Sleep Apnea and Atrial Fibrillation: Clinical Features and Screening Diagnostic Options
Abstract
1. Introduction
2. Materials and Methods
- Patients aged 18 to 80 years old.
- Confirmed diagnosis of AF.
- Signed informed consent form to participate in the study.
- Myocardial infarction within the past 3 months prior to study inclusion.
- Concomitant severe somatic diseases (endocrine pathology, renal and/or hepatic insufficiency, oncological diseases) with an estimated life expectancy of less than 1 year.
- Pregnancy or breastfeeding.
- Mental illness (severe dementia, schizophrenia, severe depression, manic-depressive psychosis).
Statistical Analysis
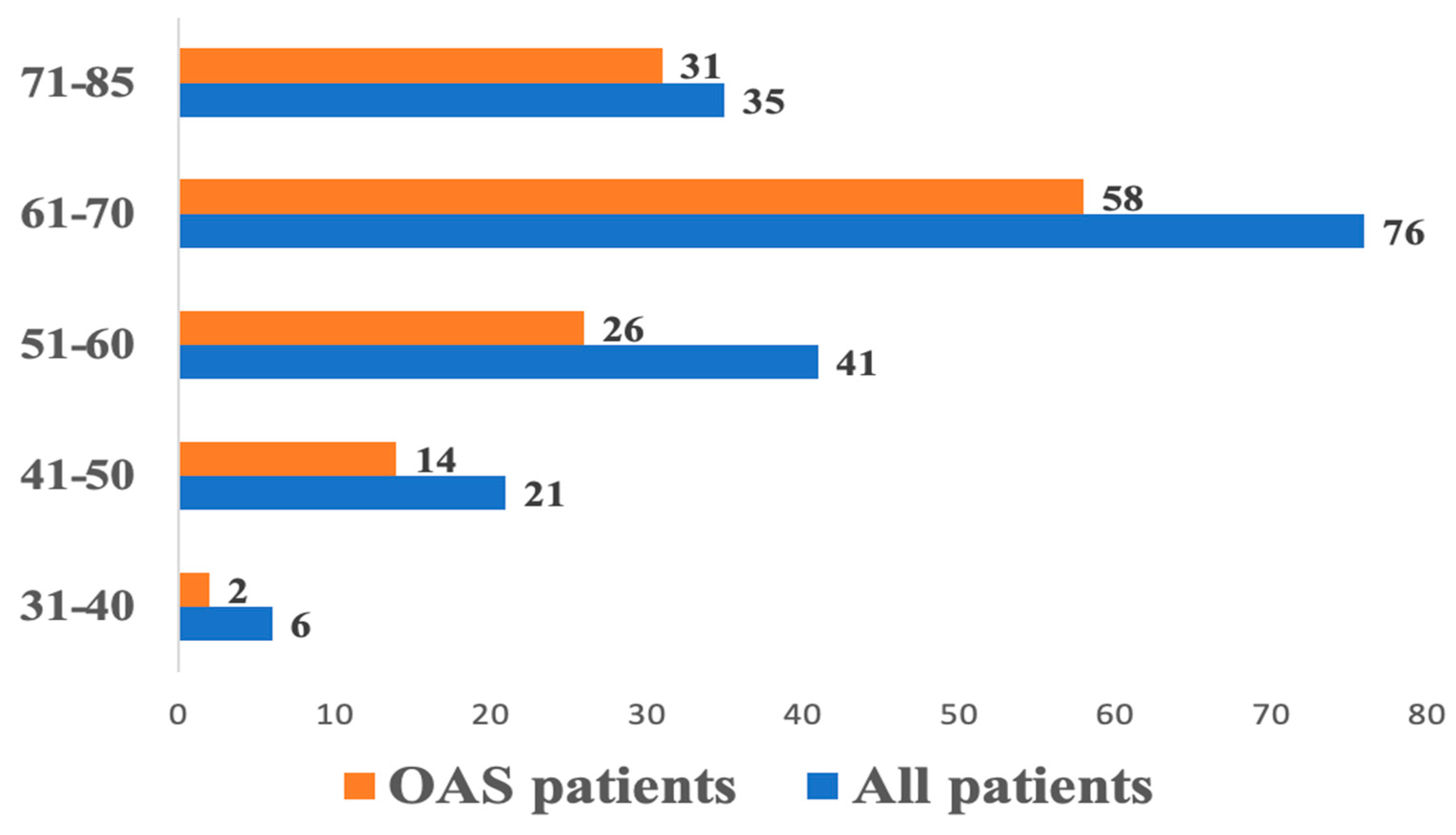
3. Results
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; McEvoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Levy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea with Atrial Fibrillation and Continuous Positive Airway Pressure Treatment A Review. JAMA Cardiol. 2018, 3, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, Y.; Po, S.S. Obstructive Sleep Apnoea and Atrial Fibrillation. Arrhythmia Electrophysiol. Rev. 2015, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- AHAS Update. Heart Disease and Stroke Statistics—2021 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar]
- Gottlieb, D.J.; Whitney, C.W.; Bonekat, W.H.; Iber, C.; James, G.D.; Lebowitz, M.; Nieto, F.J.; Rosenberg, C.E. Relation of Sleepiness to Respiratory Disturbance Index the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 1999, 159, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Tarzimanova, A.I. Obesity and atrial fibrillation: Mechanisms of occurence and current treatment guidelines. Therapy 2022, 8, 141–146. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, R.; Jiang, M.; Wang, W.; Chai, Y.; Liu, Q.; Tao, Z.; Wu, Q.; Yue, J.; Ma, J.; et al. Myocardial Tissue-Level Characteristics of Adults with Metabolically Healthy Obesity. Cardiovasc. Imaging 2023, 16, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive Sleep Apnea, Obesity, and the Risk of Incident Atrial Fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Stone, K.L.; Varosy, P.D.; Hoffman, A.R.; Marcus, G.M.; Blackwell, T.; Ibrahim, O.A.; Salem, R.; Redline, S. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: Outcomes of sleep disorders in older men (MrOS sleep) study. Arch. Intern. Med. 2009, 169, 1147–1155. [Google Scholar] [CrossRef]
- Abuyassin, B.; Sharma, K.; Ayas, N.T.; Laher, I. Obstructive Sleep Apnea and Kidney Disease: A Potential Bidirectional Relationship? Journal of Clinical Sleep Medicine. Am. Acad. Sleep Med. 2015, 11, 915–924. [Google Scholar]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. Available online: www.jhypertension.com (accessed on 7 May 2024). [PubMed]
- Javaheri, S.; Peker, Y.; Yaggi, H.K.; Bassetti, C.L. Obstructive sleep apnea and stroke: The mechanisms, the randomized trials, and the road ahead. Sleep Med. Rev. 2022, 61, 101568. [Google Scholar] [CrossRef] [PubMed]
- Mc Farlane, S.I. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: Epidemiology and pathophysiologic insights. Sleep Med. Disord. Int. J. 2018, 2, 52–58. [Google Scholar] [CrossRef]
- Javaheri, S.; Brown, L.K.; Abraham, W.T.; Khayat, R. Apneas of Heart Failure and Phenotype-Guided Treatments: Part One: OSA. Chest 2020, 157, 394–402. [Google Scholar] [CrossRef]
- Polecka, A.; Olszewska, N.; Danielski, Ł.; Olszewska, E. Association between Obstructive Sleep Apnea and Heart Failure in Adults—A Systematic Review. J. Clin. Med. 2023, 12, 6139. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.; Bjerre, J.; Zareini, B.; Koch, H.; Tønnesen, P.; Gislason, G.H.; Nielsen, O.W.; Schou, M.; Lamberts, M. Sleep apnea, the risk of developing heart failure, and potential benefits of continuous positive airway pressure (CPAP) therapy. J. Am. Heart Assoc. 2018, 7, e008684. [Google Scholar] [CrossRef]
- Khalil, M.; Power, N.; Graham, E.; Deschênes, S.S.; Schmitz, N. The association between sleep and diabetes outcomes—A systematic review. Diabetes Res. Clin. Pract. 2020, 161, 108035. [Google Scholar] [CrossRef]
- Qie, R.; Zhang, D.; Liu, L.; Ren, Y.; Zhao, Y.; Liu, D.; Liu, F.; Chen, X.; Cheng, C.; Guo, C.; et al. Obstructive sleep apnea and risk of type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of cohort studies. J. Diabetes 2020, 12, 455–464. [Google Scholar] [CrossRef]
- Amra, B.; Rahmati, B.; Soltaninejad, F.; Feizi, A. Screening questionnaires for obstructive sleep apnea: An updated systematic review. Oman Med. J. 2018, 33, 184–192. [Google Scholar] [CrossRef]
- Traaen, G.M.; Øverland, B.; Aakerøy, L.; Hunt, T.E.; Bendz, C.; Sande, L.; Aakhus, S.; Zaré, H.; Steinshamn, S.; Anfinsen, O.G.; et al. Prevalence, risk factors, and type of sleep apnea in patients with paroxysmal atrial fibrillation. IJC Heart Vasc. 2020, 26, 100447. [Google Scholar] [CrossRef]
- Albuquerque, F.N.; Calvin, A.D.; Kuniyoshi, F.H.S.; Konecny, T.; Lopez-Jimenez, F.; Pressman, G.S.; Kara, T.; Friedman, P.; Ammash, N.; Somers, V.K.; et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest 2012, 141, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, K.; Middeldorp, M.E.; Elliott, A.D.; Jones, D.; Hendriks, J.M.; Gallagher, C.; Arzt, M.; Jones, D.; Hendriks, J.M.L.; Gallagher, C.; et al. Self-Reported Daytime Sleepiness and Sleep-Disordered Breathing in Patients with Atrial Fibrillation: SNOozE-AF. Can. J. Cardiol. 2019, 35, 1457–1464. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | With OSA (n = 131) | Without OSA (n = 48) | p Value |
|---|---|---|---|
| Male, n (%) Female, n (%) | 82 (62.6%) 49 (37.4%) | 23 (47.9%) 25 (52.1%) | 0.079 |
| Age (years) | 65 (58–70) | 57 (51.2–66) | 0.001 |
| Height (cm) | 173 (165.5–178.5) | 174 (164–178.5) | 0.812 |
| Weight (kg) | 98 (88.5–112.6) | 92.5 (82.5–105) | 0.025 |
| BMI (kg/m2) | 33.6 (30.3–37.9) | 31.3 (27.5–35.2) | 0.020 |
| Abdominal circumference (cm) | 114 (108–124.5) | 107.5 (100–112) | <0.001 |
| Neck circumference (cm) | 42 (40.8–46) | 41 (38–42.5) | <0.001 |
| Hemoglobin (g/L) | 146 (137–159.5) | 148 (138–160) | 0.839 |
| Glycated hemoglobin (%) | 6.1 (5.7–6.6) | 5.9 (5.4–6.4) | 0.118 |
| GFR (mL/min/1.73 m2) | 64 (55.15–76.8) | 71.15 (61.4–78.5) | 0.367 |
| HDL cholesterol (mmol/L) | 1.08 (0.89–1.22) | 1.15 (0.97–1.36) | 0.011 |
| LDL cholesterol (mmol/L) | 2.11 (1.53–2.71) | 2.37 (1.82–3.13) | 0.831 |
| CHA2DS2-VASc (score) | 2.5 (2–3) | 2 (1–3) | 0.085 |
| LV EDV | 110 (95–128) | 110 (98–129) | 0.892 |
| LV ejection fraction (%) | 55 (48.35–61.75) | 56 (55–63) | 0.154 |
| LA AP | 42.9 (40–46.25) | 42 (39.7–45) | 0.229 |
| LAVI | 34.99 (18.3–43.1) | 37.35 (21.6–45.7) | 0.667 |
| Characteristics | Mild OSA (n = 39) | Moderate OSA (n = 49) | Severe OSA (n = 43) | p Value |
|---|---|---|---|---|
| Age (Years) | 64 (57–70) | 66 (60–71) | 66 (55–70) | 0.342 |
| Height (cm) | 172 (163–178) | 175 (168–180) | 174 (165.5–178) | 0.347 |
| Weight (kg) | 93 (86–103) | 102 (95–115) | 105 (87.5–120.5) | 0.021 p1–2 = 0.047 p1–3 = 0.041 p2–3 = 1.000 |
| BMI (kg/m2) | 31.9 (28.1–35.1) | 34.1 (30.9–38) | 35.5 (31.9–39.7) | 0.014 p1–2 = 0.374 p1–3 = 0.010 p2–3 = 0.385 |
| Abdominal circumference (cm) | 110 (101–118) | 114 (109–127) | 118 (110–128.5) | 0.001 p1–2 = 0.040 p1–3 = 0.001 p2–3 = 0.492 |
| Neck circumference (cm) | 42 (40–45) | 42 (41–45) | 44 (40.8–48.5) | 0.062 |
| GFR (mL/min/1.73 m2) | 72 (59.5–85.4) | 65.2 (57–75.2) | 61.6 (54–72) | 0.027 p1–2 = 0.179 p1–3 = 0.025 p2–3 = 1.000 |
| Disease | With OSA | Without OSA | p Value | OR; 95% CI | ||
|---|---|---|---|---|---|---|
| Absolute | % | Absolute | % | |||
| Hypertension | 117 | 75.5 | 38 | 24.5 | 0.087 | 2.9; 0.9–5.3 |
| Heart failure | 68 | 84 | 13 | 16 | 0.004 | 2.9; 1.4–5.9 |
| Myocardial infarction | 10 | 90.9 | 1 | 9.1 | 0.293 | 3.8; 0.48–31.1 |
| Type 2 diabetes | 55 | 87.3 | 8 | 12.7 | 0.001 | 3.6; 1.5–8.3 |
| Questionnaires/Scales | With OSA | Without OSA | p Value | OR; 95% CI | ||
|---|---|---|---|---|---|---|
| Absolute | % | Absolute | % | |||
| STOP-BANG | 101 | 77.1 | 24 | 50 | <0.001 | 3.3; 1.67–6.76 |
| Berlin | 104 | 79.4 | 20 | 41.7 | <0.001 | 5.3; 2.6–11.02 |
| Epworth | 19 | 14.7 | 5 | 10 | 0.473 | 1.5; 0.54–4.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baymukanov, A.M.; Weissman, Y.D.; Bulavina, I.A.; Ilyich, I.L.; Termosesov, S.A. Sleep Apnea and Atrial Fibrillation: Clinical Features and Screening Diagnostic Options. J. Pers. Med. 2024, 14, 618. https://doi.org/10.3390/jpm14060618
Baymukanov AM, Weissman YD, Bulavina IA, Ilyich IL, Termosesov SA. Sleep Apnea and Atrial Fibrillation: Clinical Features and Screening Diagnostic Options. Journal of Personalized Medicine. 2024; 14(6):618. https://doi.org/10.3390/jpm14060618
Chicago/Turabian StyleBaymukanov, Azamat Maratovich, Yuliya Dmitrievna Weissman, Irina Andreevna Bulavina, Ilya Leonidovich Ilyich, and Sergey Arturovich Termosesov. 2024. "Sleep Apnea and Atrial Fibrillation: Clinical Features and Screening Diagnostic Options" Journal of Personalized Medicine 14, no. 6: 618. https://doi.org/10.3390/jpm14060618
APA StyleBaymukanov, A. M., Weissman, Y. D., Bulavina, I. A., Ilyich, I. L., & Termosesov, S. A. (2024). Sleep Apnea and Atrial Fibrillation: Clinical Features and Screening Diagnostic Options. Journal of Personalized Medicine, 14(6), 618. https://doi.org/10.3390/jpm14060618





