In Vitro Investigation of Pulsed Electromagnetic Field Stimulation (PEMF) with MAGCELL® ARTHRO on the Regulatory Expression of Soluble and Membrane-Bound Complement Factors and Inflammatory Cytokines in Immortalized Synovial Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Culture of K4IM
2.2. PEMF Stimulation of the Synovial Fibroblasts
2.3. Gene Expression Analysis
2.3.1. RNA Isolation and cDNA Synthesis
2.3.2. qPCR
2.4. Protein Expression Analysis
Immunofluorescence Labeling of the Synovial Fibroblasts
2.5. Statistical Analysis
3. Results
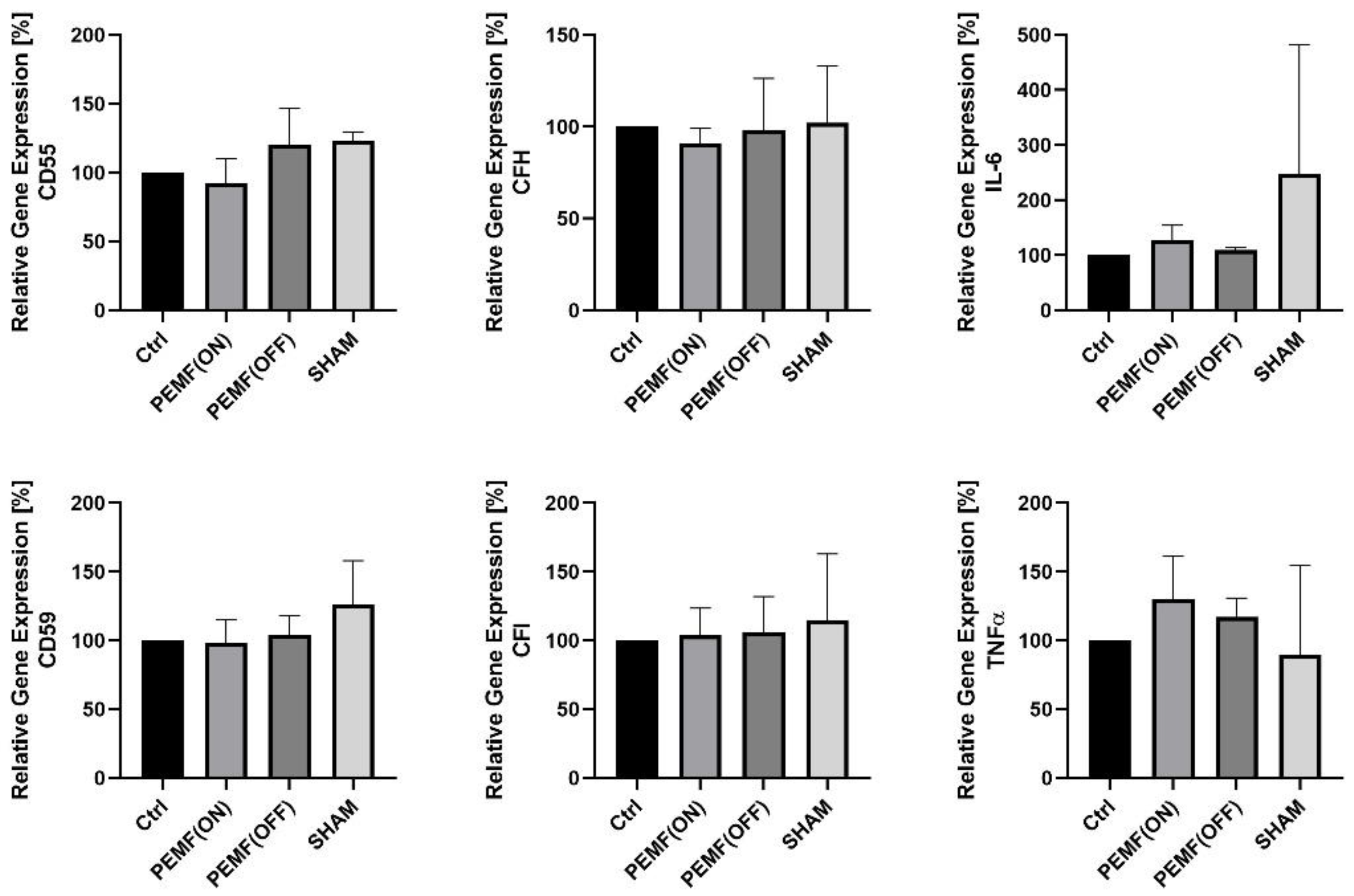
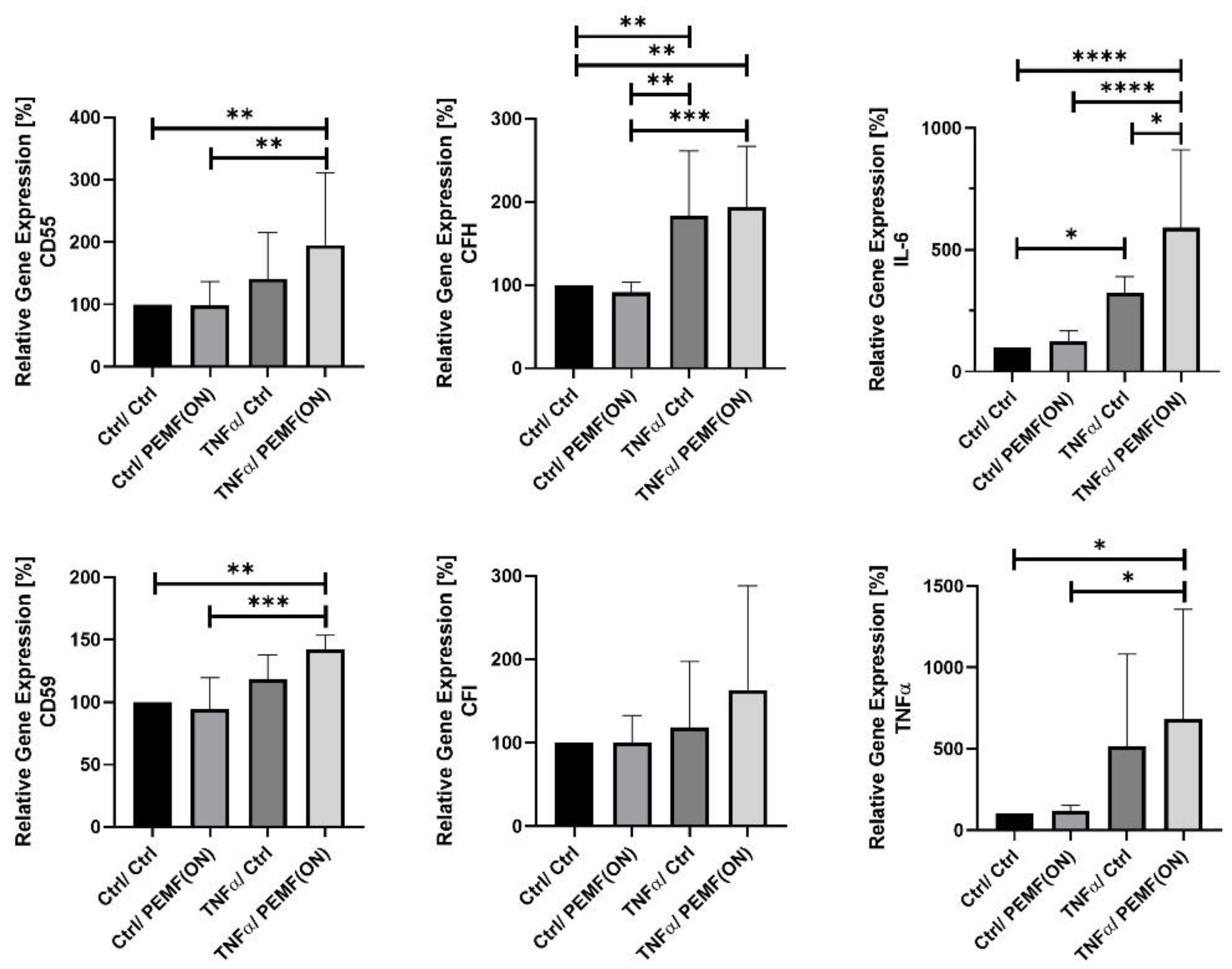
3.1. Gene Expression
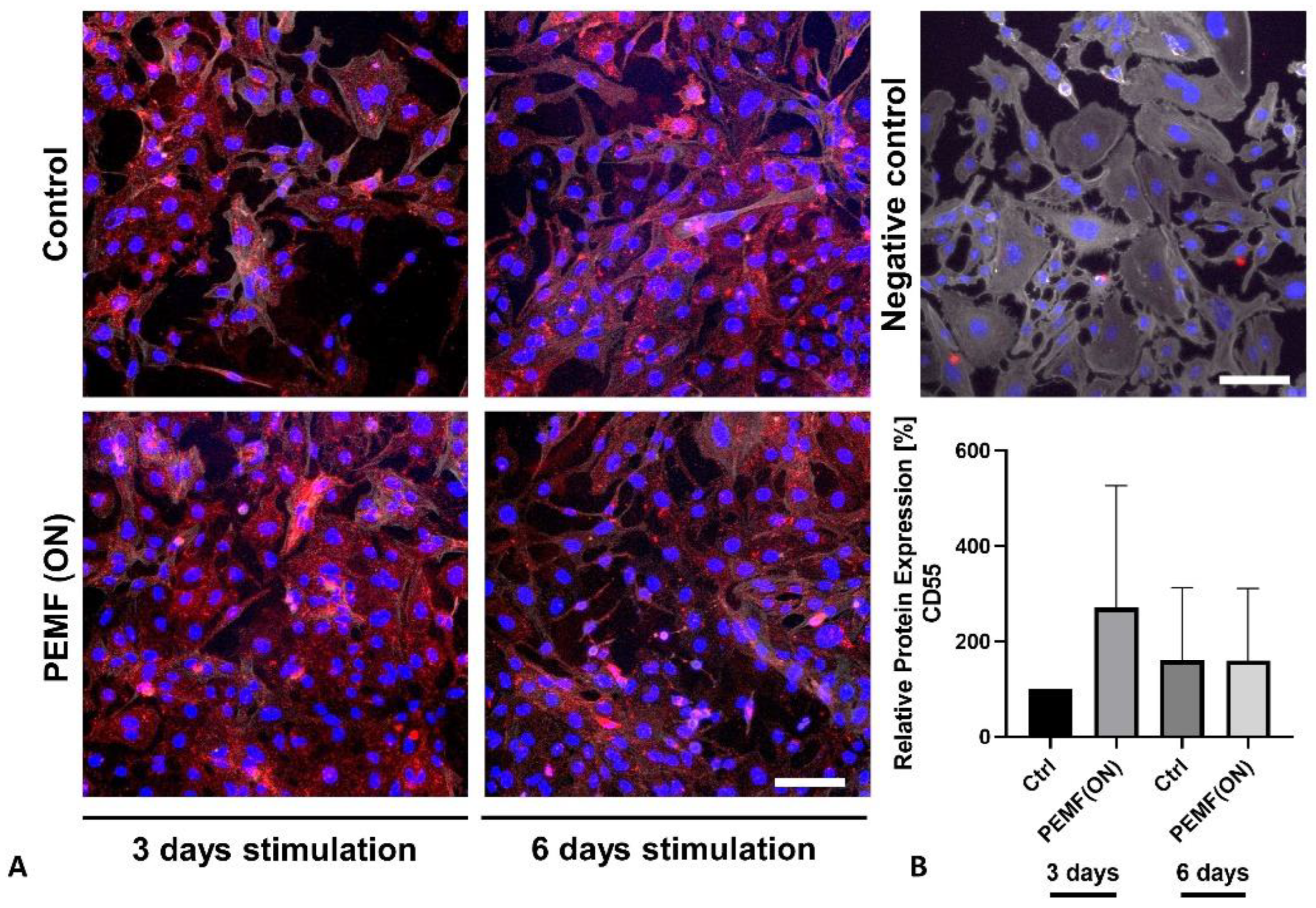
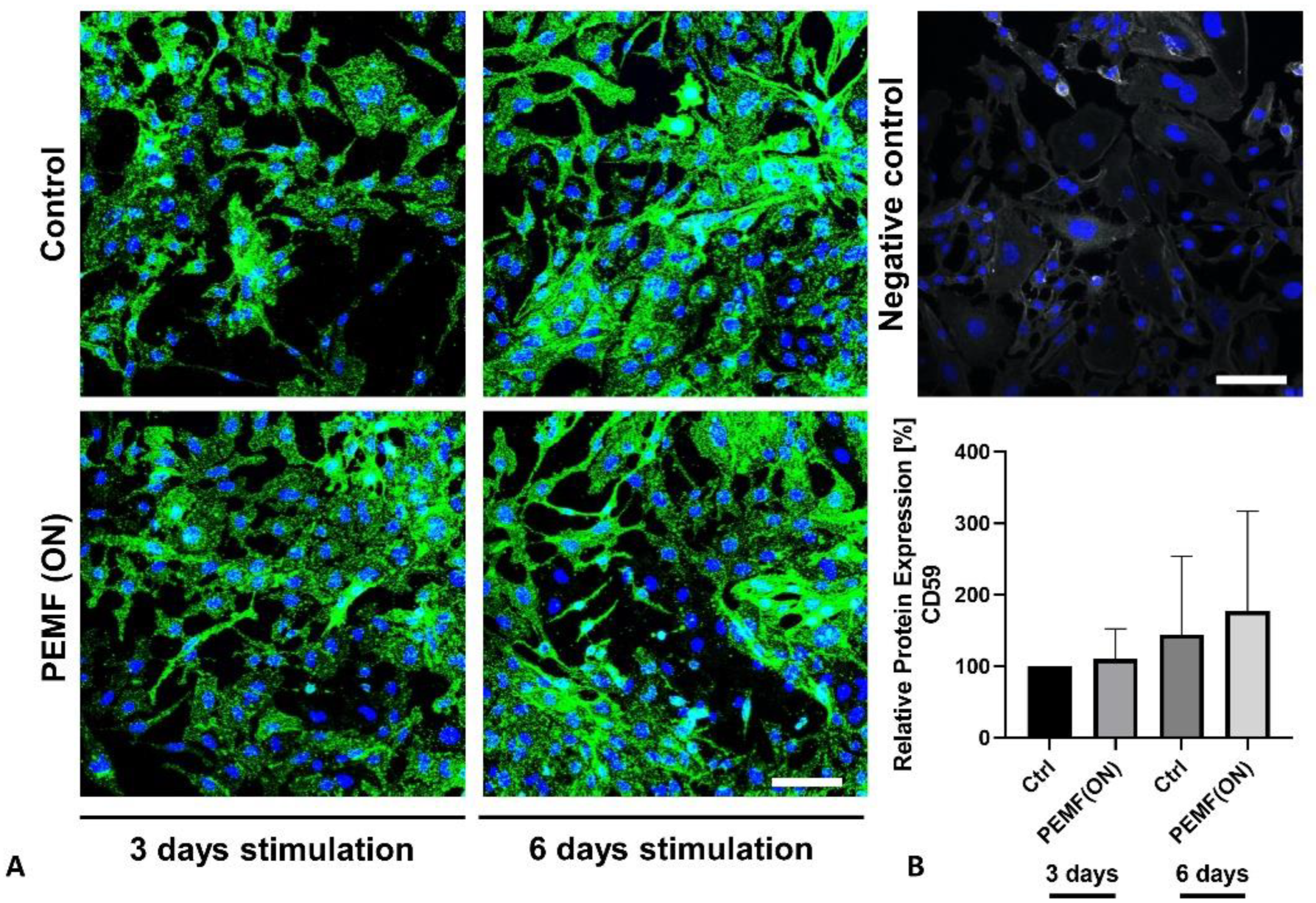
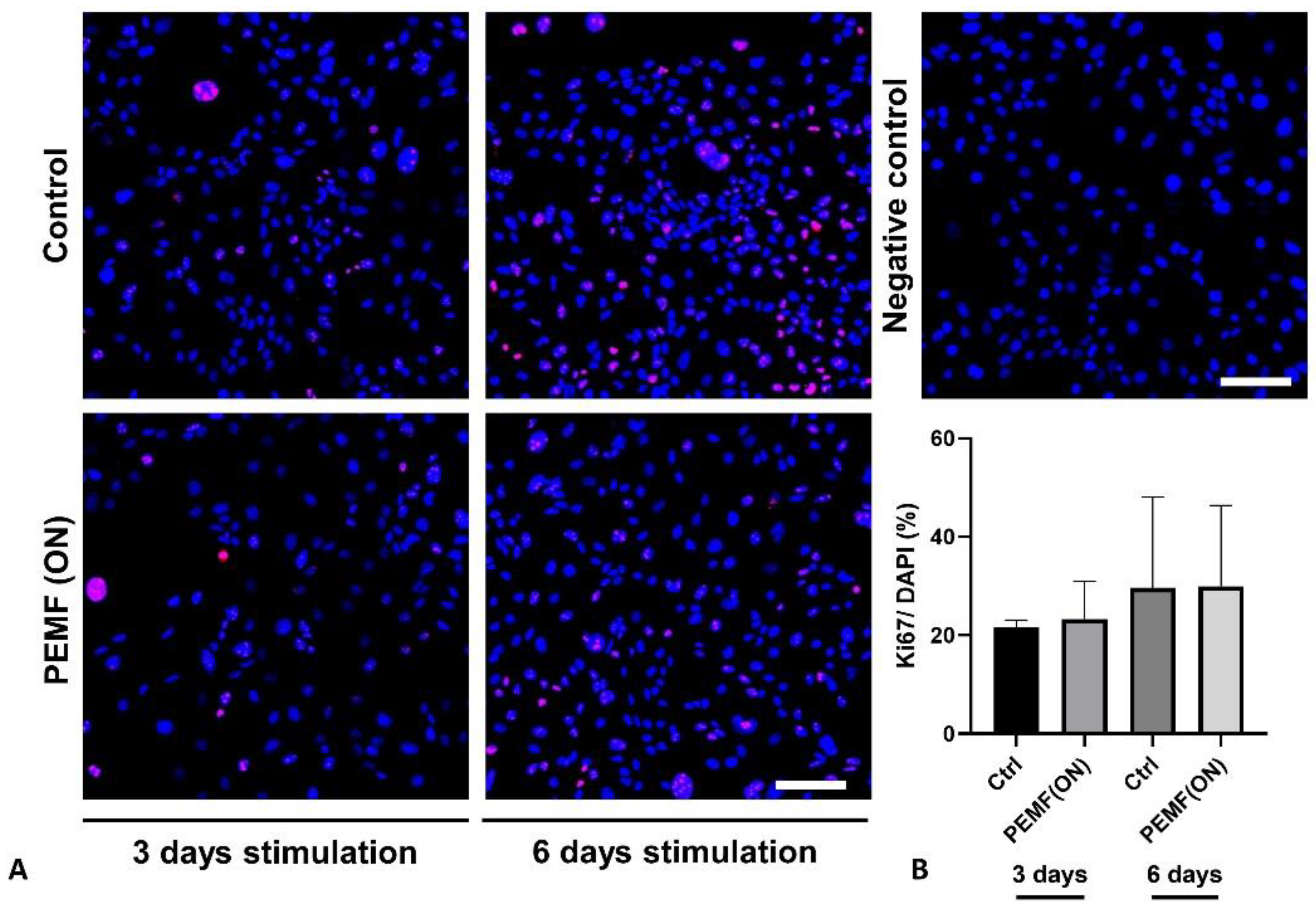
3.2. Protein Expression
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, E.; Pulsatelli, L.; Dolzani, P.; Mariani, E.; Lisignoli, G.; Addimanda, O.; Meliconi, R. Complement Expression and Activation in Osteoarthritis Joint Compartments. Front. Immunol. 2020, 11, 535010. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Sethi, V.; Anand, C.; Della Pasqua, O. Clinical Assessment of Osteoarthritis Pain: Contemporary Scenario, Challenges, and Future Perspectives. Pain Ther. 2024, 13, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.; Hinterberger, T.; Szymski, D.; Alt, V.; Rupp, M. Psychological comorbidities in osteoarthritis in Germany. Sci. Rep. 2023, 13, 2905. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Fekadu, G.; Jiang, X.; Bekele, F.; Tolossa, T.; Turi, E.; Fetensa, G.; Fanta, K. An up to date on clinical prospects and management of osteoarthritis. Ann. Med. Surg. 2021, 72, 103077. [Google Scholar] [CrossRef]
- Markovic, L.; Wagner, B.; Crevenna, R. Effects of pulsed electromagnetic field therapy on outcomes associated with osteoarthritis. Wien. Klin. Wochenschr. 2022, 134, 425–433. [Google Scholar] [CrossRef]
- Wang, T.; Xie, W.; Ye, W.; He, C. Effects of electromagnetic fields on osteoarthritis. Biomed. Pharmacother. 2019, 118, 109282. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.L.; Miceli, G.; Marino, N.; Sciortino, D.; Bagnato, G.F. Pulsed electromagnetic fields in knee osteoarthritis: A double blind, placebo-controlled, randomized clinical trial. Rheumatology 2016, 55, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed. Pharmacother. 2020, 131, 110767. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Duan, X.; Xing, F.; Liu, G.; Gong, M.; Li, L.; Chen, R.; Xiang, Z. Effects of pulsed electromagnetic field therapy on pain, stiffness and physical function in patients with knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2019, 51, 821–827. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.J.; Callaghan, M.J.; Oldham, J.A. Pulsed electromagnetic energy treatment offers no clinical benefit in reducing the pain of knee osteoarthritis: A systematic review. BMC Musculoskelet. Disord. 2006, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Wuschech, H.; von Hehn, U.; Mikus, E.; Funk, R.H. Effects of PEMF on patients with osteoarthritis: Results of a prospective, placebo-controlled, double-blind study. Bioelectromagnetics 2015, 36, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Hallam, T.M.; Sharp, S.J.; Andreadi, A.; Kavanagh, D. Complement factor I: Regulatory nexus, driver of immunopathology, and therapeutic. Immunobiology 2023, 228, 152410. [Google Scholar] [CrossRef] [PubMed]
- Härdig, Y.; Hillarp, A.; Dahlbäck, B. The amino-terminal module of the C4b-binding protein alpha-chain is crucial for C4b binding and factor I-cofactor function. Biochem. J. 1997, 323 Pt 2, 469–475. [Google Scholar] [CrossRef]
- Schwaeble, W.; Zwirner, J.; Schulz, T.F.; Linke, R.P.; Dierich, M.P.; Weiss, E.H. Human complement factor H: Expression of an additional truncated gene product of 43 kDa in human liver. Eur. J. Immunol. 1987, 17, 1485–1489. [Google Scholar] [CrossRef]
- Lucientes-Continente, L.; Márquez-Tirado, B.; Goicoechea de Jorge, E. The Factor H protein family: The switchers of the complement alternative pathway. Immunol. Rev. 2023, 313, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, I.; Gómez-Ochoa, P.; Gómez-Casal, F.; Cativiela, E.; Larrad-Mur, L. Pulsed electromagnetic fields decrease proinflammatory cytokine secretion (IL-1β and TNF-α) on human fibroblast-like cell culture. Rheumatol. Int. 2011, 31, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, Y.; Qian, J.; Yang, H. Effect of a low-frequency pulsed electromagnetic field on expression and secretion of IL-1β and TNF-α in nucleus pulposus cells. J. Int. Med. Res. 2017, 45, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Silawal, S.; Wagner, M.; Roth, D.; Bertsch, T.; Schwarz, S.; Willauschus, M.; Gesslein, M.; Triebel, J.; Schulze-Tanzil, G. Complement Proteins C5/C5a, Cathepsin D and Prolactin in Chondrocytes: A Possible Crosstalk in the Pathogenesis of Osteoarthritis. Cells 2022, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. Quantification strategies in real-time PCR. AZ Quant. PCR 2004, 1, 89–113. [Google Scholar]
- Franke, V.; Meyer, S.; Schulze-Tanzil, G.G.; Braun, T.; Kokozidou, M.; Fischlein, T.; Silawal, S. Complement Regulation in Immortalized Fibroblast-like Synoviocytes and Primary Human Endothelial Cells in Response to SARS-CoV-2 Nucleocapsid Protein and Pro-Inflammatory Cytokine TNFα. Life 2022, 12, 1527. [Google Scholar] [CrossRef] [PubMed]
- Criado-García, O.; Fernaud-Espinosa, I.; Bovolenta, P.; de la Cuesta, R.S.; de Córdoba, S.R. Expression of the ß-chain of the complement regulator C4b-binding protein in human ovary. Eur. J. Cell Biol. 1999, 78, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pernaute, O.; Esparza-Gordillo, J.; Largo, R.; Calvo, E.; Alvarez-Soria, M.A.; Marcos, M.E.; Herrero-Beaumont, G.; de Córdoba, S.R. Expression of the peptide C4b-binding protein β in the arthritic joint. Ann. Rheum. Dis. 2006, 65, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A.; Di Benedetto, M.G.; Giacobbe, J.; Pariante, C.M. Pro- and anti-inflammatory properties of interleukin (IL6) in vitro: Relevance for major depression and for human hippocampal neurogenesis. Int. J. Neuropsychopharmacol. 2020, 23, 738–750. [Google Scholar] [CrossRef]
- Orfei, C.P.; Lovati, A.B.; Lugano, G.; Viganò, M.; Bottagisio, M.; D’Arrigo, D.; Sansone, V.; Setti, S.; Girolamo, L.d. Pulsed electromagnetic fields improve the healing process of Achilles tendinopathy. Bone Joint Res. 2020, 9, 613–622. [Google Scholar] [CrossRef]
- Karpus, O.N.; Kiener, H.P.; Niederreiter, B.; Yilmaz-Elis, A.S.; van der Kaa, J.; Ramaglia, V.; Arens, R.; Smolen, J.S.; Botto, M.; Tak, P.P.; et al. CD55 deposited on synovial collagen fibers protects from immune complex-mediated arthritis. Arthritis Res. Ther. 2015, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Friese, M.A.; Manuelian, T.; Junnikkala, S.; Hellwage, J.; Meri, S.; Peter, H.H.; Gordon, D.L.; Eibel, H.; Zipfel, P.F. Release of endogenous anti-inflammatory complement regulators FHL-1 and factor H protects synovial fibroblasts during rheumatoid arthritis. Clin. Exp. Immunol. 2003, 132, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Sakhrani, N.; Stefani, R.M.; Setti, S.; Cadossi, R.; Ateshian, G.A.; Hung, C.T. Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro. Appl. Sci. 2022, 12, 12406. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. [Google Scholar] [CrossRef]
- Stefani, R.M.; Barbosa, S.; Tan, A.R.; Setti, S.; Stoker, A.M.; Ateshian, G.A.; Cadossi, R.; Vunjak-Novakovic, G.; Aaron, R.K.; Cook, J.L.; et al. Pulsed electromagnetic fields promote repair of focal articular cartilage defects with engineered osteochondral constructs. Biotechnol. Bioeng. 2020, 117, 1584–1596. [Google Scholar] [CrossRef]
- De Mattei, M.; Caruso, A.; Pezzetti, F.; Pellati, A.; Stabellini, G.; Sollazzo, V.; Traina, G.C. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect Tissue Res. 2001, 42, 269–279. [Google Scholar] [CrossRef]


| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Day 1 | Ctrl Medium 1 | Ctrl Medium 1 | Ctrl Medium 1 | Ctrl Medium 1 |
| Day 2 | Ctrl Medium 1 | Ctrl Medium 1 | Ctrl Medium 1 | Ctrl Medium 1 |
| Day 3 | Ctrl Medium 2 | PEMF (ON) | PEMF (OFF) | Sham |
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Day 1 | Ctrl Medium 1 | Ctrl Medium 1 | Ctrl Medium 1 | Ctrl Medium 1 |
| Day 2 | Ctrl Medium 1 | Ctrl Medium 1 | TNFα | TNFα |
| Day 3 | Ctrl Medium 2 | PEMF (ON) | Ctrl Medium 2 | PEMF (ON) |
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Day 1 | Ctrl Medium 1 | Ctrl Medium 1 | Ctrl Medium 1 | Ctrl Medium 1 |
| Day 2 | Ctrl Medium 2 | PEMF (ON) | Ctrl Medium 2 | PEMF (ON) |
| Day 3 | Ctrl Medium 2 | PEMF (ON) | Ctrl Medium 2 | PEMF (ON) |
| Day 4 | Ctrl Medium 2 | PEMF (ON) | Ctrl Medium 2 | PEMF (ON) |
| Day 5 | - | - | Ctrl Medium 2 | PEMF (ON) |
| Day 6 | - | - | Ctrl Medium 2 | PEMF (ON) |
| Day 7 | - | - | Ctrl Medium 2 | PEMF (ON) |
| Primer | Company | Sequence | Assay ID | Amplicon Length (bp) |
|---|---|---|---|---|
| GAPDH | ABI | * | Hs99999905_m1 | 122 |
| CD55 | ABI | * | Hs00167090_m1 | 62 |
| CD59 | ABI | * | Hs00174141_m1 | 70 |
| C4bpα | ABI | * | Hs00426339_m1 | 105 |
| C4bpβ | ABI | * | Hs01103672_m1 | 74 |
| CFH | ABI | * | Hs00962373_m1 | 72 |
| CFI | ABI | * | Hs00989715_m1 | 75 |
| TNFα | ABI | * | Hs00174128_m1 | 80 |
| IL-6 | ABI | * | Hs00174131_m1 | 95 |
| Specificity and Species | Company | Catalog Number | Stock Concentration | Used Dilution |
|---|---|---|---|---|
| Goat anti-human CD55 | R&D systems, Minneapolis, MN, USA | AF2009 | 200 µg/mL | 1:50 |
| Mouse anti-human CD59 | Bio-Rad, Bio-Rad, Feldkirchen, Germany | MCA1054 | 1 mg/mL | 1:50 |
| Mouse anti-human Ki67 | Chemicon International Inc., Temecula, CA, USA | MAB4190 | 1 mg/mL | 1:50 |
| Donkey anti-mouse- Alexa Fluor 488 | Invitrogen, Waltham, MA, USA | A21202 | 2 mg/mL | 1:200 |
| Donkey anti-goat- cyanine (Cy)3 | Dianova, Hamburg, Germany | 705-165-147 | 1.5 mg/mL | 1:200 |
| Donkey anti-mouse-Cy3 | Dianova, Hamburg, Germany | 715-166-150 | 1.5 mg/mL | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silawal, S.; Gesslein, M.; Willauschus, M.; Schulze-Tanzil, G. In Vitro Investigation of Pulsed Electromagnetic Field Stimulation (PEMF) with MAGCELL® ARTHRO on the Regulatory Expression of Soluble and Membrane-Bound Complement Factors and Inflammatory Cytokines in Immortalized Synovial Fibroblasts. J. Pers. Med. 2024, 14, 701. https://doi.org/10.3390/jpm14070701
Silawal S, Gesslein M, Willauschus M, Schulze-Tanzil G. In Vitro Investigation of Pulsed Electromagnetic Field Stimulation (PEMF) with MAGCELL® ARTHRO on the Regulatory Expression of Soluble and Membrane-Bound Complement Factors and Inflammatory Cytokines in Immortalized Synovial Fibroblasts. Journal of Personalized Medicine. 2024; 14(7):701. https://doi.org/10.3390/jpm14070701
Chicago/Turabian StyleSilawal, Sandeep, Markus Gesslein, Maximilian Willauschus, and Gundula Schulze-Tanzil. 2024. "In Vitro Investigation of Pulsed Electromagnetic Field Stimulation (PEMF) with MAGCELL® ARTHRO on the Regulatory Expression of Soluble and Membrane-Bound Complement Factors and Inflammatory Cytokines in Immortalized Synovial Fibroblasts" Journal of Personalized Medicine 14, no. 7: 701. https://doi.org/10.3390/jpm14070701





