Personalized Approach to Olfactory Neuroblastoma Care
Abstract
:1. Introduction
2. Patient- and Tumor-Specific Factors
3. Primary Tumor Treatment
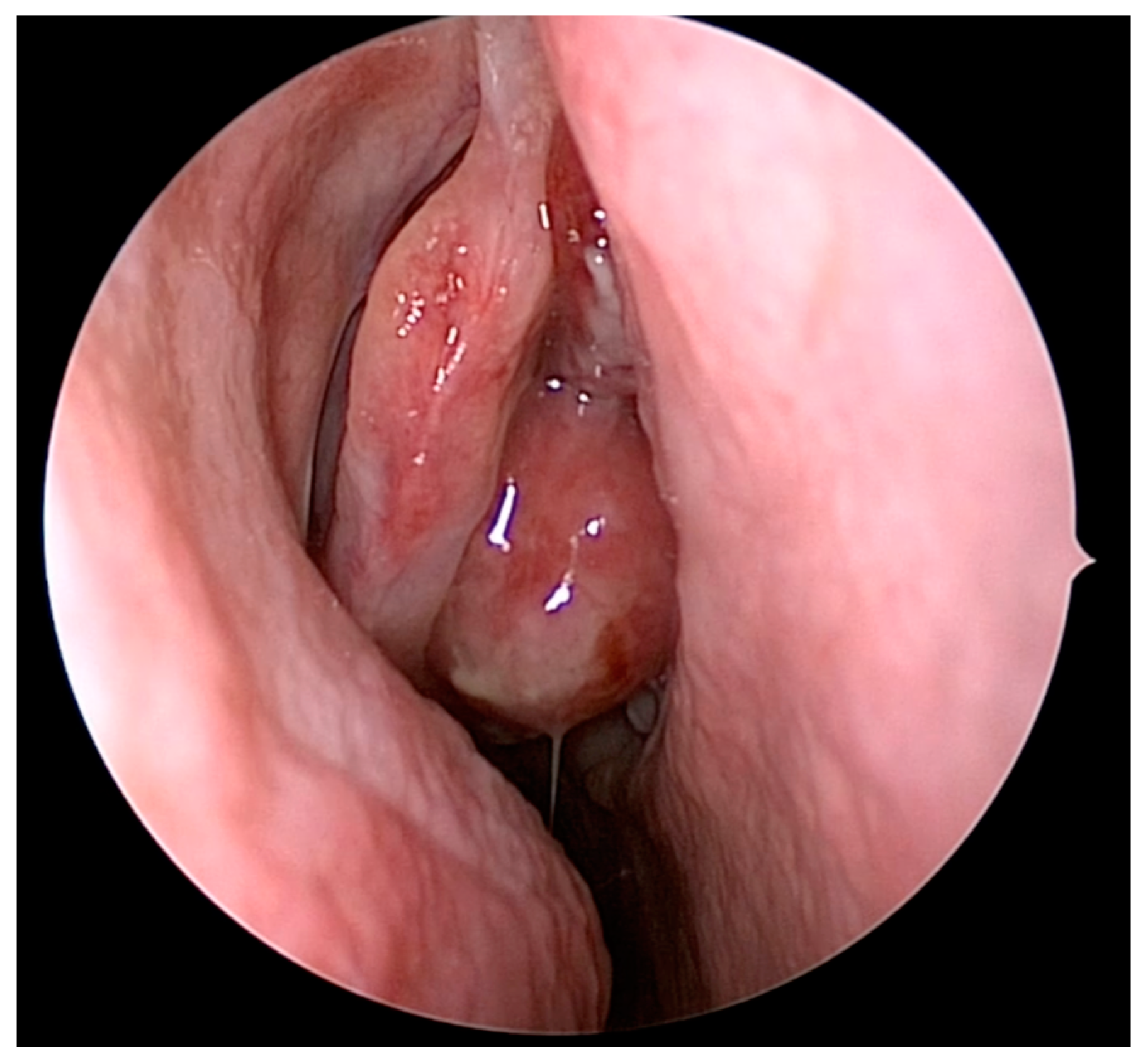
4. Olfactory Preservation and Surgical Resection of ONB
5. Treatment of the Neck
6. Posttreatment Surveillance and Salvage Therapy for Recurrence
7. Adjuvant Treatment
8. Discussion
9. Pediatric ONB
10. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, J.S.; Palmer, J.N.; Adappa, N.D. Contemporary management of esthesioneuroblastoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 63–69. [Google Scholar] [CrossRef]
- Kuan, E.C.; Wang, E.W.; Adappa, N.D.; Beswick, D.M.; London, N.R., Jr.; Su, S.Y.; Wang, M.B.; Abuzeid, W.M.; Alexiev, B.; Alt, J.A.; et al. International Consensus Statement on Allergy and Rhinology: Sinonasal Tumors. Int. Forum Allergy Rhinol. 2023, 14, 149–608. [Google Scholar] [CrossRef] [PubMed]
- Tosoni, A.; Di Nunno, V.; Gatto, L.; Corradi, G.; Bartolini, S.; Ranieri, L.; Franceschi, E. Olfactory neuroblastoma: Diagnosis, management, and current treatment options. Front. Oncol. 2023, 13, 1242453. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Hanna, E.Y.; Weber, R.S.; DeMonte, F.; Triantafyllou, A.; Lewis, J.S., Jr.; Cardesa, A.; Slootweg, P.J.; Stenman, G.; Gnepp, D.R.; et al. Neuroendocrine neoplasms of the sinonasal region. Head Neck 2016, 38 (Suppl. 1), E2259–E2266. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.H.; Lehrich, B.M.; Yasaka, T.M.; Fong, B.M.; Hsu, F.P.K.; Kuan, E.C. Characteristics and overall survival in pediatric versus adult esthesioneuroblastoma: A population-based study. Int. J. Pediatr. Otorhinolaryngol. 2021, 144, 110696. [Google Scholar] [CrossRef] [PubMed]
- Geltzeiler, M.; Choby, G.W.; Ji, K.S.Y.; JessMace, C.; Almeida, J.P.; de Almeida, J.; Champagne, P.O.; Chan, E.; Ciporen, J.N.; Chaskes, M.B.; et al. Radiographic predictors of occult intracranial involvement in olfactory neuroblastoma patients. Int. Forum Allergy Rhinol. 2023, 13, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Zafereo, M.E.; Fakhri, S.; Prayson, R.; Batra, P.S.; Lee, J.; Lanza, D.C.; Citardi, M.J. Esthesioneuroblastoma: 25-year experience at a single institution. Otolaryngol. Head Neck Surg. 2008, 138, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Dublin, A.B.; Bobinski, M. Imaging Characteristics of Olfactory Neuroblastoma (Esthesioneuroblastoma). J. Neurol. Surg. B Skull Base 2016, 77, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.R.; Husain, Q.; Roman, B.R.; Cracchiolo, J.; Yu, Y.; Tsai, J.; Kang, J.; McBride, S.; Lee, N.Y.; Morris, L.; et al. Comparing Kadish, TNM, and the modified Dulguerov staging systems for esthesioneuroblastoma. J. Surg. Oncol. 2019, 119, 130–142. [Google Scholar] [CrossRef]
- Lechner, M.; Takahashi, Y.; Turri-Zanoni, M.; Liu, J.; Counsell, N.; Hermsen, M.; Kaur, R.P.; Zhao, T.; Ramanathan, M., Jr.; Schartinger, V.H.; et al. Clinical outcomes, Kadish-INSICA staging and therapeutic targeting of somatostatin receptor 2 in olfactory neuroblastoma. Eur. J. Cancer 2022, 162, 221–236. [Google Scholar] [CrossRef]
- Su, S.Y.; Bell, D.; Ferrarotto, R.; Phan, J.; Roberts, D.; Kupferman, M.E.; Frank, S.J.; Fuller, C.D.; Gunn, G.B.; Kies, M.S.; et al. Outcomes for olfactory neuroblastoma treated with induction chemotherapy. Head Neck 2017, 39, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Akay, S.; Pollard, J.H.; Saad Eddin, A.; Alatoum, A.; Kandemirli, S.; Gholamrezanezhad, A.; Menda, Y.; Graham, M.M.; Shariftabrizi, A. PET/CT Imaging in Treatment Planning and Surveillance of Sinonasal Neoplasms. Cancers 2023, 15, 3759. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Goldrich, D.Y.; Ninan, S.J.; Filimonov, A.; Lam, H.; Govindaraj, S.; Iloreta, A.M. The value of (68) Gallium-DOTATATE PET/CT in sinonasal neuroendocrine tumor management: A case series. Head Neck 2021, 43, E30–E40. [Google Scholar] [CrossRef] [PubMed]
- Cracolici, V.; Wang, E.W.; Gardner, P.A.; Snyderman, C.; Gargano, S.M.; Chiosea, S.; Singhi, A.D.; Seethala, R.R. SSTR2 Expression in Olfactory Neuroblastoma: Clinical and Therapeutic Implications. Head Neck Pathol. 2021, 15, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Roytman, M.; Tassler, A.B.; Kacker, A.; Schwartz, T.H.; Dobri, G.A.; Strauss, S.B.; Capalbo, A.M.; Magge, R.S.; Barbaro, M.; Lin, E.; et al. [68Ga]-DOTATATE PET/CT and PET/MRI in the diagnosis and management of esthesioneuroblastoma: Illustrative cases. J. Neurosurg. Case Lessons 2021, 1, CASE2058. [Google Scholar] [CrossRef] [PubMed]
- Sanli, Y.; Garg, I.; Kandathil, A.; Kendi, T.; Zanetti, M.J.B.; Kuyumcu, S.; Subramaniam, R.M. Neuroendocrine Tumor Diagnosis and Management: (68)Ga-DOTATATE PET/CT. AJR Am. J. Roentgenol. 2018, 211, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Goshtasbi, K.; Abiri, A.; Abouzari, M.; Sahyouni, R.; Wang, B.Y.; Tajudeen, B.A.; Hsu, F.P.K.; Cadena, G.; Kuan, E.C. Hyams grading as a predictor of metastasis and overall survival in esthesioneuroblastoma: A meta-analysis. Int. Forum Allergy Rhinol. 2019, 9, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Jethanamest, D.; Morris, L.G.; Sikora, A.G.; Kutler, D.I. Esthesioneuroblastoma: A population-based analysis of survival and prognostic factors. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 276–280. [Google Scholar] [CrossRef]
- Carey, R.M.; Godovchik, J.; Workman, A.D.; Kuan, E.C.; Parasher, A.K.; Chen, J.; Palmer, J.N.; Adappa, N.D.; Newman, J.G.; Brant, J.A. Patient, disease, and treatment factors associated with overall survival in esthesioneuroblastoma. Int. Forum Allergy Rhinol. 2017, 7, 1186–1194. [Google Scholar] [CrossRef]
- Stokes, W.A.; Camilon, P.R.; Banglawala, S.M.; Nguyen, S.A.; Harvey, R.; Vandergrift, W.A.; Schlosser, R.J. Is sex an independent prognostic factor in esthesioneuroblastoma? Am. J. Rhinol. Allergy 2015, 29, 369–372. [Google Scholar] [CrossRef]
- Saade, R.E.; Hanna, E.Y.; Bell, D. Prognosis and biology in esthesioneuroblastoma: The emerging role of Hyams grading system. Curr. Oncol. Rep. 2015, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Meerwein, C.M.; Nikolaou, G.; Binz, G.H.A.; Soyka, M.B.; Holzmann, D. Surgery as Single-Modality Treatment for Early-Stage Olfactory Neuroblastoma: An Institutional Experience, Systematic Review and Meta-analysis. Am. J. Rhinol. Allergy 2021, 35, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Ziai, H.; Yu, E.; Weinreb, I.; Perez-Ordonez, B.; Yao, C.; Xu, W.; Yang, D.; Witterick, I.J.; Monteiro, E.; Gilbert, R.W.; et al. Regional Recurrences and Hyams Grade in Esthesioneuroblastoma. J. Neurol. Surg. B Skull Base 2021, 82, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.R.; Blakaj, D.; London, N.; Blakaj, A.; Klamer, B.; Pan, J.; Wakely, P.; Prevedello, L.; Bonomi, M.; Bhatt, A.; et al. Clinical Outcomes and Multidisciplinary Patterns of Failure for Olfactory Neuroblastoma: The Ohio State Experience. J. Neurol. Surg. B Skull Base 2020, 81, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.S.R.; Subramaniam, T.; Leader, M.; McConn-Walsh, R.; O’Neill, J.P.; Lacy, P.D. Changing Trends in the Management of Esthesioneuroblastoma: Irish and International Perspectives. J. Neurol. Surg. B Skull Base 2018, 79, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kadish, S.; Goodman, M.; Wang, C.C. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer 1976, 37, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Ebersold, M.J.; Olsen, K.D.; Foote, R.L.; Lewis, J.E.; Quast, L.M. Esthesioneuroblastoma: Prognosis and management. Neurosurgery 1993, 32, 706–714, discussion 714–705. [Google Scholar] [CrossRef] [PubMed]
- Dulguerov, P.; Calcaterra, T. Esthesioneuroblastoma: The UCLA experience 1970–1990. Laryngoscope 1992, 102, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Ugiliweneza, B.; Boakye, M.; Andaluz, N.; Williams, B.J. Feasibility of Bundled Payments in Anterior, Middle, and Posterior Cranial Fossa Skull Base Meningioma Surgery: MarketScan Analysis of Health Care Utilization and Outcomes. World Neurosurg. 2019, 131, e116–e127. [Google Scholar] [CrossRef]
- Orton, A.; Boothe, D.; Evans, D.; Lloyd, S.; Monroe, M.M.; Jensen, R.; Shrieve, D.C.; Hitchcock, Y.J. Esthesioneuroblastoma: A Patterns-of-Care and Outcomes Analysis of the National Cancer Database. Neurosurgery 2018, 83, 940–947. [Google Scholar] [CrossRef]
- Konuthula, N.; Iloreta, A.M.; Miles, B.; Rhome, R.; Ozbek, U.; Genden, E.M.; Posner, M.; Misiukiewicz, K.; Govindaraj, S.; Shrivastava, R.; et al. Prognostic significance of Kadish staging in esthesioneuroblastoma: An analysis of the National Cancer Database. Head Neck 2017, 39, 1962–1968. [Google Scholar] [CrossRef]
- Fu, T.S.; Monteiro, E.; Muhanna, N.; Goldstein, D.P.; de Almeida, J.R. Comparison of outcomes for open versus endoscopic approaches for olfactory neuroblastoma: A systematic review and individual participant data meta-analysis. Head Neck 2016, 38 (Suppl. 1), E2306–E2316. [Google Scholar] [CrossRef]
- Harvey, R.J.; Nalavenkata, S.; Sacks, R.; Adappa, N.D.; Palmer, J.N.; Purkey, M.T.; Schlosser, R.J.; Snyderman, C.; Wang, E.W.; Woodworth, B.A.; et al. Survival outcomes for stage-matched endoscopic and open resection of olfactory neuroblastoma. Head Neck 2017, 39, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Barinsky, G.L.; Azmy, M.C.; Kilic, S.; Grube, J.G.; Baredes, S.; Hsueh, W.D.; Eloy, J.A. Comparison of Open and Endoscopic Approaches in the Resection of Esthesioneuroblastoma. Ann. Otol. Rhinol. Laryngol. 2021, 130, 136–141. [Google Scholar] [CrossRef]
- Abdelmeguid, A.S.; Bell, D.; Roberts, D.; Ferrarotto, R.; Phan, J.; Su, S.Y.; Kupferman, M.; Raza, S.; DeMonte, F.; Hanna, E. Long-Term Outcomes of Olfactory Neuroblastoma: MD Anderson Cancer Center Experience and Review of the Literature. Laryngoscope 2022, 132, 290–297. [Google Scholar] [CrossRef]
- Wu, K.; Avila, S.A.; Bhuyan, R.; Matloob, A.; Del Signore, A.G.; Hadjipanayis, C.; Chelnis, J. Orbital invasion by Esthesioneuroblastoma: A comparative case series and review of literature. Orbit 2022, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.A.; Van Gompel, J.J.; Link, M.J.; Moore, E.J.; Price, D.L.; Stokken, J.K.; Van Abel, K.M.; O’Byrne, J.; Giannini, C.; Chintakuntlawar, A.; et al. Long-term oncologic outcomes in esthesioneuroblastoma: An institutional experience of 143 patients. Int. Forum Allergy Rhinol. 2022, 12, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, J.; Lund, V.J.; Beale, T.; Wei, W.I.; Howard, D. Olfactory neuroblastoma: A 35-year experience and suggested follow-up protocol. Laryngoscope 2014, 124, 1542–1549. [Google Scholar] [CrossRef]
- Wang, E.W.; Zanation, A.M.; Gardner, P.A.; Schwartz, T.H.; Eloy, J.A.; Adappa, N.D.; Bettag, M.; Bleier, B.S.; Cappabianca, P.; Carrau, R.L.; et al. ICAR: Endoscopic skull-base surgery. Int. Forum Allergy Rhinol. 2019, 9, S145–S365. [Google Scholar] [CrossRef]
- Herr, M.W.; Gray, S.T.; Erman, A.B.; Curry, W.T.; Deschler, D.G.; Lin, D.T. Orbital preservation in patients with esthesioneuroblastoma. J. Neurol. Surg. B Skull Base 2013, 74, 142–145. [Google Scholar] [CrossRef]
- Li, R.; Tian, S.; Zhu, Y.; Yan, L.; Zhu, W.; Quan, H.; Wang, S. Management of orbital invasion in esthesioneuroblastoma: 14 years’ experience. Radiat. Oncol. 2019, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.C.; Marinelli, J.P.; Janus, J.R.; Chintakuntlawar, A.V.; Foote, R.L.; Link, M.J.; Choby, G.; Van Gompel, J.J. Induction Therapy Prior to Surgical Resection for Patients Presenting with Locally Advanced Esthesioneuroblastoma. J. Neurol. Surg. B Skull Base 2021, 82, e131–e137. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, B.A.; Adappa, N.D.; Kuan, E.C.; Schwartz, J.S.; Suh, J.D.; Wang, M.B.; Palmer, J.N. Smell preservation following endoscopic unilateral resection of esthesioneuroblastoma: A multi-institutional experience. Int. Forum Allergy Rhinol. 2016, 6, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Gompel, J.J.V.; Janus, J.R.; Hughes, J.D.; Stokken, J.K.; Moore, E.J.; Ryan, T.; Price, D.L.; Link, M.J. Esthesioneuroblastoma and Olfactory Preservation: Is it Reasonable to Attempt Smell Preservation? J. Neurol. Surg. B Skull Base 2018, 79, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kodama, S.; Kobayashi, M.; Sanuki, T.; Tanaka, S.; Hanai, N.; Hanazawa, T.; Monobe, H.; Yokoi, H.; Suzuki, M.; et al. Endoscopic endonasal management of esthesioneuroblastoma: A retrospective multicenter study. Auris Nasus Larynx 2018, 45, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Gomez Galarce, M.; Yanez-Siller, J.C.; Carrau, R.L.; Montaser, A.; Lima, L.R.; Servian, D.; Otto, B.A.; Prevedello, D.M.; Naudy, C.A. Endonasal anatomy of the olfactory neural network: Surgical implications. Laryngoscope 2018, 128, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Nalavenkata, S.B.; Sacks, R.; Adappa, N.D.; Palmer, J.N.; Purkey, M.T.; Feldman, M.D.; Schlosser, R.J.; Snyderman, C.H.; Wang, E.W.; Woodworth, B.A.; et al. Olfactory Neuroblastoma: Fate of the Neck--A Long-term Multicenter Retrospective Study. Otolaryngol. Head Neck Surg. 2016, 154, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kuan, E.C.; Nasser, H.B.; Carey, R.M.; Workman, A.D.; Alonso, J.E.; Wang, M.B.; John, M.A.S.; Palmer, J.N.; Adappa, N.D.; Tajudeen, B.A. A Population-Based Analysis of Nodal Metastases in Esthesioneuroblastomas of the Sinonasal Tract. Laryngoscope 2019, 129, 1025–1029. [Google Scholar] [CrossRef]
- Ni, G.; Pinheiro-Neto, C.D.; Iyoha, E.; Van Gompel, J.J.; Link, M.J.; Peris-Celda, M.; Moore, E.J.; Stokken, J.K.; Gamez, M.; Choby, G. Recurrent Esthesioneuroblastoma: Long-Term Outcomes of Salvage Therapy. Cancers 2023, 15, 1506. [Google Scholar] [CrossRef]
- Bao, C.; Hu, W.; Hu, J.; Dong, Y.; Lu, J.J.; Kong, L. Intensity-Modulated Radiation Therapy for Esthesioneuroblastoma: 10-Year Experience of a Single Institute. Front. Oncol. 2020, 10, 1158. [Google Scholar] [CrossRef]
- Nakamura, N.; Zenda, S.; Tahara, M.; Okano, S.; Hayashi, R.; Hojo, H.; Hotta, K.; Kito, S.; Motegi, A.; Arahira, S.; et al. Proton beam therapy for olfactory neuroblastoma. Radiother. Oncol. 2017, 122, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Duo, G.S.; Feng, J.L.; Zhang, Z.Y.; Wang, L.J. Survival impact of postoperative radiotherapy in patients with olfactory neuroblastoma: 513 cases from the SEER database. Cancer Radiother. 2022, 26, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Brisson, R.J.; Quinn, T.J.; Deraniyagala, R.L. The role of chemotherapy in the management of olfactory neuroblastoma: A 40-year surveillance, epidemiology, and end results registry study. Health Sci. Rep. 2021, 4, e257. [Google Scholar] [CrossRef] [PubMed]
- Cranmer, L.D.; Chau, B.; Rockhill, J.K.; Ferreira, M., Jr.; Liao, J.J. Chemotherapy in Esthesioneuroblastoma/Olfactory Neuroblastoma: An Analysis of the Surveillance Epidemiology and End Results (SEER) 1973-2015 Database. Am. J. Clin. Oncol. 2020, 43, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Romani, C.; Bignotti, E.; Mattavelli, D.; Bozzola, A.; Lorini, L.; Tomasoni, M.; Ardighieri, L.; Rampinelli, V.; Paderno, A.; Battocchio, S.; et al. Gene Expression Profiling of Olfactory Neuroblastoma Helps Identify Prognostic Pathways and Define Potentially Therapeutic Targets. Cancers 2021, 13, 2527. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Spielman, D.; Otten, M.; Bruce, J.N.; Feldstein, N.; Overdevest, J.B.; Gudis, D.A. Treatment Strategies and Outcomes of Pediatric Esthesioneuroblastoma: A Systematic Review. Front. Oncol. 2020, 10, 1247. [Google Scholar] [CrossRef] [PubMed]
- Venkatramani, R.; Pan, H.; Furman, W.L.; Marron, J.M.; Haduong, J.; Friedrich-Medina, P.; Mahajan, A.; Bavle, A.; Wu, H.; Chintagumpala, M. Multimodality Treatment of Pediatric Esthesioneuroblastoma. Pediatr. Blood Cancer 2016, 63, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Fallon, R.J.; Hill, J.S.; Davis, M.M. Esthesioneuroblastoma in children. J. Pediatr. Hematol. Oncol. 2002, 24, 482–487. [Google Scholar] [CrossRef] [PubMed]
- El Kababri, M.; Habrand, J.L.; Valteau-Couanet, D.; Gaspar, N.; Dufour, C.; Oberlin, O. Esthesioneuroblastoma in children and adolescent: Experience on 11 cases with literature review. J. Pediatr. Hematol. Oncol. 2014, 36, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Classe, M.; Yao, H.; Mouawad, R.; Creighton, C.J.; Burgess, A.; Allanic, F.; Wassef, M.; Leroy, X.; Verillaud, B.; Mortuaire, G.; et al. Integrated Multi-omic Analysis of Esthesioneuroblastomas Identifies Two Subgroups Linked to Cell Ontogeny. Cell Rep. 2018, 25, 811–821.e5. [Google Scholar] [CrossRef]


| Stage A | Tumor confined to the nasal cavity |
| Stage B | Tumor confined to the nasal cavity and paranasal sinuses |
| Stage C | Tumor extent beyond nasal cavity and paranasal sinuses, including involvement of the cribriform plate, base of the skull, orbit, or intracranial cavity |
| Stage D | Tumor with metastasis to cervical lymph nodes or distant sites |
| T1 | Tumor involving the nasal cavity and/or paranasal sinuses (excluding sphenoid), sparing the most superior ethmoidal cells |
| T2 | Tumor involving the nasal cavity and/or paranasal sinuses (including the sphenoid) with extension to or erosion of the cribriform plate |
| T3 | Tumor extending into the orbit or protruding into the anterior cranial fossa |
| T4 | Tumor involving the brain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerner, D.K.; Palmer, J.N. Personalized Approach to Olfactory Neuroblastoma Care. J. Pers. Med. 2024, 14, 423. https://doi.org/10.3390/jpm14040423
Lerner DK, Palmer JN. Personalized Approach to Olfactory Neuroblastoma Care. Journal of Personalized Medicine. 2024; 14(4):423. https://doi.org/10.3390/jpm14040423
Chicago/Turabian StyleLerner, David K., and James N. Palmer. 2024. "Personalized Approach to Olfactory Neuroblastoma Care" Journal of Personalized Medicine 14, no. 4: 423. https://doi.org/10.3390/jpm14040423
APA StyleLerner, D. K., & Palmer, J. N. (2024). Personalized Approach to Olfactory Neuroblastoma Care. Journal of Personalized Medicine, 14(4), 423. https://doi.org/10.3390/jpm14040423




