfusion due to postpartum hemorrhage: A comparison over time between two nationwide cohort studies. Acta Obstet. Gynecol. Scand. 2019, 98, 795–804. [Google Scholar] [CrossRef]
- Mhyre, J.M.; Shilkrut, A.; Kuklina, E.V.; Callaghan, W.M.; Creanga, A.A.; Kaminsky, S.; Bateman, B.T. Massive blood transfusion during hospitalization for delivery in New York State, 1998–2007. Obstet. Gynecol. 2013, 122, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Lee, H.S.; Jeon, S.Y.; You, J.S.; Lee, J.W.; Chung, H.S.; Chung, S.P. Delta neutrophil index and shock index can stratify risk for the requirement for massive transfusion in patients with primary postpartum hemorrhage in the emergency department. PLoS ONE 2021, 16, e0258619. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Regan, F.; Paterson-Brown, S. Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. Bjog 2006, 113, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Stafford, I.A.; Moaddab, A.; Dildy, G.A.; Klassen, M.; Berra, A.; Watters, C.; Belfort, M.A.; Romero, R.; Clark, S.L. Amniotic fluid embolism syndrome: Analysis of the Unites States International Registry. Am. J. Obstet. Gynecol. MFM 2020, 2, 100083. [Google Scholar] [CrossRef]
- Hofer, S.; Blaha, J.; Collins, P.W.; Ducloy-Bouthors, A.S.; Guasch, E.; Labate, F.; Lança, F.; Nyfløt, L.T.; Steiner, K.; Van de Velde, M. Haemostatic support in postpartum haemorrhage: A review of the literature and expert opinion. Eur. J. Anaesthesiol. 2023, 40, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Charbit, B.; Mandelbrot, L.; Samain, E.; Baron, G.; Haddaoui, B.; Keita, H.; Sibony, O.; Mahieu-Caputo, D.; Hurtaud-Roux, M.F.; Huisse, M.G.; et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J. Thromb. Haemost. 2007, 5, 266–273. [Google Scholar] [CrossRef] [PubMed]
- de Lloyd, L.; Bovington, R.; Kaye, A.; Collis, R.E.; Rayment, R.; Sanders, J.; Rees, A.; Collins, P.W. Standard haemostatic tests following major obstetric haemorrhage. Int. J. Obstet. Anesth. 2011, 20, 135–141. [Google Scholar] [CrossRef]
- Collins, P.W.; Lilley, G.; Bruynseels, D.; Laurent, D.B.; Cannings-John, R.; Precious, E.; Hamlyn, V.; Sanders, J.; Alikhan, R.; Rayment, R.; et al. Fibrin-based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage: A prospective study. Blood 2014, 124, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.F.; Collis, R.E.; Collins, P.W. Comparison of haematological indices and transfusion management in severe and massive postpartum haemorrhage: Analysis of a two-year national prospective observational study. Int. J. Obstet. Anesth. 2022, 50, 103547. [Google Scholar] [CrossRef]
- Hiippala, S.T.; Myllylä, G.J.; Vahtera, E.M. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth. Analg. 1995, 81, 360–365. [Google Scholar] [CrossRef]
- Collins, P.W.; Cannings-John, R.; Bruynseels, D.; Mallaiah, S.; Dick, J.; Elton, C.; Weeks, A.D.; Sanders, J.; Aawar, N.; Townson, J.; et al. Viscoelastometric-guided early fibrinogen concentrate replacement during postpartum haemorrhage: OBS2, a double-blind randomized controlled trial. Br. J. Anaesth. 2017, 119, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.D.; De Lloyd, L.; Bell, S.F.; Cohen, L.; James, D.; Ridgway, A.; Jenkins, V.; Field, V.; Collis, R.E.; Collins, P.W. Utility of viscoelastography with TEG 6s to direct management of haemostasis during obstetric haemorrhage: A prospective observational study. Int. J. Obstet. Anesth. 2021, 47, 103192. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.F.; Roberts, T.C.D.; Freyer Martins Pereira, J.; De Lloyd, L.; Amir, Z.; James, D.; Jenkins, P.V.; Collis, R.E.; Collins, P.W. The sensitivity and specificity of rotational thromboelastometry (ROTEM) to detect coagulopathy during moderate and severe postpartum haemorrhage: A prospective observational study. Int. J. Obstet. Anesth. 2022, 49, 103238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Saxena, R. A novel thromboelastographic score to identify overt disseminated intravascular coagulation resulting in a hypocoagulable state. Am. J. Clin. Pathol. 2010, 134, 97–102. [Google Scholar] [CrossRef] [PubMed]
- de Lange, N.M.; Lancé, M.D.; de Groot, R.; Beckers, E.A.; Henskens, Y.M.; Scheepers, H.C. Obstetric hemorrhage and coagulation: An update. Thromboelastography, thromboelastometry, and conventional coagulation tests in the diagnosis and prediction of postpartum hemorrhage. Obstet. Gynecol. Surv. 2012, 67, 426–435. [Google Scholar] [CrossRef]
- Cho, H.Y.; Na, S.; Kim, M.D.; Park, I.; Kim, H.O.; Kim, Y.H.; Park, Y.W.; Chun, J.H.; Jang, S.Y.; Chung, H.K.; et al. Implementation of a multidisciplinary clinical pathway for the management of postpartum hemorrhage: A retrospective study. Int. J. Qual. Health Care 2015, 27, 459–465. [Google Scholar] [CrossRef]
| Variables | MT Group (n = 34) | Non-MT Group (n = 150) | p Value |
|---|---|---|---|
| Age, years | 34.1 ± 4.2 | 32.9 ± 4.1 | 0.127 |
| Parity | 0.085 | ||
| Primipara | 16 (47.1) | 95 (63.3) | |
| Multipara | 18 (52.9) | 55 (36.7) | |
| Delivery type | 0.442 | ||
| Vaginal delivery | 23 (67.6) | 90 (60.0) | |
| Cesarean section | 11 (32.4) | 60 (40.0) | |
| Initial mental status | <0.001 | ||
| Alert | 28 (82.4) | 149 (99.3) | |
| Verbal | 4 (11.8) | 1 (0.7) | |
| Painful | 1 (2.9) | 0 (0.0) | |
| Unresponsive | 1 (2.9) | 0 (0.0) | |
| Initial vital signs | |||
| Systolic blood pressure, mmHg | 103.0 (75.0–121.0) | 115.0 (105.0–129.0) | 0.001 |
| Diastolic blood pressure, mmHg | 63.0 (50.0–72.0) | 73.0 (62.0–84.0) | 0.003 |
| Heart rate, beats/min | 114.0 (100.0–124.0) | 90.0 (80.0–101.0) | <0.001 |
| Body temperature, °C | 37.5 (37.0–38.0) | 37.4 (36.9–38.0) | 0.655 |
| Shock index | 1.1 (0.8–1.5) | 0.8 (0.7–0.9) | <0.001 |
| Variables | MT Group (n = 34) | Non-MT Group (n = 150) | p Value |
|---|---|---|---|
| Initial laboratory findings | |||
| Lactate, mmol/L | 2.8 (1.9–4.2) | 2.1 (1.6–3.0) | 0.006 |
| Hemoglobin, g/dL | 8.4 (6.7–10.3) | 10.2 (9.1–11.7) | <0.001 |
| Hematocrit, % | 26.6 (20.9–31.2) | 31.3 (27.7–35.2) | <0.001 |
| Platelets, ×103/µL | 113.0 (95.0–143.0) | 160.0 (133.0–201.0) | <0.001 |
| Prothrombin time (INR) | 1.4 (1.2–1.9) | 1.1 (1.0–1.2) | <0.001 |
| Fibrinogen, mg/dL | 104.0 (50.0–167.0) | 245.0 (169.0–325.0) | <0.001 |
| FDP, µg/mL | 115.0 (38.0–120.0) | 48.0 (22.0–120.0) | 0.13 |
| D-dimer, µg/mL | 35.2 (14.9–35.5) | 13.4 (7.6–35.2) | 0.018 |
| Pregnancy-modified ISTH DIC score | 51.0 (32.8–51.8) | 26.0 (7.0–27.8) | <0.001 |
| Amount of blood transfusion, units | |||
| Packed red blood cells | 12.0 (11.0–15.0) | 3.0 (2.0–5.0) | <0.001 |
| Fresh frozen plasma | 11.0 (8.0–12.0) | 2.0 (0.0–4.0) | <0.001 |
| Platelet concentrates | 10.0 (8.0–16.0) | 0.0 (0.0–0.0) | <0.001 |
| Clinical outcomes | |||
| Embolization | 30 (88.2) | 61 (40.7) | <0.001 |
| Hysterectomy | 2 (5.9) | 0 (0.0) | 0.033 |
| Length of hospital stay, days | 4.0 (2.0–5.0) | 2.0 (1.0–3.0) | <0.001 |
| ICU admission | 2 (5.9) | 5 (3.3) | 0.615 |
| In-hospital mortality | 1 (2.9) | 0 (0.0) | 0.185 |
| Variables | MT Group (n = 34) | Non-MT Group (n = 150) | AUC | p Value |
|---|---|---|---|---|
| R, min | 4.3 (3.2–6.4) | 3.8 (3.2–4.4) | 0.621 | 0.028 |
| K, min | 3.9 (2.2–6.4) | 1.5 (1.2–2.3) | 0.813 | <0.001 |
| Alpha angle, degrees | 47.5 (33.1–61.1) | 68.0 (58.4–73.1) | 0.794 | <0.001 |
| MA, mm | 48.9 (34.7–59.3) | 64.1 (56.1–68.6) | 0.801 | <0.001 |
| LY30, % | 0.0 (0.0–1.0) | 0.0 (0.0–0.3) | 0.527 | 0.58 |
| Variables | Adjusted Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Lactate, mmol/L | 1.674 | 1.218–2.300 | 0.001 |
| Shock index > 0.9 | 4.638 | 1.784–12.056 | 0.002 |
| Alpha angle < 60 degrees | 7.769 | 2.736–22.062 | <0.001 |
| Variables | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| K > 1.5 | 88.2 (72.6–96.7) | 52.0 (43.7–60.2) | 29.4 (25.3–33.9) | 95.1 (88.5–98.0) |
| Angle < 60 | 73.5 (55.6–87.1) | 72.0 (64.1–79.0) | 37.3 (30.0–45.2) | 92.3 (87.2–95.5) |
| MA < 63 | 85.3 (68.9–95.1) | 54.7 (46.3–62.8) | 29.9 (25.4–34.8) | 94.3 (87.8–97.4) |
| SI > 0.9 | 67.6 (49.5–82.6) | 72.7 (64.8–79.6) | 35.9 (28.3–44.3) | 90.8 (85.8–94.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.M.; Sohn, C.H.; Kwon, H.; Ryoo, S.M.; Ahn, S.; Seo, D.W.; Kim, W.Y. Prognostic Role of Initial Thromboelastography in Emergency Department Patients with Primary Postpartum Hemorrhage: Association with Massive Transfusion. J. Pers. Med. 2024, 14, 422. https://doi.org/10.3390/jpm14040422
Kim SM, Sohn CH, Kwon H, Ryoo SM, Ahn S, Seo DW, Kim WY. Prognostic Role of Initial Thromboelastography in Emergency Department Patients with Primary Postpartum Hemorrhage: Association with Massive Transfusion. Journal of Personalized Medicine. 2024; 14(4):422. https://doi.org/10.3390/jpm14040422
Chicago/Turabian StyleKim, Sang Min, Chang Hwan Sohn, Hyojeong Kwon, Seung Mok Ryoo, Shin Ahn, Dong Woo Seo, and Won Young Kim. 2024. "Prognostic Role of Initial Thromboelastography in Emergency Department Patients with Primary Postpartum Hemorrhage: Association with Massive Transfusion" Journal of Personalized Medicine 14, no. 4: 422. https://doi.org/10.3390/jpm14040422
APA StyleKim, S. M., Sohn, C. H., Kwon, H., Ryoo, S. M., Ahn, S., Seo, D. W., & Kim, W. Y. (2024). Prognostic Role of Initial Thromboelastography in Emergency Department Patients with Primary Postpartum Hemorrhage: Association with Massive Transfusion. Journal of Personalized Medicine, 14(4), 422. https://doi.org/10.3390/jpm14040422





