Vasculopathy Augments Cardiovascular Risk in Community-Dwelling Elderly with Left Ventricular Hypertrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Demographic, Clinical, and Biological Data
2.3. Echocardiography and Definition of LV Geometry
2.4. Definition of Vasculopathy
2.5. Clinical Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
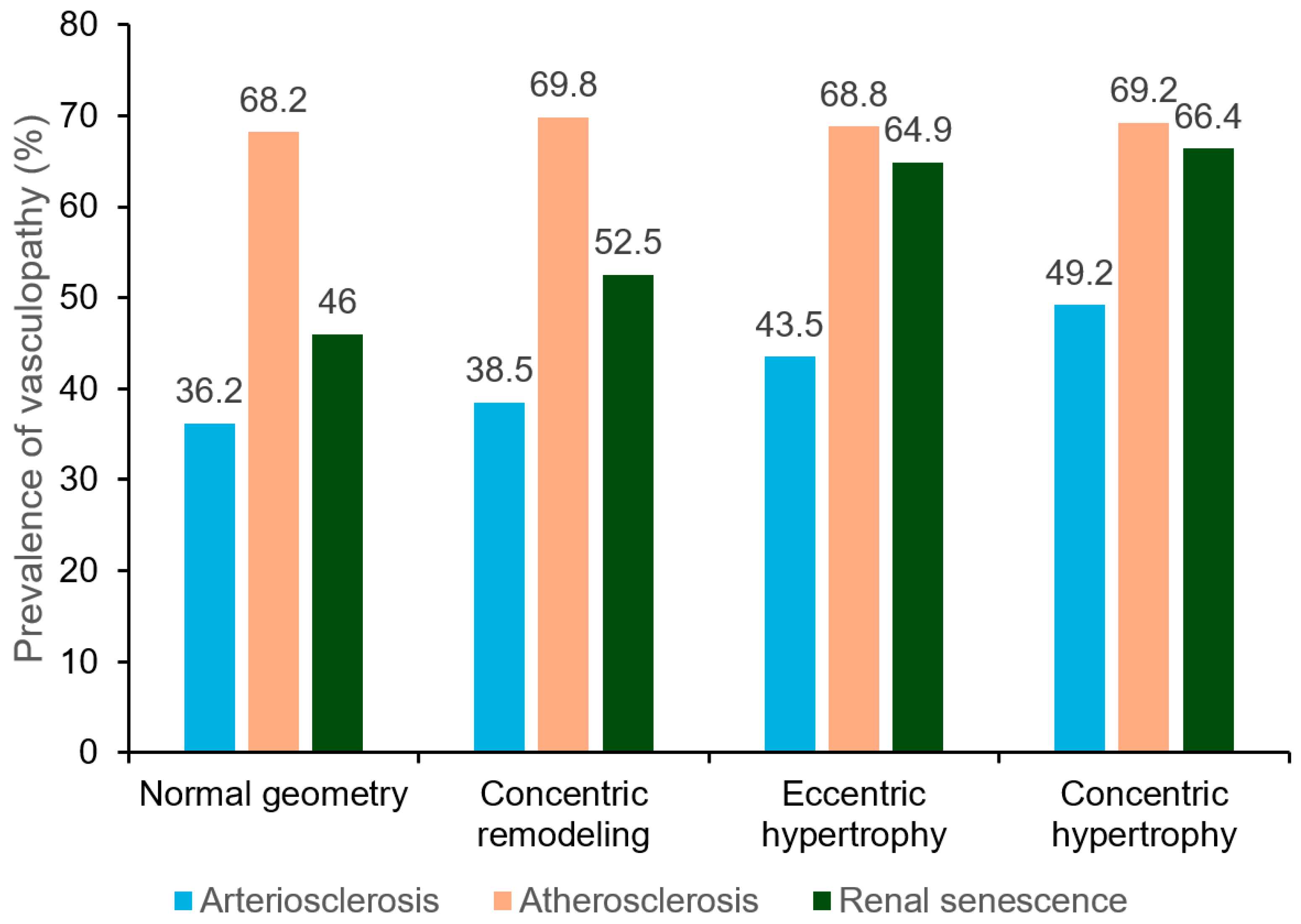
3.2. Prevalence of Vasculopathy Stratified by LV Geometry
3.3. Association of Left Ventricular Geometry with MACEs
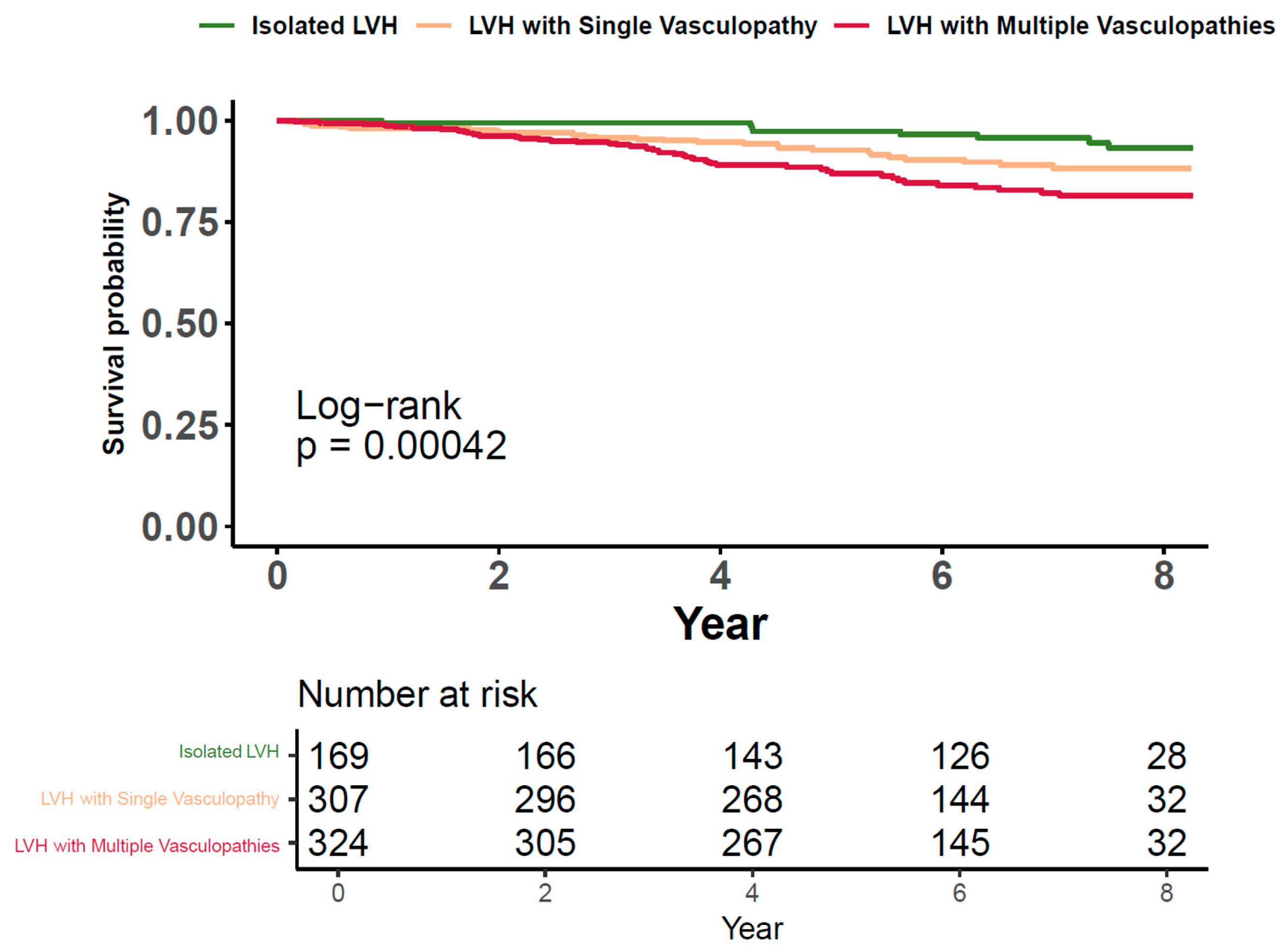
3.4. Cardiovascular Risk of LVH along with Vasculopathies
3.5. Cardiovascular Risks Associated with LVH in the Presence of Concurrent Vasculopathies
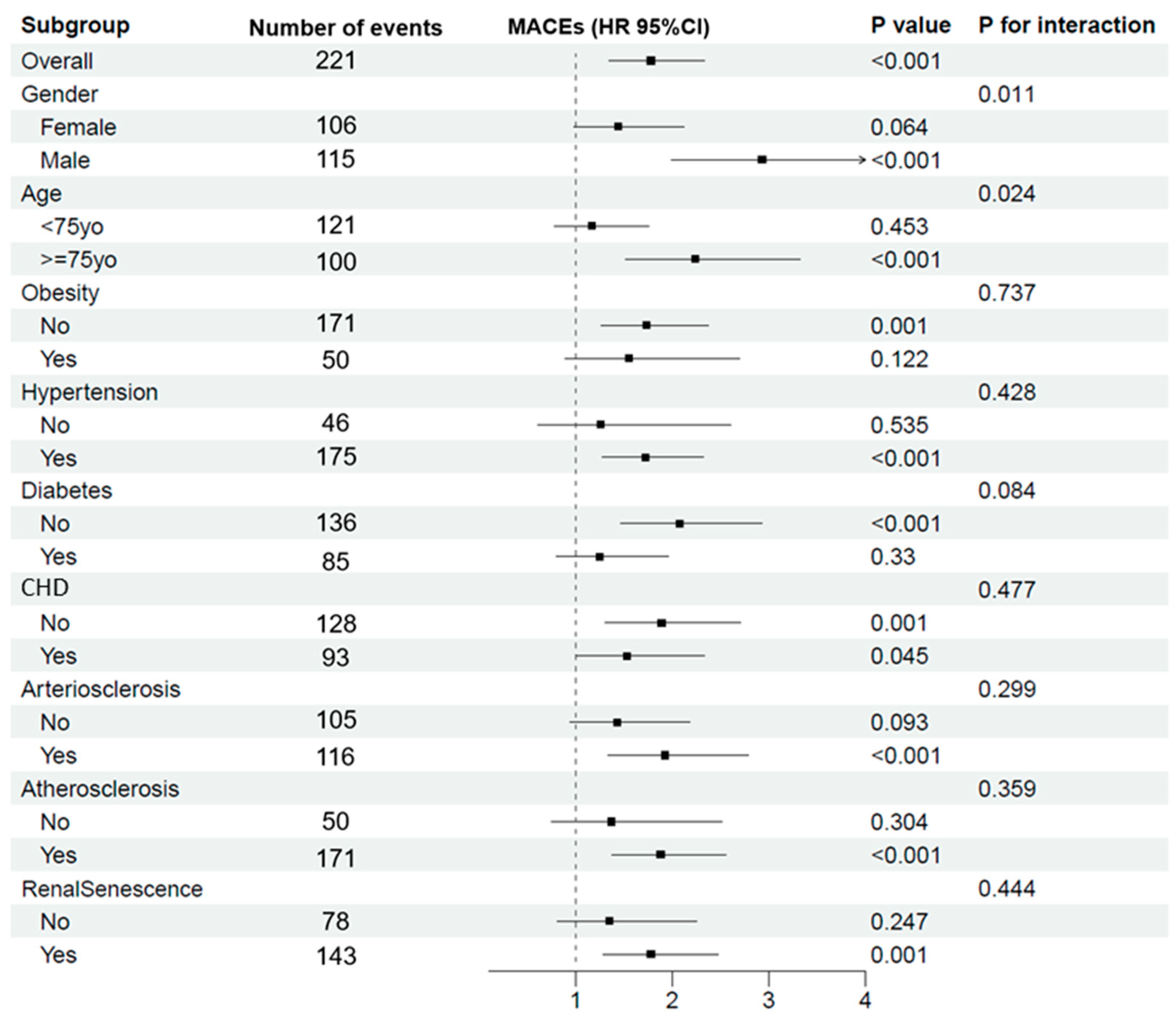
3.6. Impact of LVH on MACEs across Various Subgroups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganau, A.; Orru, M.; Floris, M.; Saba, P.S.; Loi, F.; Sanna, G.D.; Marongiu, M.; Balaci, L.; Curreli, N.; Ferreli, L.A.P.; et al. Echocardiographic heart ageing patterns predict cardiovascular and non-cardiovascular events and reflect biological age: The SardiNIA study. Eur. J. Prev. Cardiol. 2024, 31, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Donekal, S.; Venkatesh, B.A.; Wu, C.O.; Liu, C.Y.; Souto Nacif, M.; Armstrong, A.; Gomes, A.S.; Hundley, W.G.; McClelland, R.L.; et al. Natural History of Myocardial Function in an Adult Human Population: Serial Longitudinal Observations From MESA. JACC Cardiovasc. Imaging 2016, 9, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Hardy, R.J.; Francis, D.P.; Chaturvedi, N.; Pellerin, D.; Deanfield, J.; Kuh, D.; Mayet, J.; Hughes, A.D.; Medical Research Council National Survey of Health and Development (NHSD) Scientific and Data Collection Team; et al. Midlife blood pressure change and left ventricular mass and remodelling in older age in the 1946 British Birth Cohort Study. Eur. Heart J. 2014, 35, 3287–3295. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; McEniery, C.M. Central Versus Peripheral Artery Stiffening and Cardiovascular Risk. Arter. Thromb. Vasc. Biol. 2020, 40, 1028–1033. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef]
- van Sloten, T.T.; Sedaghat, S.; Laurent, S.; London, G.M.; Pannier, B.; Ikram, M.A.; Kavousi, M.; Mattace-Raso, F.; Franco, O.H.; Boutouyrie, P.; et al. Carotid stiffness is associated with incident stroke: A systematic review and individual participant data meta-analysis. J. Am. Coll. Cardiol. 2015, 66, 2116–2125. [Google Scholar] [CrossRef]
- Lavallee, P.C.; Charles, H.; Albers, G.W.; Caplan, L.R.; Donnan, G.A.; Ferro, J.M.; Hennerici, M.G.; Labreuche, J.; Molina, C.; Rothwell, P.M.; et al. Effect of atherosclerosis on 5-year risk of major vascular events in patients with transient ischaemic attack or minor ischaemic stroke: An international prospective cohort study. Lancet Neurol. 2023, 22, 320–329. [Google Scholar] [CrossRef]
- Sigvant, B.; Hasvold, P.; Thuresson, M.; Jernberg, T.; Janzon, M.; Nordanstig, J. Myocardial infarction and peripheral arterial disease: Treatment patterns and long-term outcome in men and women results from a Swedish nationwide study. Eur. J. Prev. Cardiol. 2021, 28, 1426–1434. [Google Scholar] [CrossRef]
- El Nahas, M. Cardio-Kidney-Damage: A unifying concept. Kidney Int. 2010, 78, 14–18. [Google Scholar] [CrossRef]
- Nayor, M.; Larson, M.G.; Wang, N.; Santhanakrishnan, R.; Lee, D.S.; Tsao, C.W.; Cheng, S.; Benjamin, E.J.; Vasan, R.S.; Levy, D.; et al. The association of chronic kidney disease and microalbuminuria with heart failure with preserved vs. reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 615–623. [Google Scholar] [CrossRef]
- Carvalho-Romano, L.; Bonafe, R.P.; Paim, L.R.; Marques, E.R.; Vegian, C.F.L.; Pio-Magalhaes, J.A.; Mello, D.S.S.; de Rossi, G.; Coelho-Filho, O.R.; Schreiber, R.; et al. Association of carotid wall layers with atherosclerotic plaques and cardiac hypertrophy in hypertensive subjects. J. Hum. Hypertens. 2022, 36, 732–737. [Google Scholar] [CrossRef]
- Bell, V.; Sigurdsson, S.; Westenberg, J.J.; Gotal, J.D.; Torjesen, A.A.; Aspelund, T.; Launer, L.J.; Harris, T.B.; Gudnason, V.; de Roos, A.; et al. Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility--Reykjavik Study. Circ. Cardiovasc. Imaging 2015, 8, e003039. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ambale-Venkatesh, B.; Noda, C.; Chugh, A.R.; Teixido-Tura, G.; Kim, J.Y.; Donekal, S.; Yoneyama, K.; Gjesdal, O.; Redheuil, A.; et al. Association of Aortic Stiffness with Left Ventricular Remodeling and Reduced Left Ventricular Function Measured by Magnetic Resonance Imaging: The Multi-Ethnic Study of Atherosclerosis. Circ. Cardiovasc. Imaging 2016, 9, e004426. [Google Scholar] [CrossRef]
- Agarwal, R.; Song, R.J.; Vasan, R.S.; Xanthakis, V. Left Ventricular Mass and Incident Chronic Kidney Disease. Hypertension 2020, 75, 702–706. [Google Scholar] [CrossRef]
- Ikeda, S.; Shinohara, K.; Tagawa, K.; Tohyama, T.; Kishimoto, J.; Kazurayama, M.; Tanaka, S.; Yamaizumi, M.; Nagayoshi, H.; Toyama, K.; et al. Association of baseline electrocardiographic left ventricular hypertrophy with future renal function decline in the general population. Sci. Rep. 2024, 14, 301. [Google Scholar] [CrossRef]
- Taddei, S.; Nami, R.; Bruno, R.M.; Quatrini, I.; Nuti, R. Hypertension, left ventricular hypertrophy and chronic kidney disease. Heart Fail. Rev. 2011, 16, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Lin, F.; Vittinghoff, E.; Peralta, C.; Lima, J.; Kramer, H.; Shlipak, M.; Bibbins-Domingo, K. Estimated GFR and Subsequent Higher Left Ventricular Mass in Young and Middle-Aged Adults with Normal Kidney Function: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Kidney Dis. 2016, 67, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Reffelmann, T.; Dorr, M.; Volzke, H.; Friedrich, N.; Krebs, A.; Ittermann, T.; Felix, S.B. Urinary albumin excretion, even within the normal range, predicts an increase in left ventricular mass over the following 5 years. Kidney Int. 2010, 77, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Xiong, J.; Yu, S.; Chi, C.; Fan, X.; Bai, B.; Zhou, Y.; Teliewubai, J.; Lu, Y.; Xu, H.; et al. Northern Shanghai Study: Cardiovascular risk and its associated factors in the Chinese elderly-a study protocol of a prospective study design. BMJ Open 2017, 7, e013880. [Google Scholar] [CrossRef]
- Chen, C.; Lu, F.C.; Department of Disease Control Ministry of Health, P.R.C. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004, 17, 1–36. [Google Scholar]
- Mancia, G.; Kreutz, R.; Brunstrom, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Teichholz, L.E.; Kreulen, T.; Herman, M.V.; Gorlin, R. Problems in echocardiographic volume determinations: Echocardiographic-angiographic correlations in the presence of absence of asynergy. Am. J. Cardiol. 1976, 37, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- Allison, M.A.; Aboyans, V.; Granston, T.; McDermott, M.M.; Kamineni, A.; Ni, H.; Criqui, M.H. The relevance of different methods of calculating the ankle-brachial index: The multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2010, 171, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Gamble, G.; MacMahon, S.; Culpan, A.; Ciobo, C.; Whalley, G.; Sharpe, N. Atherosclerosis and left ventricular hypertrophy: Persisting problems in treated hypertensive patients. J. Hypertens. 1998, 16, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Drawz, P.; Rahman, M. Chronic kidney disease. Ann. Intern. Med. 2015, 162, ITC1-16. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, S.; Hao, H.; Li, Q.; Bao, X.; Wang, K.; Gu, R.; Li, G.; Kang, L.; Wu, H.; et al. The predictive value of relative wall thickness on the prognosis of the patients with ST-segment elevation myocardial infarction. BMC Cardiovasc. Disord. 2023, 23, 383. [Google Scholar] [CrossRef]
- Bae, S.; Park, S.M.; Kim, S.R.; Kim, M.N.; Cho, D.H.; Kim, H.D.; Yoon, H.J.; Kim, M.A.; Kim, H.L.; Hong, K.S.; et al. Early menopause is associated with abnormal diastolic function and poor clinical outcomes in women with suspected angina. Sci. Rep. 2024, 14, 6306. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Magalhaes, T.A.; George, R.T.; Dewey, M.; Laham, R.J.; Niinuma, H.; Friedman, L.A.; Cox, C.; Tanami, Y.; Schuijf, J.D.; et al. Relationship of left ventricular mass to coronary atherosclerosis and myocardial ischaemia: The CORE320 multicenter study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.L.; Miao, H.; Wang, Y.N.; Liu, F.; Li, P.; Zhao, Y.Y. TGF-beta as A Master Regulator of Aging-Associated Tissue Fibrosis. Aging Dis. 2023, 14, 1633–1650. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, C.; Copur, S.; Mutlu, A.; Peltek, I.B.; Galassi, A.; Ciceri, P.; Cozzolino, M.; Kanbay, M. Early aging and premature vascular aging in chronic kidney disease. Clin. Kidney J. 2023, 16, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Fularski, P.; Krzeminska, J.; Lewandowska, N.; Mlynarska, E.; Saar, M.; Wronka, M.; Rysz, J.; Franczyk, B. Statins in Chronic Kidney Disease-Effects on Atherosclerosis and Cellular Senescence. Cells 2023, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Lentini, P.; Briet, M.; Castellino, P.; House, A.A.; London, G.M.; Malatino, L.; McCullough, P.A.; Mikhailidis, D.P.; Boutouyrie, P. Arterial Stiffness in the Heart Disease of CKD. J. Am. Soc. Nephrol. 2019, 30, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, M.; Paini, A.; Bertacchini, F.; Stassaldi, D.; Aggiusti, C.; Agabiti Rosei, C.; Bassetti, D.; Agabiti-Rosei, E.; Muiesan, M.L. Changes in left ventricular geometry during antihypertensive treatment. Pharmacol. Res. 2018, 134, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, W.H.; Zile, M.R. Left ventricular structural remodeling in health and disease: With special emphasis on volume, mass, and geometry. J. Am. Coll. Cardiol. 2011, 58, 1733–1740. [Google Scholar] [CrossRef]
- van der Harst, P.; van Setten, J.; Verweij, N.; Vogler, G.; Franke, L.; Maurano, M.T.; Wang, X.; Mateo Leach, I.; Eijgelsheim, M.; Sotoodehnia, N.; et al. 52 Genetic Loci Influencing Myocardial Mass. J. Am. Coll. Cardiol. 2016, 68, 1435–1448. [Google Scholar] [CrossRef]
| Left Ventricular Geometry | LVMI (g/m2) | RWT |
|---|---|---|
| Normal | <115 for male <95 for female | ≤0.42 |
| Concentric remodeling | <115 for male <95 for female | >0.42 |
| Eccentric hypertrophy | ≥115 for male ≥95 for female | ≤0.42 |
| Concentric hypertrophy | ≥115 for male ≥95 for female | >0.42 |
| Normal Geometry (n = 1761) | Concentric Remodeling (n = 775) | Eccentric Hypertrophy (n = 552) | Concentric Hypertrophy (n = 251) | p for Trend | ||
|---|---|---|---|---|---|---|
| Basic characteristics | ||||||
| Age (years) | 69 (66–73) | 69 (67–74) | 70 (67–76) | 71 (67–78) | <0.001 | |
| Male (n, %) | 859 (48.8) | 378 (48.8) | 154 (27.9) | 62 (24.8) | <0.001 | |
| BMI (kg/m2) | 24 (22–26) | 24 (21–26) | 25 (22–27) | 24 (22–27) | <0.001 | |
| Alcohol history (n, %) | 322 (18.3) | 135 (17.4) | 70 (12.7) | 30 (12.0) | <0.001 | |
| Smoking history (n, %) | 488 (27.7) | 210 (27.1) | 92 (16.7) | 33 (13.2) | <0.001 | |
| Blood pressure | ||||||
| Systolic blood pressure (mm Hg) | 134 (122–145) | 131 (120–145) | 139 (127–150) | 140 (126–151) | <0.001 | |
| Diastolic blood pressure (mm Hg) | 80 (72–86) | 80 (71–84) | 80 (71–85) | 80 (73–85) | 0.275 | |
| Laboratory blood results | ||||||
| FBG (mmol/L) | 5.2 (4.8–5.9) | 5.3 (4.8–6.1) | 5.3 (4.8–6.1) | 5.3 (4.8–6.5) | 0.026 | |
| Total cholesterol (mmol/L) | 5.1 (4.4–5.7) | 5.0 (4.4–5.7) | 5.1 (4.5–5.7) | 5.3 (4.5–5.9) | 0.193 | |
| Triglyceride (mmol/L) | 1.4 (1.0–1.9) | 1.4 (1.0–1.9) | 1.4 (1.1–1.9) | 1.4 (1.1–2.0) | 0.126 | |
| Uric acid (mmol/L) | 325 (274–379) | 322 (277–380) | 321 (276–374) | 323 (264–382) | 0.940 | |
| Left ventricular parameters | ||||||
| LVMI (g/m2) | 76 (64–88) | 77 (64–88) | 120 (105–133) | 119 (107–133) | <0.001 | |
| RWT | 0.35 (0.32–0.38) | 0.47 (0.44–0.51) | 0.36 (0.32–0.39) | 0.47 (0.44–0.52) | <0.001 | |
| LVEF (%) | 59 (51–68) | 57 (50–66) | 56 (50–63) | 58 (51–64) | <0.001 | |
| Pre-existing diseases | ||||||
| Hypertension (n, %) | 1132 (64.2) | 485 (62.6) | 432 (78.3) | 197 (78.8) | <0.001 | |
| Diabetes (n, %) | 399 (22.6) | 199 (25.7) | 160 (29.0) | 80 (32.0) | <0.001 | |
| CHD (n, %) | 519 (29.5) | 245 (31.6) | 216 (39.3) | 91 (36.4) | <0.001 | |
| Vasculopathy parameters | ||||||
| UACR (mg/g) | 25 (12–51) | 30 (16–57) | 42 (21–76) | 38 (23–79) | <0.001 | |
| cfPWV (m/s) | 8.8 (7.6–10.2) | 8.9 (7.9–10.6) | 9.5 (8.1–10.9) | 9.7 (8.3–11.2) | <0.001 | |
| R-ABI | 1.09 (1.02–1.15) | 1.08 (0.99–1.14) | 1.08 (1.01–1.15) | 1.06 (0.98–1.13) | <0.001 | |
| L-ABI | 1.08 (1.01–1.14) | 1.06 (0.97–1.13) | 1.08 (0.99–1.14) | 1.03 (0.96–1.10) | <0.001 | |
| eGFR (mL/min/1.73/m2) | 85 (75–96) | 84 (74–96) | 84 (73–95) | 83 (71–95) | 0.003 | |
| cIMT (mm) | 0.64 (0.55–0.77) | 0.64 (0.54–0.76) | 0.61 (0.52–0.74) | 0.64 (0.53–0.74) | 0.008 | |
| Carotid plaque (n, %) | 1169 (66.3) | 521 (66.2) | 361 (65.4) | 162 (64.8) | 0.614 | |
| Events | ||||||
| MACEs | 87 (4.9) | 52 (6.7) | 54 (9.8) | 28 (11.2) | <0.001 | |
| Normal Geometry | Concentric Remodeling | Eccentric Hypertrophy | Concentric Hypertrophy | ||||
|---|---|---|---|---|---|---|---|
| MACEs | ref | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Model 1 | 1.000 | 1.256 (0.891–1.772) | 0.193 | 1.866 (1.329–2.622) | <0.001 | 2.042 (1.333–3.127) | 0.001 |
| Model 2 | 1.000 | 1.183 (0.837–1.673) | 0.342 | 1.776 (1.249–2.525) | 0.001 | 1.868 (1.203–2.899) | 0.005 |
| Model 3 | 1.000 | 1.176 (0.831–1.663) | 0.361 | 1.675 (1.178–2.381) | 0.004 | 1.783 (1.148–2.771) | 0.010 |
| Model 4 | 1.000 | 1.167 (0.825–1.651) | 0.384 | 1.638 (1.151–2.331) | 0.006 | 1.751 (1.127–2.721) | 0.013 |
| HR | 95% CI | p | |
|---|---|---|---|
| LVH | 1.585 | 1.186–2.119 | 0.002 |
| Arteriosclerosis | 1.140 | 0.857–1.515 | 0.368 |
| Atherosclerosis | 1.187 | 0.859–1.639 | 0.299 |
| Renal senescence | 1.361 | 1.019–1.819 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maimaitiaili, R.; Zhao, S.; Teliewubai, J.; Yu, S.; Meng, W.; Zhao, Y.; Xu, Y.; Zhang, Y. Vasculopathy Augments Cardiovascular Risk in Community-Dwelling Elderly with Left Ventricular Hypertrophy. J. Pers. Med. 2024, 14, 558. https://doi.org/10.3390/jpm14060558
Maimaitiaili R, Zhao S, Teliewubai J, Yu S, Meng W, Zhao Y, Xu Y, Zhang Y. Vasculopathy Augments Cardiovascular Risk in Community-Dwelling Elderly with Left Ventricular Hypertrophy. Journal of Personalized Medicine. 2024; 14(6):558. https://doi.org/10.3390/jpm14060558
Chicago/Turabian StyleMaimaitiaili, Rusitanmujiang, Song Zhao, Jiadela Teliewubai, Shikai Yu, Weilun Meng, Yifan Zhao, Yawei Xu, and Yi Zhang. 2024. "Vasculopathy Augments Cardiovascular Risk in Community-Dwelling Elderly with Left Ventricular Hypertrophy" Journal of Personalized Medicine 14, no. 6: 558. https://doi.org/10.3390/jpm14060558








