Advances in Molecular and Genetic Technologies and the Problems Related to Their Application in Personalized Medicine
Abstract
:1. Introduction
- Investment in biotechnology related to personalized medicine catalyzes research and development. Therefore, novel technologies as well as diagnosis and treatment methods are created, in turn contributing to growth of the industry and the economy as a whole.
- Application of biotechnologies in personalized medicine allows one to develop customized treatment approaches taking into account patients’ genetic characteristics. It reduces the cost of treating complications and improves healthcare outcomes, eventually saving resources and increasing efficiency.
- Furthermore, advances in this sector will also contribute to creating new jobs due to the founding of new companies and laboratories as well as launching innovative projects.
- Countries investing in biotechnology and personalized medicine solidify their global market position. The cutting-edge medical technologies and chances to receive personalized treatment are attractive for patients, which spurs the development of medical tourism and attracts more foreign patients and medical experts to the country.
2. Methods
3. Results
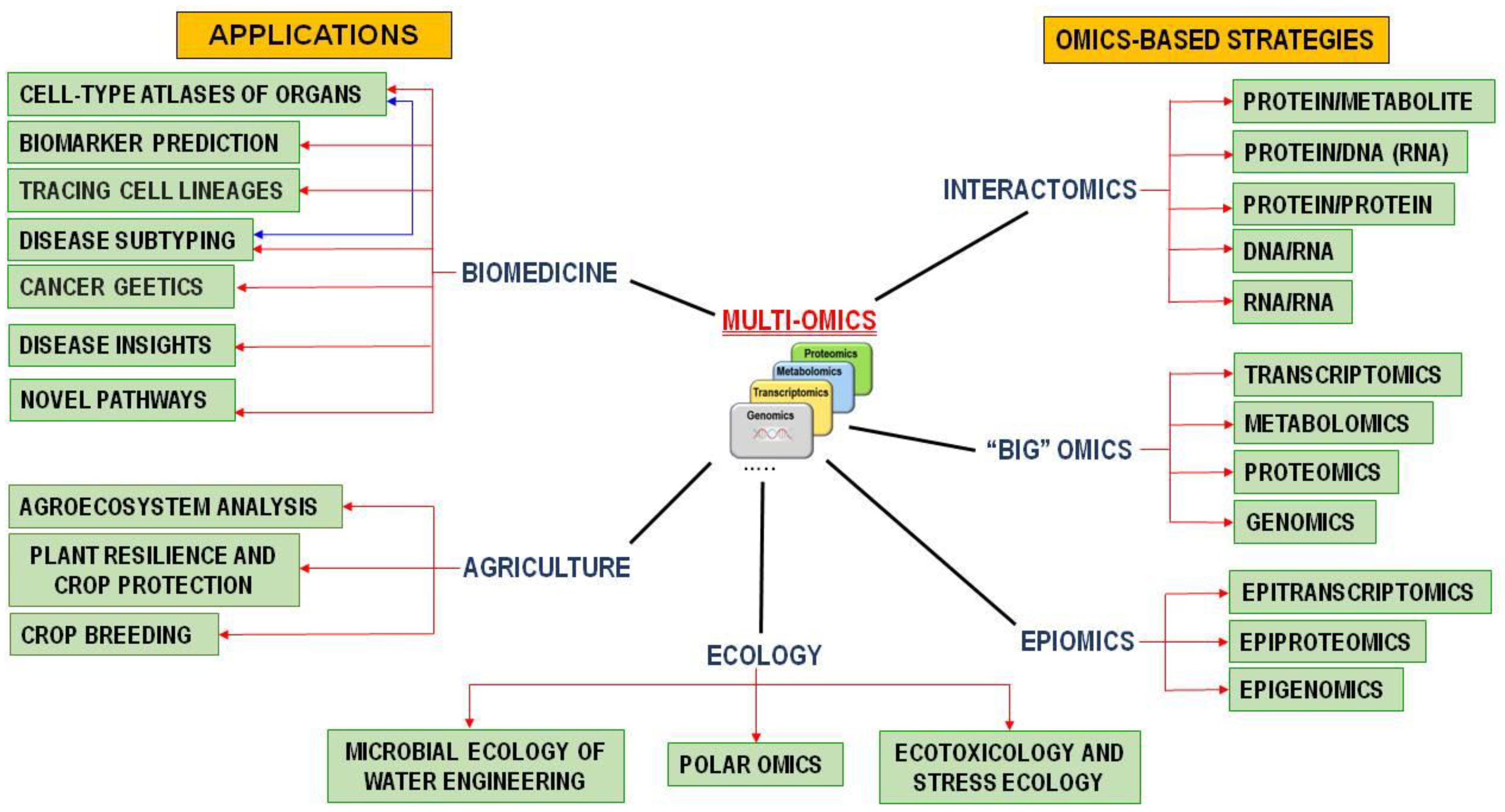
3.1. Development Trends for Molecular and Genetic Technologies
- -
- pharmaceuticals: vaccine formulations, personalized drugs, identification of novel molecular biomarkers, and molecular diagnostics;
- -
- personalized medicine: microarrays and biosensors;
- -
- agrobiotechnology: genetic engineering, biologically pure foods, etc.;
- -
- bioindustry: DNA sequencing, recombinant technologies, fermentation, tissue engineering, chromatography, PCR technologies, nanobiotechnology, and cellular analysis;
- -
- bioinformatics: elaborating algorithms for big data storage and management.
- -
- short read length (the average read length ranges from 32 to 330 base pairs) and
- -
- low accuracy compared to that for Sanger sequencing.
3.2. Developments Contributing to the Progress in Personalized Medicine
- -
- oncology: diagnosing a large number of diseases at the early (preclinical) stage;
- -
- allergology: screening across 17 mixed allergen groups (house dust, grass and tree pollen, pets, etc.), as well as inhalant and food allergens;
- -
- cardiology: rapid diagnosis of cardiovascular diseases outside of specialized medical settings;
- -
- infectious diseases: early symptoms of some infectious diseases are often almost identical, while their therapies differ fundamentally, so efficient segmentation of such diseases is a very relevant area;
- -
- dentistry: microbiological analysis of the oral cavity to detect various types of bacteria;
- -
- transfusion medicine: in the near future, laboratory screening for all the parameters (blood group, serology, bacteriology and virology testing) in blood biorepositories will be based on the DNA microarray technology [34];
- -
3.3. Nanomedicine—Medical Applications of Nanotechnology
- highly sensitive biosensors and nanoparticles with a functionalized surface for phishing and recognition of biomarkers in the body for early diagnosis of diseases [60];
- nanoparticles for delivering drugs directly to affected tissues or organs in order to increase the effectiveness of treatment, as well as reduce negative side effects [61];
- nanodevices for monitoring various health indicators (pulse, glucose and oxygen levels in the blood) in real time. The collected data can be transferred to a smartphone or other device to warn of possible problems [62];
- nanomaterials for the needs of regenerative medicine, used in the creation of biocompatible materials [63].
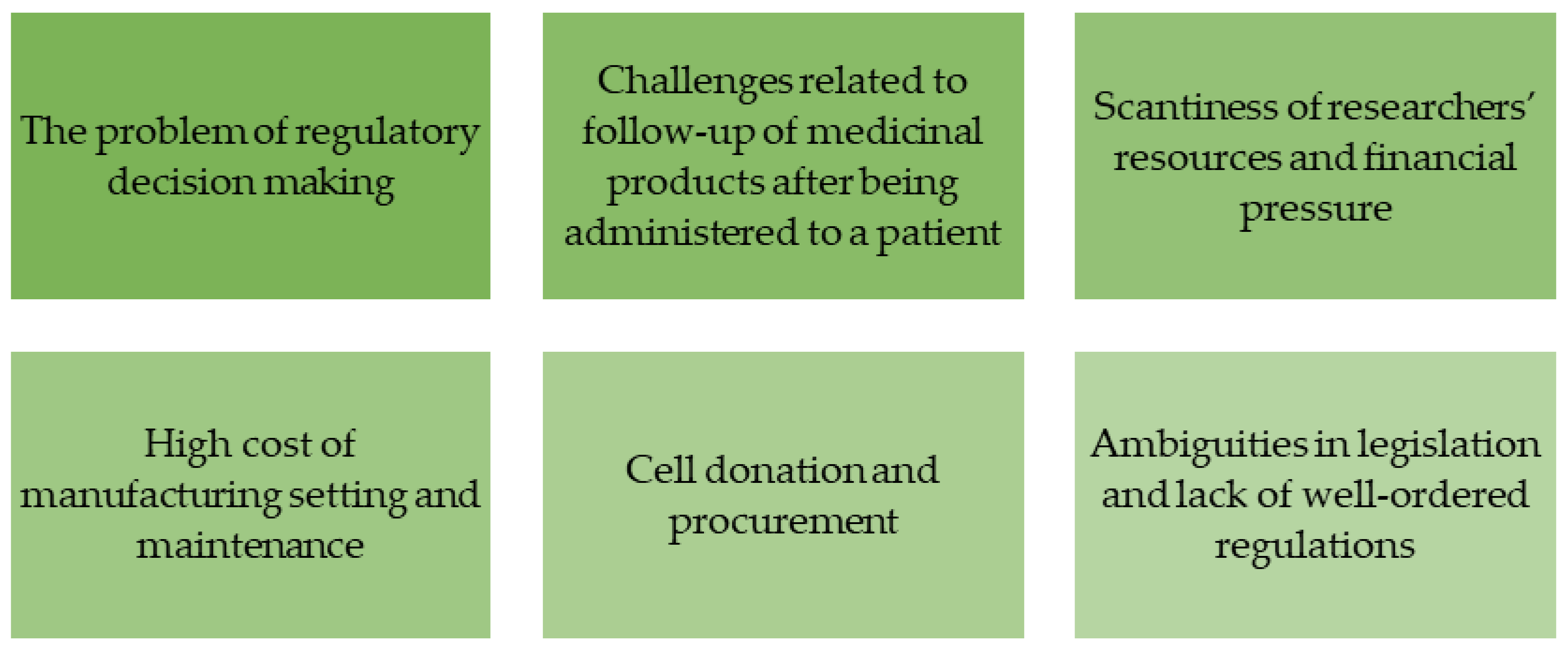
3.4. The Problems Related to Application of Molecular and Genetic Technologies
3.5. The Needs of the Development of Molecular and Genetic Technologies
- -
- cell engineering;
- -
- bioengineering;
- -
- medicine;
- -
- targeted drug delivery;
- -
- pharmaceutics;
- -
- molecular biosensors;
- -
- research into the origin of life, etc.
- -
- DNA microarrays (identification of genes and mutations in them, monitoring gene expression, diagnosing diseases and assessing therapy effectiveness, as well as screening of microorganisms);
- -
- protein microarrays (identification and quantification of proteins during the development of various pathologies, assessment of the effectiveness of pharmaceuticals);
- -
- cellular microarrays (analysis of interactions of protein molecules in the cell);
- -
- tissue microarrays (analysis of tissue samples);
- -
- small-molecule microarrays (one-step screening of a large number of potential drugs);
- -
- microfluidic microarrays (repeated examination of biological objects).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biotechnology Conference|Biotechnology Congress|Biotechnology Engineering Conference|Chicago|USA|2023. Available online: https://www.lexismeeting.com/biotechnologycongress (accessed on 30 November 2023).
- Biotechnology Market Size, Share & Trends Analysis Report by Technology (Nanobiotechnology, DNA Sequencing, Cell-Based Assays), By Application (Health, Bioinformatics), By Region, And Segment Forecasts, 2023–2030. Available online: https://www.researchandmarkets.com/reports/4396357/biotechnology-market-size-share-and-trends#sp-pos-39 (accessed on 30 November 2023).
- Ugalmugle, S.; Swain, R. Biotechnology Market; Global Market In Sights, Inc.: Selbyville, DE, USA, 2019. [Google Scholar]
- Valieva, O.V. Biotechnology Market Development: Global Trends and the Place of Russia. World Econ. Manag. 2021, 21, 82–102. [Google Scholar] [CrossRef]
- Prendergast, S.C.; Strobl, A.-C.; Cross, W.; Pillay, N.; Strauss, S.J.; Ye, H.; Lindsay, D.; Tirabosco, R.; Chalker, J.; Mahamdallie, S.S.; et al. Sarcoma and the 100,000 Genomes Project: Our Experience and Changes to Practice. J. Pathol. Clin. Res. 2020, 6, 297–307. [Google Scholar] [CrossRef]
- Thomas, R.; Pontius, J.U.; Borst, L.B.; Breen, M. Development of a Genome-Wide Oligonucleotide Microarray Platform for Detection of DNA Copy Number Aberrations in Feline Cancers. Vet. Sci. 2020, 7, 88. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Hossain, M.U.; Islam, M.N.; Rahman, M.H.; Ahmed, I.; Rahman, T.A.; Bhattacharjee, A.; Amin, M.R.; Rashed, A.; Keya, C.A.; et al. Coding-Complete Genome Sequence of SARS-CoV-2 Isolate from Bangladesh by Sanger Sequencing. Microbiol. Resour. Announc. 2020, 9, e00626-20. [Google Scholar] [CrossRef]
- Chen, X.; Dong, Y.; Huang, Y.; Fan, J.; Yang, M.; Zhang, J. Whole-Genome Resequencing Using next-Generation and Nanopore Sequencing for Molecular Characterization of T-DNA Integration in Transgenic Poplar 741. BMC Genom. 2021, 22, 329. [Google Scholar] [CrossRef]
- Ou, Y.-J.; Ren, Q.-Q.; Fang, S.-T.; Wu, J.-G.; Jiang, Y.-X.; Chen, Y.-R.; Zhong, Y.; Wang, D.-D.; Zhang, G.-X. Complete Genome Insights into Lactococcus Petauri CF11 Isolated from a Healthy Human Gut Using Second- and Third-Generation Sequencing. Front. Genet. 2020, 11, 119. [Google Scholar] [CrossRef]
- Painter, H.J.; Altenhofen, L.M.; Kafsack, B.F.C.; Llinás, M. Whole-Genome Analysis of Plasmodium Spp. Utilizing a New Agilent Technologies DNA Microarray Platform. Methods Mol. Biol. 2013, 923, 213–219. [Google Scholar] [CrossRef]
- Graham, N.S.; May, S.T.; Daniel, Z.C.T.R.; Emmerson, Z.F.; Brameld, J.M.; Parr, T. Use of the Affymetrix Human GeneChip Array and Genomic DNA Hybridisation Probe Selection to Study Ovine Transcriptomes. Animal 2011, 5, 861–866. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, N.; Li, M.; Chen, F.; Niu, H. Applications of Next-Generation Sequencing in Systemic Autoimmune Diseases. Genom. Proteom. Bioinform. 2015, 13, 242–249. [Google Scholar] [CrossRef]
- Sahu, S.; Gupta, P.; Dey, P. Molecular Testing on Serous Effusion: An Update. Cytojournal 2021, 18, 35. [Google Scholar] [CrossRef]
- Singh, R.R. Target Enrichment Approaches for Next-Generation Sequencing Applications in Oncology. Diagnostics 2022, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, F.; Shuai, Y.; Huang, L.; Zhang, X. Integrated Analysis of Single-Molecule Real-Time Sequencing and Next-Generation Sequencing Eveals Insights into Drought Tolerance Mechanism of Lolium Multiflorum. Int. J. Mol. Sci. 2022, 23, 7921. [Google Scholar] [CrossRef] [PubMed]
- Pruneri, G.; De Braud, F.; Sapino, A.; Aglietta, M.; Vecchione, A.; Giusti, R.; Marchiò, C.; Scarpino, S.; Baggi, A.; Bonetti, G.; et al. Next-Generation Sequencing in Clinical Practice: Is It a Cost-Saving Alternative to a Single-Gene Testing Approach? PharmacoEconomics-Open 2021, 5, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Helgason, H.; Gudjonsson, S.A.; Zink, F.; Oddson, A.; Gylfason, A.; Besenbacher, S.; Magnusson, G.; Halldorsson, B.V.; Hjartarson, E.; et al. Large-Scale Whole-Genome Sequencing of the Icelandic Population. Nat. Genet. 2015, 47, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Adewale, B.A. Will Long-Read Sequencing Technologies Replace Short-Read Sequencing Technologies in the next 10 Years? Afr. J. Lab. Med. 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Fei, Z.; Xiao, P. Methods to Improve the Accuracy of Next-Generation Sequencing. Front. Bioeng. Biotechnol. 2023, 11, 982111. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, R.; Siquier-Pernet, K.; Rio, M.; Guimier, A.; Ollivier, E.; Nitschke, P.; Bole-Feysot, C.; Romana, S.; Hastie, A.; Cantagrel, V.; et al. 16p13.11p11.2 Triplication Syndrome: A New Recognizable Genomic Disorder Characterized by Optical Genome Mapping and Whole Genome Sequencing. Eur. J. Hum. Genet. 2022, 30, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryoo, N.-H.; Ha, J.-S.; Park, S.; Kim, K.; Hee, K.; Kim, D.-H. Next Generation Sequencing Is a Reliable Tool for Detecting BRCA1/2 Mutations, Including Large Genomic Rearrangements. Clin. Lab. 2022, 68. [Google Scholar] [CrossRef]
- Kuchinski, K.S.; Nguyen, J.; Lee, T.D.; Hickman, R.; Jassem, A.N.; Hoang, L.M.N.; Prystajecky, N.A.; Tyson, J.R. Mutations in Emerging Variant of Concern Lineages Disrupt Genomic Sequencing of SARS-CoV-2 Clinical Specimens. Int. J. Infect. Dis. 2022, 114, 51–54. [Google Scholar] [CrossRef]
- Hong, J.H.; Cho, H.W.; Ouh, Y.-T.; Lee, J.K.; Chun, Y.; Gim, J.-A. Genomic Landscape of Advanced Endometrial Cancer Analyzed by Targeted Next-Generation Sequencing and the Cancer Genome Atlas (TCGA) Dataset. J. Gynecol. Oncol. 2022, 33, e29. [Google Scholar] [CrossRef]
- Loginova, M.; Smirnova, D.; Druzhinina, S.; Paramonov, I. Genomic Full-length Sequence of the HLA-B*44:348 Allele Was Identified by next Generation Sequencing. HLA 2022, 100, 160–161. [Google Scholar] [CrossRef]
- Liacini, A.; Peters, L.; Pfau, K.; Mancini, S.; Geier, S. Full Genomic Sequence of the HLA-DPA1*02:46 Allele Identified by next Generation Sequencing. HLA 2022, 100, 99–101. [Google Scholar] [CrossRef]
- DNA Sequencing Market Size to Surpass US$ 37.99 Bn by 2032. Available online: https://www.precedenceresearch.com/dna-sequencing-market (accessed on 30 November 2023).
- Boo, S.H.; Kim, Y.K. The Emerging Role of RNA Modifications in the Regulation of mRNA Stability. Exp. Mol. Med. 2020, 52, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Olabi, M.; Stein, M.; Wätzig, H. Affinity Capillary Electrophoresis for Studying Interactions in Life Sciences. Methods 2018, 146, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.N.; Falcone, G.J.; Rajpurkar, P.; Topol, E.J. Multimodal Biomedical AI. Nat. Med. 2022, 28, 1773–1784. [Google Scholar] [CrossRef]
- Crown, W.; Buyukkaramikli, N.; Sir, M.Y.; Thokala, P.; Morton, A.; Marshall, D.A.; Tosh, J.C.; Ijzerman, M.J.; Padula, W.V.; Pasupathy, K.S. Application of Constrained Optimization Methods in Health Services Research: Report 2 of the ISPOR Optimization Methods Emerging Good Practices Task Force. Value Health 2018, 21, 1019–1028. [Google Scholar] [CrossRef]
- O’Shea, K.; Misra, B.B. Software Tools, Databases and Resources in Metabolomics: Updates from 2018 to 2019. Metabolomics 2020, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The Future of NMR-Based Metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Global Metabolomics Market Size by Indication (Cancer, Cardiovascular Disorders, Neurological Disorders), by Application (Biomarker Discovery, Drug Discovery, Toxicology Testing), by Product And Services (Metabolomics Instruments, Metabolomics Bioinformatics Tools and Services), by Geographic Scope and Forecast. Available online: https://goo.su/MBAYL (accessed on 30 March 2024).
- Eltorai, A.E.M.; Fox, H.; McGurrin, E.; Guang, S. Microchips in Medicine: Current and Future Applications. Biomed. Res. Int. 2016, 2016, 1743472. [Google Scholar] [CrossRef]
- Santini, J.T., Jr.; Sheppard, N.F., Jr.; Young, C.C.; Langer, R.S. Medical Device with Reservoir-Based Sensors. U.S. Patent No. 8,442,611, 14 May 2013. [Google Scholar]
- Quick Read on Your Genetics. Available online: https://www.wired.com/2004/09/quick-read-on-your-genetics (accessed on 30 March 2024).
- Nikitina, V.N.; Karastsialiova, A.R.; Karyakin, A.A. Glucose Test Strips with the Largest Linear Range Made via Single Step Modification by Glucose Oxidase-Hexacyanoferrate-Chitosan Mixture. Biosens. Bioelectron. 2023, 220, 114851. [Google Scholar] [CrossRef]
- Randox Food Diagnostics Home Page. Available online: https://www.randoxfood.com (accessed on 30 March 2024).
- Archer Commences Development of A1 Biochip for Simplifying Disease Detection. Available online: https://www.zdnet.com/article/archer-commences-development-of-a1-biochip-for-simplifying-disease-detection (accessed on 30 March 2024).
- Malsagova, K.A.; Pleshakova, T.O.; Galiullin, R.A.; Kozlov, A.F.; Romanova, T.S.; Shumov, I.D.; Popov, V.P.; Tikhonenko, F.V.; Glukhov, A.V.; Smirnov, A.Y.; et al. SOI-Nanowire Biosensor for the Detection of Glioma-Associated miRNAs in Plasma. Chemosensors 2020, 8, 95. [Google Scholar] [CrossRef]
- Technology. Microarrays. Available online: https://akonni.com/technology/microarrays (accessed on 30 March 2024).
- OciChip™ Microarrays. Available online: https://ocimumbio.com/microarrays (accessed on 30 March 2024).
- Vogtmann, E.; Goedert, J.J. Epidemiologic Studies of the Human Microbiome and Cancer. Br. J. Cancer 2016, 114, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, N.J.; McRae, M.P.; Abram, T.J.; Simmons, G.W.; McDevitt, J.T. Innovative Programmable Bio-Nano-Chip Digitizes Biology Using Sensors That Learn Bridging Biomarker Discovery and Clinical Implementation. Front. Public Health 2017, 5, 110. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Li, M.; Lin, J.; Zhang, G.; Chen, M.; Moazzam, N.F.; Qian, W. Preliminary Study on the Sequencing of Whole Genomic Methylation and Transcriptome-Related Genes in Thyroid Carcinoma. Cancers 2022, 14, 1163. [Google Scholar] [CrossRef] [PubMed]
- Mass Spectrometry Market Size, Share, Trends and Revenue Forecast, 2028. Available online: https://www.marketsandmarkets.com/Market-Reports/mass-spectrometry-market-437.html (accessed on 2 May 2024).
- Saito, T.; Momiyama, M.; Manri, C.; Yu, H.; Choudhury, K.; Osterfeld, S.J.; Nakasawa, T. Effects of Serum Matrix on Molecular Interactions between Drugs and Target Proteins Revealed by Giant Magneto-Resistive Bio-Sensing Techniques. J. Pharm. Biomed. Anal. 2021, 198, 114015. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of Gold Nanoparticles in Biomedical and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-Ray, CT, and Multimodal Imaging Contrast Agents: Formulation, Targeting, and Methodology. J. Nanomater. 2018, 2018, e5837276. [Google Scholar] [CrossRef]
- Yoon, H.-J.; Kim, S.-W. Nanogenerators to Power Implantable Medical Systems. Joule 2020, 4, 1398–1407. [Google Scholar] [CrossRef]
- Kimoto, A.; Watanabe, A.; Yamamoto, E.; Higashi, T.; Kato, M. Rapid Analysis of DOXIL Stability and Drug Release from DOXIL by HPLC Using a Glycidyl Methacrylate-Coated Monolithic Column. Chem. Pharm. Bull. 2017, 65, 945–949. [Google Scholar] [CrossRef]
- Zhang, H. Onivyde for the Therapy of Multiple Solid Tumors. OncoTargets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef]
- Nanoparticle Albumin-Bound Paclitaxel (Abraxane®)|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-981-10-2116-9_6 (accessed on 3 May 2024).
- Hama, M.; Ishima, Y.; Chuang, V.T.G.; Ando, H.; Shimizu, T.; Ishida, T. Evidence for Delivery of Abraxane via a Denatured-Albumin Transport System. ACS Appl. Mater. Interfaces 2021, 13, 19736–19744. [Google Scholar] [CrossRef] [PubMed]
- Virosome-Based Nanovaccines. A Promising Bioinspiration and Biomimetic Approach for Preventing Viral Diseases: A Review–PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8049750/ (accessed on 3 May 2024).
- Petkar, K.C.; Patil, S.M.; Chavhan, S.S.; Kaneko, K.; Sawant, K.K.; Kunda, N.K.; Saleem, I.Y. An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics 2021, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Thapa, R.K.; Kim, J.O. Nanomedicine-Based Commercial Formulations: Current Developments and Future Prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current Approaches of Nanomedicines in the Market and Various Stage of Clinical Translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.-W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.-H.; Nguyen, C.M.; Huynh, M.A.; Vu, H.H.; Nguyen, T.-K.; Nguyen, N.-T. Field Effect Transistor Based Wearable Biosensors for Healthcare Monitoring. J. Nanobiotechnology 2023, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Meng, Q.; Xie, E.; Li, K.; Hu, J.; Chen, Q.; Li, J.; Han, F. Engineered Biomimetic Micro/Nano-Materials for Tissue Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1205792. [Google Scholar] [CrossRef]
- NANOMED–Centro Di Ricerca in Nanomedicina e Nanotecnologia Farmaceutica|Department of Drug and Health Sciences. Available online: https://www.dsf.unict.it/en/node/2722 (accessed on 3 May 2024).
- European Center For Personalized Medicine Home Page. Available online: https://ecpm.center (accessed on 30 March 2024).
- Medizin, Z.-Z. Für P. Centers for Personalized Medicine. Available online: https://zpm-verbund.de/en (accessed on 3 May 2024).
- Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the Deliberate Release into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC—Commission Declaration. Official Journal L 106, 17/04/2001 P. 0001 – 0039. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32001L0018 (accessed on 30 March 2024).
- Good Practice on the Assessment of GMO-Related Aspects in the Context of Clinical Trials with Human Cells Genetically Modified. Available online: https://health.ec.europa.eu/latest-updates/updated-version-good-practice-assessment-gmo-related-aspects-context-clinical-trials-human-cells-2021-11-06_en (accessed on 30 March 2024).
- Medicinal Products for Human Use Containing or Consisting of GMOs: Interplay between the EU-Legislation on Medicinal Products and GMOs. Available online: https://health.ec.europa.eu/system/files/2019-10/gmcells_qa_en_0.pdf (accessed on 30 March 2024).
- Olesti, E.; Nuevo, Y.; Bachiller, M.; Guillen, E.; Bascuas, J.; Varea, S.; Saez-Peñataro, J.; Calvo, G. Academic Challenges on Advanced Therapy Medicinal Products’ Development: A Regulatory Perspective. Cytotherapy 2024, 26, 221–230. [Google Scholar] [CrossRef]
- Regulation 2007/1394–Advanced Therapy Medicinal Products–EU Monitor. Available online: https://www.eumonitor.eu/9353000/1/j4nvk6yhcbpeywk_j9vvik7m1c3gyxp/vitgbgiqbgzp (accessed on 20 February 2024).
- Bludau, I.; Aebersold, R. Proteomic and Interactomic Insights into the Molecular Basis of Cell Functional Diversity. Nat. Rev. Mol. Cell Biol. 2020, 21, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Monk, D.; Mackay, D.J.G.; Eggermann, T.; Maher, E.R.; Riccio, A. Genomic Imprinting Disorders: Lessons on How Genome, Epigenome and Environment Interact. Nat. Rev. Genet. 2019, 20, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, S.; Zaleta-Rivera, K. RNA: Interactions Drive Functionalities. Mol. Biol. Rep. 2020, 47, 1413–1434. [Google Scholar] [CrossRef] [PubMed]
- Khurana, E.; Fu, Y.; Chakravarty, D.; Demichelis, F.; Rubin, M.A.; Gerstein, M. Role of Non-Coding Sequence Variants in Cancer. Nat. Rev. Genet. 2016, 17, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Gevaert, K.; De Smet, I. Protein Language: Post-Translational Modifications Talking to Each Other. Trends Plant Sci. 2018, 23, 1068–1080. [Google Scholar] [CrossRef]
- van der Oost, J.; Patinios, C. The Genome Editing Revolution. Trends Biotechnol. 2023, 41, 396–409. [Google Scholar] [CrossRef]
| Items | Specification |
|---|---|
| Databases and other sources searched | PubMed, Scopus, and Web of Science |
| Search terms used | “molecular technologies”, “genetic technologies”, “personalized medicine”, “biotechnology market”, “microarray”, “sequencing”, “omics technologies”, “mass spectrometry”, “gene therapy”, “RNA modifications”, “metabolomics”, “single cell” |
| Timeframe | 2015–2024 |
| Inclusion criteria | English language Peer-reviewed journals Full text |
| Included in analysis | 77 manuscripts |
| Product/Technology | Company | Characteristics | Reference |
|---|---|---|---|
| Medical device with reservoir-based sensors | MicroCHIPS, Inc. (Southfield, MI, USA) | Storing chemicals (reagents) and protecting them against environmental factors until the procedure of chemical reaction initiation and/or assay or biospecific recognition (sensing) is performed | United States Patent US8442611B2 [35] |
| DNA microarray based on a quartz ball (“geneball”) | University of Queensland (Brisbane, Australia) | The microarray surface contains channels 10 nm in diameter, with specific tagged DNA fragments. The microarray works like a barcode and aims to analyze human genetic information | [36] |
| Next-generation microarray based on the PHENOMICS® technology | Proteus (Nîmes, France) | Ensures analysis of the proteome, genome, or its fragments | [37] |
| Biochip Array Technology | Randox Food Diagnostics (Crumlin, Co. Antrim, Ireland) | A biochip array sized 9 × 9 mm consisting of 44 test spots that enable screening of up to 48 food or fodder samples within less than 3 hrs | [38] |
| A1 Biochip | Archer Materials (Sydney, Australia) | Miniaturization of medical laboratory tests on the integrated circuit of a single array several millimeters in size. The array contains integrated microfluidic channels enabling gas or liquid sampling and miniaturized electrodes within the sensing regions made using gold and titanium metals | [39] |
| GeneChip | Affymetrix (Santa Clara, CA, USA) | The high-density array allows one to perform multiple independent measurements for each transcript call and genotype. The GeneChip system offers a comprehensive solution for elucidating the complex mechanisms and networks underlying the biological processes and diseases | [11] |
| SOI–NW biosensor | Institute of Biomedical Chemistry, Institute of Semiconductor Physics, Siberian Branch of the Russian Academy of Sciences (Moscow, Novosibirsk, Russia) | The microarrays forming this biosensor contain nano-sized silicon sensing elements. The NW biosensor is a molecular detector making it possible to register individual biological molecules in the counting mode, thus ensuring high concentration sensitivity of the analysis | [40] |
| Biosensor for simultaneous detection of glucose and lactate in blood | Moscow State University (Moscow, Russia) | A planar biosensor for detection of glucose and lactate in a liquid sample consists of a silver/silver chloride reference electrode and two working electrodes on a substrate, as well as a counter electrode placed between the working electrodes | [37] |
| A unique diagnostic platform | Akonni Biosystems, Inc. (Frederick, MD, USA) | The solution is based on the TruArray technology employing 3D gel microarrays for rapid simultaneous screening of the sample to detect hundreds of disease markers. The 3D gel droplet arrays have a sterically favorable distance between the immobilized molecules over the entire bulk of a gel droplet and the 99% aqueous environment surrounding the covalently linked probes | [41] |
| OciChip™ microarrays | Ocimum Biosolutions (Madhapur, India) | The process of microarray design and production involves two stages: (1) high-specificity and high-sensitivity microarrays; (2) oligonucleotides are manufactured and purified using strict quality control procedures | [42] |
| HOMIM | Foresight Institute (San Francisco, CA, USA) | The technology enables microbiological analysis of 300 species of the main bacteria that live in the oral cavity using 16S rRNA sequencing | [43] |
| Disease Groups | Description | Commercial Name | Ref. |
|---|---|---|---|
| Diagnostic tools and health monitoring | |||
| Cancer | Hardware and software complex for multiplex immunoassay. Target molecules are caught on the surface of the sensor areas of the chip. Next, the target molecules are recognized by antibodies labeled with magnetic nanoparticles. Magnetic nanosensors detect the magnetic field formed by such “sandwich” immune complexes | MagArray (Milpitas, CA, USA) | [47] |
| Cancer, kidney diseases, bone defects, etc. | Gold nanoparticles as imaging contrast agents for X-ray/CT; gold nanoparticles with inclusion of heavy metals (gadolinium, iron oxide) for MRI; nanoparticles with radioisotopes (64Cu, 111In) for nuclear imaging. The size of particles can range from 1 nm to over 100 nm | AuroVist™ (Nanoprobes, Yaphank, NY, USA) | [48,49] |
| Cardiovascular diseases, neurological diseases, endoscopy | Nanogenerators are a class of self-powered and implantable medical nanosensors. Used for pacemakers, neurostimulators, and drug delivery systems | – | [50] |
| Medicines and vaccines | |||
| Cancer | Iposomal injection doxorubicin hydrochloride—doxorubicin encapsulated in a closed lipid sphere | Doxil (San Francisco, CA, USA) | [51] |
| Liposomal formulation of irinotecan, which is used in the treatment of malignant neoplasms | Onivyde (Roma, Italy) | [52] | |
| Albumin-bound 130 nm particle form of paclitaxel | Abraxane (Basking Ridge, NJ, USA) | [53,54] | |
| Infectious diseases | Virosome-based adjuvanted influenza vaccine licensed for all age groups (from 6 months) | Inflexal V (Site Berne, Switzerland) | [55,56] |
| Consists of formalin-inactivated hepatitis A virus (HAV) | Epaxal (Site Berne, Switzerland) | [55,56] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhod, V.; Krivenko, A.; Butkova, T.; Malsagova, K.; Kaysheva, A. Advances in Molecular and Genetic Technologies and the Problems Related to Their Application in Personalized Medicine. J. Pers. Med. 2024, 14, 555. https://doi.org/10.3390/jpm14060555
Nakhod V, Krivenko A, Butkova T, Malsagova K, Kaysheva A. Advances in Molecular and Genetic Technologies and the Problems Related to Their Application in Personalized Medicine. Journal of Personalized Medicine. 2024; 14(6):555. https://doi.org/10.3390/jpm14060555
Chicago/Turabian StyleNakhod, Valeriya, Anton Krivenko, Tatiana Butkova, Kristina Malsagova, and Anna Kaysheva. 2024. "Advances in Molecular and Genetic Technologies and the Problems Related to Their Application in Personalized Medicine" Journal of Personalized Medicine 14, no. 6: 555. https://doi.org/10.3390/jpm14060555






