Genetic Variation in ABCB1, ADRB1, CYP3A4, CYP3A5, NEDD4L and NR3C2 Confers Differential Susceptibility to Resistant Hypertension among South Africans
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Study Approval
2.2. Study Design and Setting
2.3. Sample Size Calculation
2.4. Genetic Characterization
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Genotype or Allele Frequency Distributions and Associations with Resistant Hypertension
3.3. Haplotype Frequency Distributions and Associations with Resistant Hypertension
3.4. Effect of Confounding Variables on Associations of Genetic Variables with Resistant Hypertension
3.5. Association of Genetic Variables with Resistant Hypertension Related Adverse Outcomes: Chronic Kidney Disease (CKD) and Left Ventricular Hypertrophy (LVH)
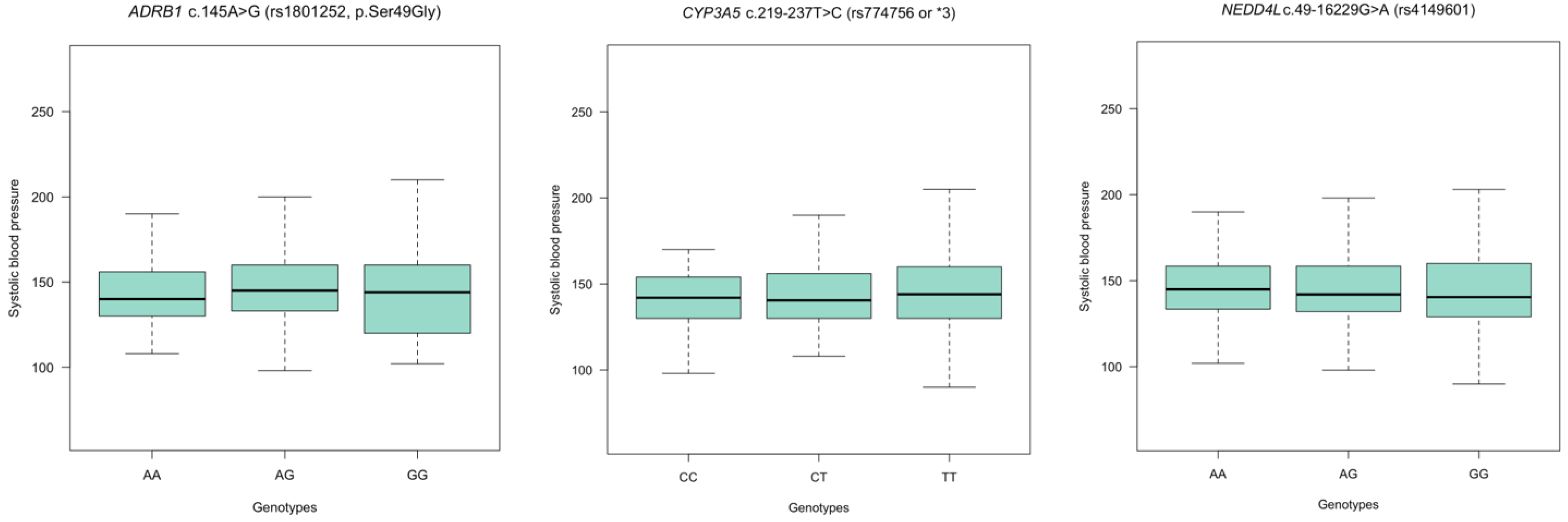
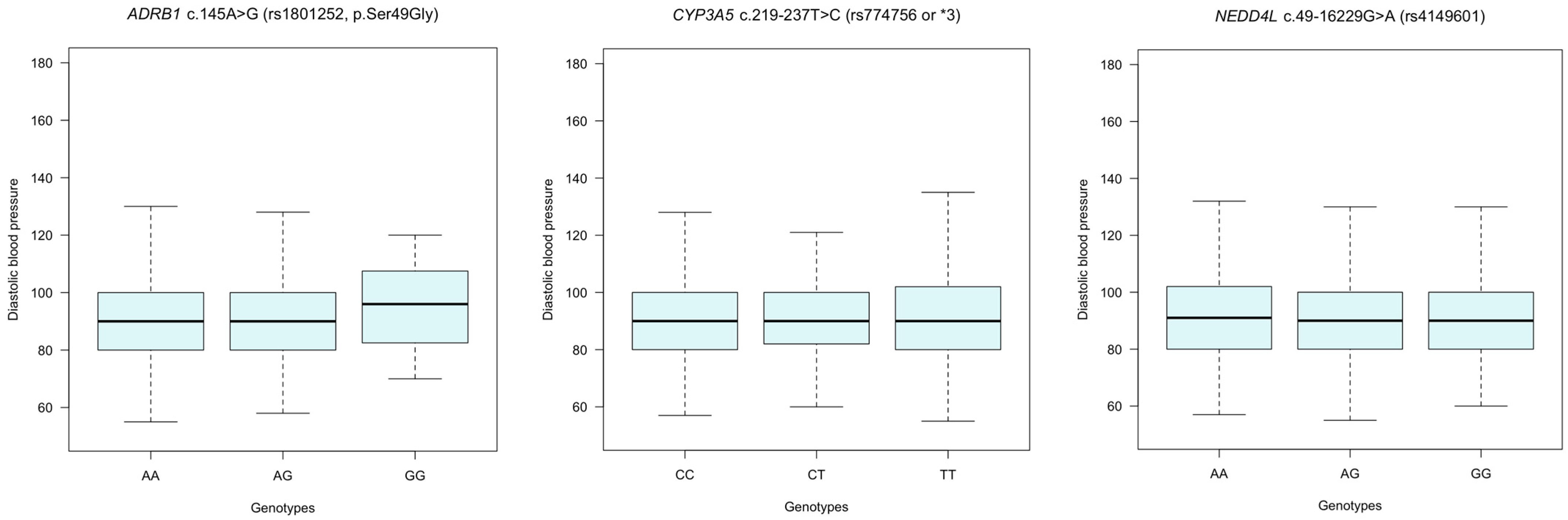
3.6. Assessing Blood Pressure (BP) Variation according to Genotypes in ADRB1, CYP3A5, and NEDD4L Polymorphisms in Hypertension
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. More Than 700 Million People with Untreated Hypertension: Number of People Living with Hypertension Has Doubled to 1.28 Billion Since 1990. Available online: https://www.who.int/news/item/25-08-2021-more-than-700-million-people-with-untreated-hypertension (accessed on 5 April 2024).
- Brant, L.C.C.; Passaglia, L.G.; Pinto-Filho, M.M.; de Castilho, F.M.; Ribeiro, A.L.P.; Nascimento, B.R. The Burden of Resistant Hypertension Across the World. Curr. Hypertens. Rep. 2022, 24, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Nansseu, J.R.N.; Noubiap, J.J.N.; Mengnjo, M.K.; Aminde, L.N.; Essouma, M.; Jingi, A.M.; Bigna, J.J.R. The highly neglected burden of resistant hypertension in Africa: A systematic review and meta-analysis. BMJ Open 2016, 6, e011452. [Google Scholar] [CrossRef] [PubMed]
- Moosa, M.S.; Kuttschreuter, L.S.; Rayner, B.L. Evaluation and management of patients referred to a tertiary-level hypertension clinic in Cape Town, South Africa. S. Afr. Med. J. 2016, 106, 797. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Buhnerkempe, M.G.; Moore, K.T. Resistant Hypertension: Disease Burden and Emerging Treatment Options. Curr. Hypertens. Rep. 2024, 26, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Champaneria, M.K.; Patel, R.S.; Oroszi, T.L. When blood pressure refuses to budge: Exploring the complexity of resistant hypertension. Front. Cardiovasc. Med. 2023, 10, 1211199. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Pharmacogenomics of Hypertension Treatment. Int. J. Mol. Sci. 2020, 21, 4709. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Pereira, S.C.; Tanus-Santos, J.E.; Lacchini, R. Pharmacogenomics and Hypertension: Current Insights. Pharmgenom. Pers. Med. 2019, 12, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Dludla, P.; Mabhida, S.; Benjeddou, M.; Louw, J.; February, F. Pharmacogenomics of amlodipine and hydrochlorothiazide therapy and the quest for improved control of hypertension: A mini review. Heart Fail. Rev. 2019, 24, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Seeley, A.; Prynn, J.; Perera, R.; Street, R.; Davis, D.; Etyang, A.O. Pharmacotherapy for hypertension in Sub-Saharan Africa: A systematic review and network meta-analysis. BMC Med. 2020, 18, 75. [Google Scholar] [CrossRef]
- Onwukwe, S.C.; Ngene, N.C. Blood pressure control in hypertensive patients attending a rural community health centre in Gauteng Province, South Africa: A cross-sectional study. S. Afr. Fam. Pract. (2004) 2022, 64, e1–e9. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef]
- Türkmen, D.; Masoli, J.A.; Delgado, J.; Kuo, C.L.; Bowden, J.; Melzer, D.; Pilling, L.C. Calcium-channel blockers: Clinical outcome associations with reported pharmacogenetics variants in 32 000 patients. Br. J. Clin. Pharmacol. 2023, 89, 853–864. [Google Scholar] [CrossRef]
- Guerra, L.A.; Lteif, C.; Arwood, M.J.; McDonough, C.W.; Dumeny, L.; Desai, A.A.; Cavallari, L.H.; Duarte, J.D. Genetic polymorphisms in ADRB2 and ADRB1 are associated with differential survival in heart failure patients taking β-blockers. Pharmacogenom. J. 2022, 22, 62–68. [Google Scholar] [CrossRef]
- Ishigami, T.; Kino, T.; Minegishi, S.; Araki, N.; Umemura, M.; Ushio, H.; Saigoh, S.; Sugiyama, M. Regulators of Epithelial Sodium Channels in Aldosterone-Sensitive Distal Nephrons (ASDN): Critical Roles of Nedd4L/Nedd4-2 and Salt-Sensitive Hypertension. Int. J. Mol. Sci. 2020, 21, 3871. [Google Scholar] [CrossRef]
- Cunningham, P.N.; Chapman, A.B. The Future of Pharmacogenetics in the Treatment of Hypertension. Pharmacogenomics 2019, 20, 129–132. [Google Scholar] [CrossRef]
- McDonough, C.W.; Burbage, S.E.; Duarte, J.D.; Gong, Y.; Langaee, T.Y.; Turner, S.T.; Gums, J.G.; Chapman, A.B.; Bailey, K.R.; Beitelshees, A.L.; et al. Association of variants in NEDD4L with blood pressure response and adverse cardiovascular outcomes in hypertensive patients treated with thiazide diuretics. J. Hypertens. 2013, 31, 698–704. [Google Scholar] [CrossRef]
- Dumeny, L.; Vardeny, O.; Edelmann, F.; Pieske, B.; Duarte, J.D.; Cavallari, L.H. NR3C2 genotype is associated with response to spironolactone in diastolic heart failure patients from the Aldo-DHF trial. Pharmacotherapy 2021, 41, 978–987. [Google Scholar] [CrossRef]
- Sarhan, N.M.; Shahin, M.H.; El Rouby, N.M.; El-Wakeel, L.M.; Solayman, M.H.; Langaee, T.; Khorshid, H.; Schaalan, M.F.; Sabri, N.A.; Cavallari, L.H. Effect of Genetic and Nongenetic Factors on the Clinical Response to Mineralocorticoid Receptor Antagonist Therapy in Egyptians with Heart Failure. Clin. Transl. Sci. 2020, 13, 195–203. [Google Scholar] [CrossRef]
- Zilbermint, M.; Hannah-Shmouni, F.; Stratakis, C.A. Genetics of Hypertension in African Americans and Others of African Descent. Int. J. Mol. Sci. 2019, 20, 1081. [Google Scholar] [CrossRef]
- Peeters, L.E.J.; Feyz, L.; Boersma, E.; Daemen, J.; van Gelder, T.; Koch, B.C.P.; Versmissen, J. Clinical Applicability of Monitoring Antihypertensive Drug Levels in Blood. Hypertension 2020, 76, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Naing, L.; Winn, T.; Nordin, R. Pratical Issues in Calculating the Sample Size for Prevalence Studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Shi, Y.Y.; He, L. Publisher Correction: SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2023, 1, 1. [Google Scholar] [CrossRef]
- Harrison, P.W.; Amode, M.R.; Austine-Orimoloye, O.; Andrey, G.A.; Barba, M.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2024. Nucleic Acids Res. 2023, 52, D891–D899. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liu, Y.H.; Wu, D.W.; Su, H.M.; Chen, S.C. Dyslipidemia Increases the Risk of Incident Hypertension in a Large Taiwanese Population Follow-Up Study. Nutrients 2022, 14, 3277. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Ingelfinger, J.R. Aldosterone and Treatment-Resistant Hypertension. N. Engl. J. Med. 2023, 388, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Sowers, J.R. Hypertension in Diabetes: An Update of Basic Mechanisms and Clinical Disease. Hypertension 2021, 78, 1197–1205. [Google Scholar] [CrossRef]
- Mehta, N.D.; Battle, S.J.; DiPette, D.J. Cardiac structure and function in resistant hypertension: The beneficial role of blood pressure control. J. Clin. Hypertens. 2023, 25, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Antona, C.; Savieo, J.L.; Lauschke, V.M.; Sangkuhl, K.; Drögemöller, B.I.; Wang, D.; van Schaik, R.H.N.; Gilep, A.A.; Peter, A.P.; Boone, E.C.; et al. PharmVar GeneFocus: CYP3A5. Clin. Pharmacol. Ther. 2022, 112, 1159–1171. [Google Scholar] [CrossRef]
- Lee, F.Y.; Islahudin, F.; Abdul Gafor, A.H.; Wong, H.S.; Bavanandan, S.; Mohd Saffian, S.; Md Redzuan, A.; Makmor-Bakry, M. Adverse Drug Reactions of Antihypertensives and CYP3A5*3 Polymorphism Among Chronic Kidney Disease Patients. Front. Pharmacol. 2022, 13, 848804. [Google Scholar] [CrossRef]
- Soria-Chacartegui, P.; Zubiaur, P.; Ochoa, D.; Villapalos-García, G.; Román, M.; Matas, M.; Figueiredo-Tor, L.; Mejía-Abril, G.; Calleja, S.; de Miguel, A.; et al. Genetic Variation in CYP2D6 and SLC22A1 Affects Amlodipine Pharmacokinetics and Safety. Pharmaceutics 2023, 15, 404. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, V.; Garcia, E.P.; O’Connor, D.T.; Brophy, V.H.; Alcaraz, J.; Richard, E.; Bakris, G.L.; Middleton, J.P.; Norris, K.C.; Wright, J.; et al. CYP3A4 and CYP3A5 polymorphisms and blood pressure response to amlodipine among African-American men and women with early hypertensive renal disease. Am. J. Nephrol. 2010, 31, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Esp. Cardiol. (Engl. Ed.) 2019, 72, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Johnson, J.A. Pharmacogenetic factors affecting β-blocker metabolism and response. Expert Opin. Drug Metab. Toxicol. 2020, 16, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Turgut, S.; Yaren, A.; Kursunluoglu, R.; Turgut, G. MDR1 C3435T polymorphism in patients with breast cancer. Arch. Med. Res. 2007, 38, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, K.E.; Zhang, W.; Yang, D.; Press, O.A.; Gordon, M.; Vallböhmer, D.; Schultheis, A.M.; Lurje, G.; Ladner, R.D.; Fazzone, W.; et al. ABCB1, SLCO1B1 and UGT1A1 gene polymorphisms are associated with toxicity in metastatic colorectal cancer patients treated with first-line irinotecan. Drug Metab. Lett. 2007, 1, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, A.; Nishizawa, D.; Kasai, S.; Hasegawa, J.; Fukuda, K.-i.; Nagashima, M.; Katoh, R.; Ikeda, K. Association between Genetic Polymorphisms of the β1-Adrenergic Receptor and Sensitivity to Pain and Fentanyl in Patients Undergoing Painful Cosmetic Surgery. J. Pharmacol. Sci. 2013, 121, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Tavira, B.; Coto, E.; Diaz-Corte, C.; Alvarez, V.; López-Larrea, C.; Ortega, F. A search for new CYP3A4 variants as determinants of tacrolimus dose requirements in renal-transplanted patients. Pharmacogenet. Genom. 2013, 23, 445–448. [Google Scholar] [CrossRef]
- van Schaik, R.H.; van der Heiden, I.P.; van den Anker, J.N.; Lindemans, J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin. Chem. 2002, 48, 1668–1671. [Google Scholar] [CrossRef]
| Variable(s) | Cases (N = 190) | Controls (N = 189) | p-Value |
|---|---|---|---|
| Age years | 43.8 (20–54.2) | 42.2 (26–52.6) | 0.05 |
| Female sex | 103 (54.2%) | 102 (54.0%) | 0.99 |
| Ethnicity | |||
| Black African | 58 (30.5%) | 52 (27.5%) | 0.57 |
| Mixed ancestry | 132 (69.5%) | 137 (72.5%) | |
| Alcohol consumption (frequent/occasional) | 45 (23.7%) | 47 (24.9%) | 0.90 |
| Smoking (active/past) | 63 (33.2%) | 64 (33.9%) | 0.99 |
| Positive family History of hypertension | 123 (64.7%) | 121 (64.0%) | 0.91 |
| Blood pressure (mmHg) | |||
| Uncontrolled (≥140/90) | 149 (78.4%) | 68 (36.0%) | <0.001 |
| Controlled (<140/90) | 41 (21.6%) | 121 (64.0%) | |
| Left ventricular hypertrophy a | |||
| Yes | 90 (47.4%) | 52 (27.5%) | <0.001 |
| No/unknown | 100 (52.6%) | 189 (72.5%) | |
| Aldosterone (pmol/L) | 276 (31–435) | 200 (2.3–313) | 0.002 |
| Comorbidities | |||
| Chronic kidney disease (CKD) b | 36 (19.1%) | 10 (5.3%) | <0.001 |
| Diabetes mellitus (DM) | 33 (17.6%) | 18 (9.6%) | 0.03 |
| Dyslipidemia | 30 (15.8%) | 15 (7.9%) | 0.03 |
| Ischemic heart disease (IHD) | 17 (8.9%) | 9 (4.8%) | 0.11 |
| Previous stroke | 13 (6.8%) | 5 (2.6%) | 0.09 |
| Concomitant drugs | |||
| Statin or lipid-lowering therapy | 76 (40.0%) | 45 (23.8%) | <0.001 |
| Diabetic treatment c | 23 (12.1%) | 15 (7.9%) | 0.08 |
| Analgesics | 12 (6.0%) | 10 (5.2%) | 0.69 |
| Antihypertensives drugs | |||
| Amlodipine | 172 (90.5%) | 116 (61.3%) | <0.001 |
| Hydrochlorothiazide | 148 (77.9%) | 108 (57.1%) | <0.001 |
| Atenolol | 123 (64.7%) | 27 (14.2%) | <0.001 |
| Enalapril | 150 (78.9%) | 88 (46.6%) | <0.001 |
| Spironolactone | 52 (27.3%) | 7 (3.7%) | <0.001 |
| Losartan | 39 (20.5%) | 14 (7.4%) | <0.001 |
| Single Nucleotide Polymorphism | Genotype | Genotype Frequencies, N (freq) | p-Value | OR [95% CI] | ||
|---|---|---|---|---|---|---|
| Combined | Cases | Controls | ||||
| ABCB1 rs1045642 (c.3435C>T) | C/C | 191 (0.50) | 99 (0.52) | 92 (0.49) | 1 | |
| C/T | 142 (0.37) | 70 (0.37) | 72 (0.38) | 0.66 | 0.90 [0.57–1.42] | |
| T/T | 46 (0.12) | 21 (0.11) | 25 (0.13) | 0.51 | 0.78 [0.39–1.57] | |
| ABCB1 rs2032582 (c.2677C>A) | C/C | 219 (0.59) | 112 (0.60) | 107 (0.58) | 1 | |
| A/C | 109 (0.29) | 54 (0.29) | 55 (0.30) | 0.82 | 0.93 [0.58–1.52] | |
| A/A | 46 (0.13) | 22 (0.11) | 24 (0.13) | 0.74 | 0.88 [0.44–1.74] | |
| ADRB1 rs1801252 (c.145A>G) | A/A | 190 (0.51) | 92 (0.48) | 107 (0.57) | 1 | |
| A/G | 149 (0.40) | 79 (0.42) | 70 (0.37) | 0.23 | 1.31 [0.84–2.05] | |
| G/G | 31 (0.08) | 19 (0.10) | 12 (0.06) | 0.13 | 1.83 [0.80–4.39] | |
| ADRB1 rs1801253 (c.1165G>C) | C/C | 195 (0.51) | 100 (0.53) | 95 (0.50) | 1 | |
| C/G | 153 (0.40) | 74 (0.39) | 79 (0.42) | 0.67 | 0.89 [0.57–1.39] | |
| G/G | 31 (0.08) | 16 (0.08) | 15 (0.08) | 0.98 | 1.01 [0.44–2.34] | |
| CYP3A4 rs2740574 (c.-392C>T, CYP3A4*1B) | T/T | 136 (0.36) | 59 (0.32) | 77 (0.41) | 1 | |
| C/T | 132 (0.35) | 74 (0.40) | 58 (0.31) | 0.05 | 1.30 [0.78–2.17] | |
| C/C | 105 (0.28) | 52 (0.28) | 53 (0.28) | 0.36 | 0.78 [0.47–1.30] | |
| CYP3A5 rs776746 (c.219-237T>C, CYP3A5*3) | T/T | 144 (0.38) | 78 (0.41) | 66 (0.35) | 1 | |
| C/T | 146 (0.39) | 77 (0.41) | 69 (0.37) | 0.82 | 0.94 [0.58–1.54] | |
| C/C | 89 (0.23) | 35 (0.18) | 54 (0.29) | 0.03 | 0.54 [0.31–0.97] | |
| CYP3A5 rs10264272 (c.624C>T, CYP3A5*6) | C/C | 285 (0.75) | 141 (0.74) | 144 (0.76) | 1 | |
| C/T | 86 (0.22) | 45 (0.24) | 41 (0.22) | 0.71 | 1.12 [0.67–1.87] | |
| T/T | 8 (0.10) | 4 (0.02) | 4 (0.02) | 0.99 | 1.02 [0.18–5.60] | |
| CYP3A5 rs41303343 (insT, CYP3A5*7) | A/A | 322 (0.85) | 158 (0.83) | 164 (0.87) | 1 | |
| A/T | 52 (0.14) | 30 (0.16) | 22 (0.12) | 0.29 | 1.41 [0.75–2.69] | |
| T/T | 3 (0.01) | 1 (0.01) | 2 (0.01) | 0.99 | 0.51 [0.01–10.08] | |
| NEDD4L rs4149601 (c.49-16229G>A) | G/G | 134 (0.36) | 59 (0.31) | 75 (0.40) | 1 | |
| A/G | 179 (0.47) | 95 (0.50) | 84 (0.45) | 0.14 | 1.43 [0.89–2.31] | |
| A/A | 64 (0.17) | 36 (0.19) | 28 (0.15) | 0.13 | 1.63 [0.86–3.11] | |
| NEDD4L rs292449 (c.-300G>C) | G/G | 100 (0.26) | 52 (0.28) | 48 (0.25) | 1 | |
| C/G | 190 (0.50) | 89 (0.47) | 101 (0.53) | 0.25 | 0.73 [0.42–1.26] | |
| C/C | 88 (0.23) | 48 (0.25) | 40 (0.21) | 0.77 | 0.90 [0.49–1.67] | |
| NR3C2 rs5522 (c.538G>A) | T/T | 286 (0.76) | 145 (0.76) | 141 (0.75) | 1 | |
| C/T | 90 (0.24) | 45 (0.24) | 45 (0.24) | 0.99 | 0.97 [0.59–1.61] | |
| C/C | 1 (0.003) | 0 (0) | 1 (0.01) | 0.49 | 0 [0–38.2] | |
| NR3C2 rs2070950 (c.-2-358C>G) | G/G | 171 (0.45) | 88 (0.47) | 83 (0.44) | 1 | |
| C/G | 145 (0.38) | 71 (0.38) | 74 (0.39) | 0.73 | 0.91 [0.57–1.44] | |
| C/C | 62 (0.16) | 30 (0.16) | 32 (0.17) | 0.77 | 0.88 [0.47–1.65] | |
| SNP Combination | Haplotype | Cases, N (Freq) | Controls, N (Freq) | p-Value | OR [95% CI] |
|---|---|---|---|---|---|
| ADRB1 rs1801252—rs1801253 | A-C | 157 (0.41) | 175 (0.46) | 0.17 | 0.82 [0.61–1.09] |
| A-G | 106 (0.28) | 109 (0.29) | 0.77 | 0.96 [0.69–1.31] | |
| G-C | 117 (0.31) | 94 (0.25) | 0.06 | 1.34 [0.98–1.85] | |
| CYP3A5 rs776746—rs10264272—rs41303343 | C-C-A | 144 (0.38) | 172 (0.46) | 0.03 | 0.73 [0.54–0.97] |
| T-C-A | 150 (0.40) | 130 (0.35) | 0.14 | 1.25 [0.93–1.68] | |
| T-C-T | 30 (0.08) | 25 (0.07) | 0.43 | 1.25 [0.72–2.16] | |
| T-T-A | 50 (0.13) | 46 (0.12) | 0.65 | 1.10 [0.72–1.70] | |
| NEDD4L rs4149601—rs292449 | A-C | 93 (0.25) | 72 (0.19) | 0.07 | 1.37 [0.97–1.94] |
| A-G | 73 (0.19) | 68 (0.18) | 0.70 | 1.07 [0.75–1.55] | |
| G-C | 100 (0.26) | 123 (0.33) | 0.05 | 0.73 [0.54–1.00] | |
| G-G | 112 (0.30) | 111 (0.30) | 0.99 | 0.99 [0.73–1.37] |
| SNP/SNP Combination | Genotype/Haplotype | Coefficient | p-Value | OR [95% CI] a |
|---|---|---|---|---|
| ADRB1 rs1801252 | GG | 1.19 | 0.02 | 3.30 [1.17–10.03] b |
| ADRB1 rs1801252—rs1801253 | G-C | 1.04 | 0.04 | 2.83 [1.05–8.20] b |
| CYP3A5 rs776746 | CC | −0.80 | 0.02 | 0.44 [0.22–0.89] b |
| CYP3A5 rs776746—rs1026427—rs41303343 | C-C-A | −0.14 | 0.59 | 0.86 [0.49–1.50] |
| NEDD4L rs4149601 | AA | 1.34 | 0.001 | 3.82 [1.67–9.07] b |
| NEDD4L rs4149601—rs292449 | A-C | 1.14 | 0.08 | 3.14 [0.88–12.9] |
| Chronic Kidney Disease | |||||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted a | ||||||
| Variable | Genotype | Coefficient | p-Value | OR [95% CI] | Coefficient | p-Value | OR [95% CI] |
| ADRB1 rs1801252 | G/G | −0.11 | 0.87 | 0.90 [0.20–2.83] | −0.09 | 0.83 | 0.91 [0.39–2.08] |
| CYP3A5 rs776746 | C/C | −0.99 | 0.03 | 0.37 [0.13–0.90] | −1.22 | 0.04 | 0.29 [0.08–0.93] b |
| NEDD4L rs4149601 | A/A | 0.19 | 0.71 | 1.20 [0.46–3.54] | −0.25 | 0.72 | 0.78 [0.16–3.02] |
| Left Ventricular Hypertrophy | |||||||
| ADRB1 rs1801252 | G/G | 0.26 | 0.12 | 1.29 [0.83–2.00] | 0.55 | 0.25 | 1.13 [0.57–2.25] |
| CYP3A5 rs776746 | C/C | −0.08 | 0.76 | 0.92 [0.53–1.59] | 0.12 | 0.72 | 1.74 [0.67–4.45] |
| NEDD4L rs4149601 | A/A | −0.05 | 0.85 | 0.95 [0.50–1.74] | −0.17 | 0.69 | 0.85 [0.37–1.89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsukunya, J.N.; Jones, E.; Soko, N.D.; Blom, D.; Sinxadi, P.; Rayner, B.; Dandara, C. Genetic Variation in ABCB1, ADRB1, CYP3A4, CYP3A5, NEDD4L and NR3C2 Confers Differential Susceptibility to Resistant Hypertension among South Africans. J. Pers. Med. 2024, 14, 664. https://doi.org/10.3390/jpm14070664
Katsukunya JN, Jones E, Soko ND, Blom D, Sinxadi P, Rayner B, Dandara C. Genetic Variation in ABCB1, ADRB1, CYP3A4, CYP3A5, NEDD4L and NR3C2 Confers Differential Susceptibility to Resistant Hypertension among South Africans. Journal of Personalized Medicine. 2024; 14(7):664. https://doi.org/10.3390/jpm14070664
Chicago/Turabian StyleKatsukunya, Jonathan N., Erika Jones, Nyarai D. Soko, Dirk Blom, Phumla Sinxadi, Brian Rayner, and Collet Dandara. 2024. "Genetic Variation in ABCB1, ADRB1, CYP3A4, CYP3A5, NEDD4L and NR3C2 Confers Differential Susceptibility to Resistant Hypertension among South Africans" Journal of Personalized Medicine 14, no. 7: 664. https://doi.org/10.3390/jpm14070664
APA StyleKatsukunya, J. N., Jones, E., Soko, N. D., Blom, D., Sinxadi, P., Rayner, B., & Dandara, C. (2024). Genetic Variation in ABCB1, ADRB1, CYP3A4, CYP3A5, NEDD4L and NR3C2 Confers Differential Susceptibility to Resistant Hypertension among South Africans. Journal of Personalized Medicine, 14(7), 664. https://doi.org/10.3390/jpm14070664








