Artificial Intelligence Assists in the Early Identification of Cardiac Amyloidosis
Abstract
:1. Introduction
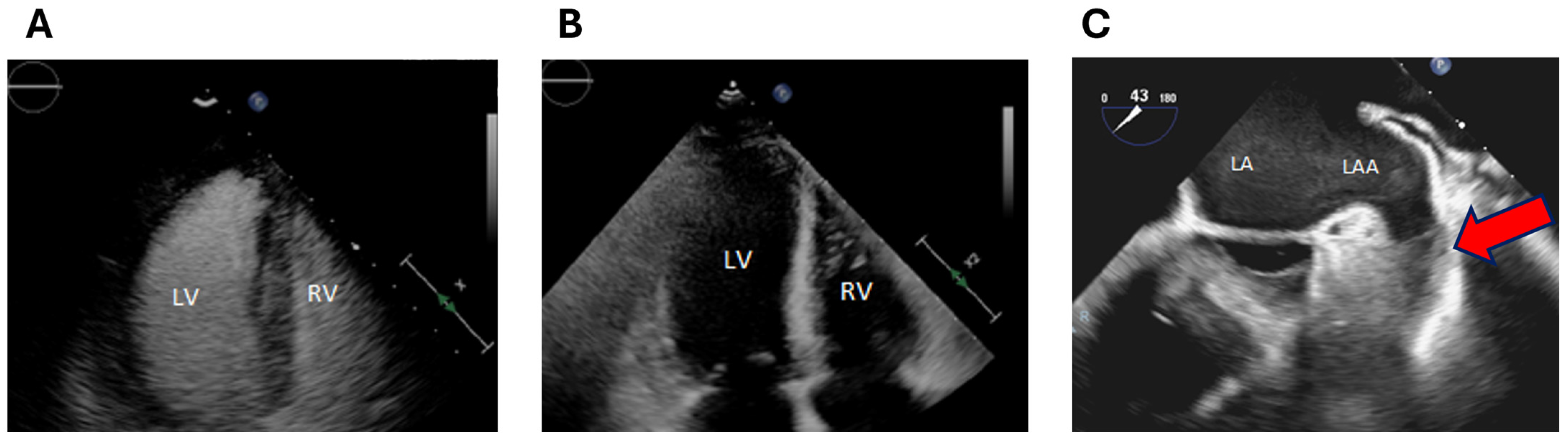
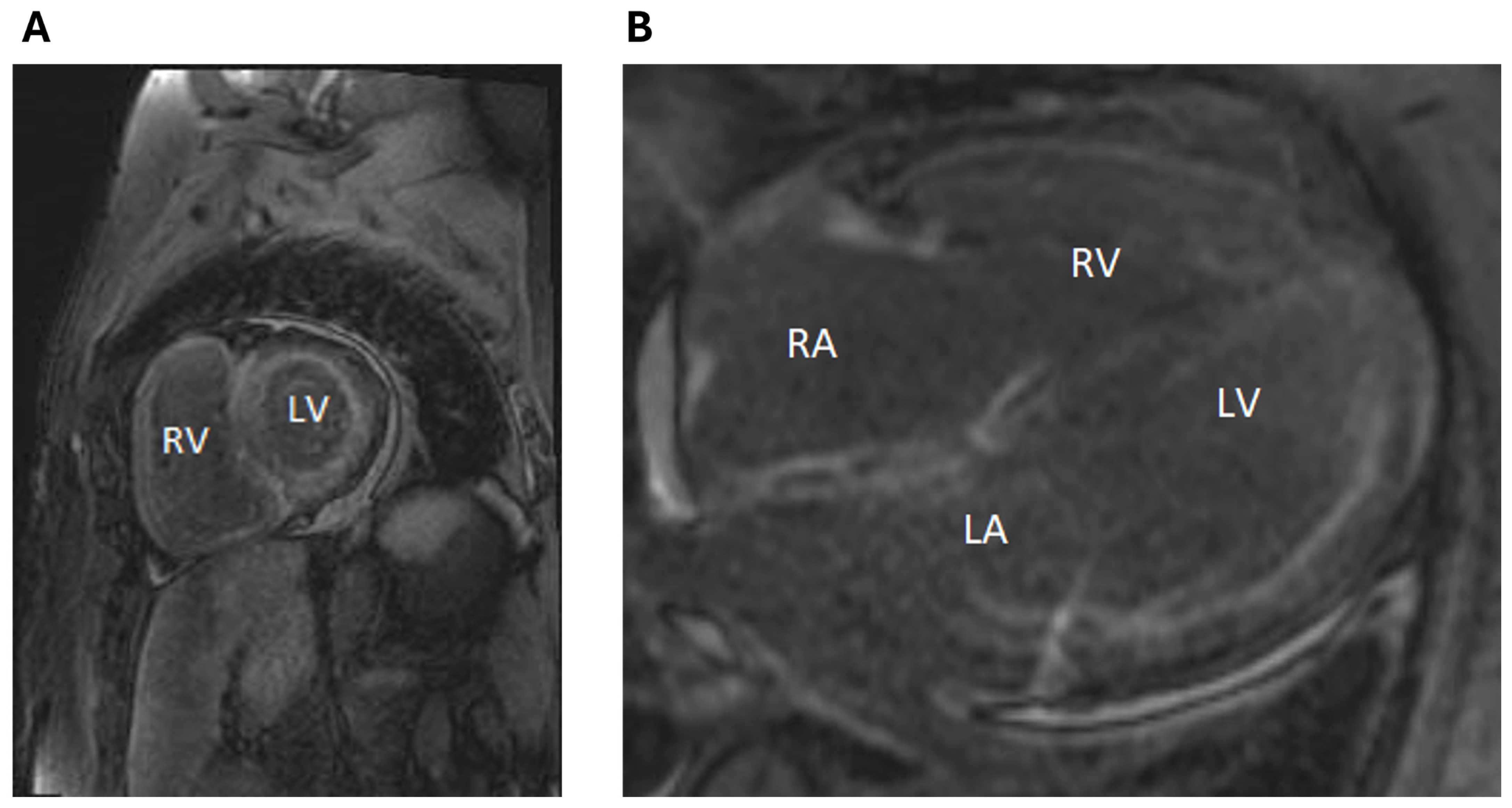
2. Clinical Case
3. Discussion
4. Learning Objectives
- To recognize that AI applied to ECG has clinical utility for the early detection of rare and difficult-to-diagnose conditions such as AL cardiac amyloidosis.
- To recognize that the early diagnosis of cardiac amyloidosis is critical as it carries poor prognosis if untreated and requires the prompt initiation of targeted therapy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | light chain amyloidosis |
| AI | artificial intelligence |
| ECG | electrocardiogram |
| EF | ejection fraction |
| LV | left ventricle |
| TEE | transesophageal echocardiogram |
| MRI | magnetic resonance imaging |
| TTE | transthoracic echocardiogram |
| LGE | late gadolinium enhancement |
| EMR | electronic medical record |
References
- Fine, N.M. Challenges and Strategies in the Diagnosis of Cardiac Amyloidosis. Can. J. Cardiol. 2020, 36, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.A.; Abbas, M.T.; Kanaan, C.N.; Awad, K.A.; Baba Ali, N.; Scalia, I.G.; Farina, J.M.; Pereyra, M.; Mahmoud, A.K.; Steidley, D.E.; et al. How Artificial Intelligence Can Enhance the Diagnosis of Cardiac Amyloidosis: A Review of Recent Advances and Challenges. J. Cardiovasc. Dev. Dis. 2024, 11, 118. [Google Scholar] [CrossRef]
- Baker, K.R. Light Chain Amyloidosis: Epidemiology, Staging, and Prognostication. Methodist. Debakey Cardiovasc. J. 2022, 18, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lousada, I.; Comenzo, R.L.; Landau, H.; Guthrie, S.; Merlini, G. Light Chain Amyloidosis: Patient Experience Survey from the Amyloidosis Research Consortium. Adv. Ther. 2015, 32, 920–928. [Google Scholar] [CrossRef]
- Grogan, M.; Lopez-Jimenez, F.; Cohen-Shelly, M.; Dispenzieri, A.; Attia, Z.I.; Abou Ezzedine, O.F.; Lin, G.; Kapa, S.; Borgeson, D.D.; Friedman, P.A.; et al. Artificial Intelligence-Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin. Proc. 2021, 96, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.M.; Mangold, K.; Baez Suarez, A.; Scott, C.G.; Murphree, D.H.; Malik, A.; Attia, Z.I.; Lopez-Jimenez, F.; Friedman, P.A.; Dispenzieri, A.; et al. Postdevelopment Performance and Validation of the Artificial Intelligence-Enhanced Electrocardiogram for Detection of Cardiac Amyloidosis. JACC Adv. 2023, 2, 100612. [Google Scholar] [CrossRef]
- Chen, Q.; Yi, Z.; Cheng, J. Atrial fibrillation in aging population. Aging Med. 2018, 1, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Vakamudi, S.; Sperry, B.; Hanna, M.; Saliba, W.; Wazni, O.; Jaber, W. ATRIAL Thrombus in Patients with Cardiac Amyloidosis: Prevalence and Implications for Therapy. J. Am. Coll. Cardiol. 2017, 69, 829. [Google Scholar] [CrossRef]
- El-Am, E.A.; Grogan, M.; Ahmad, A.; Patlolla, S.H.; Klarich, K.W.; AbouEzzeddine, O.F.; Melduni, R.M.; Maleszewski, J.J.; Dispenzieri, A.; Nkomo, V.T. Persistence of Left Atrial Appendage Thrombus in Patients With Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021, 77, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Syed, I.; Martinez, M.; Oh, J.; Jaffe, A.; Grogan, M.; Edwards, W.; Gertz, M.; Klarich, K. Intracardiac Thrombosis and Anticoagulation Therapy in Cardiac Amyloidosis. Circulation 2009, 119, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, J.A.; Abraham, R.S.; Dispenzieri, A.; Lust, J.A.; Kyle, R.A. Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin. Chem. 2005, 51, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Quock, T.P.; Yan, T.; Chang, E.; Guthrie, S.; Broder, M.S. Epidemiology of AL amyloidosis: A real-world study using US claims data. Blood Adv. 2018, 2, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J. Cardiovasc. Dev. Dis. 2023, 10, 175. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenyon, C.R.; Pietri, M.P.; Rosenthal, J.L.; Arsanjani, R.; Ayoub, C. Artificial Intelligence Assists in the Early Identification of Cardiac Amyloidosis. J. Pers. Med. 2024, 14, 559. https://doi.org/10.3390/jpm14060559
Kenyon CR, Pietri MP, Rosenthal JL, Arsanjani R, Ayoub C. Artificial Intelligence Assists in the Early Identification of Cardiac Amyloidosis. Journal of Personalized Medicine. 2024; 14(6):559. https://doi.org/10.3390/jpm14060559
Chicago/Turabian StyleKenyon, Courtney R., Milagros Pereyra Pietri, Julie L. Rosenthal, Reza Arsanjani, and Chadi Ayoub. 2024. "Artificial Intelligence Assists in the Early Identification of Cardiac Amyloidosis" Journal of Personalized Medicine 14, no. 6: 559. https://doi.org/10.3390/jpm14060559
APA StyleKenyon, C. R., Pietri, M. P., Rosenthal, J. L., Arsanjani, R., & Ayoub, C. (2024). Artificial Intelligence Assists in the Early Identification of Cardiac Amyloidosis. Journal of Personalized Medicine, 14(6), 559. https://doi.org/10.3390/jpm14060559






