Comparison of a Two (32/38 Weeks) versus One (36 Weeks) Ultrasound Protocol for the Detection of Decreased Fetal Growth and Adverse Perinatal Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Study Population
3.2. Comparison of P1 and P2 in Terms of Cost-Effectiveness
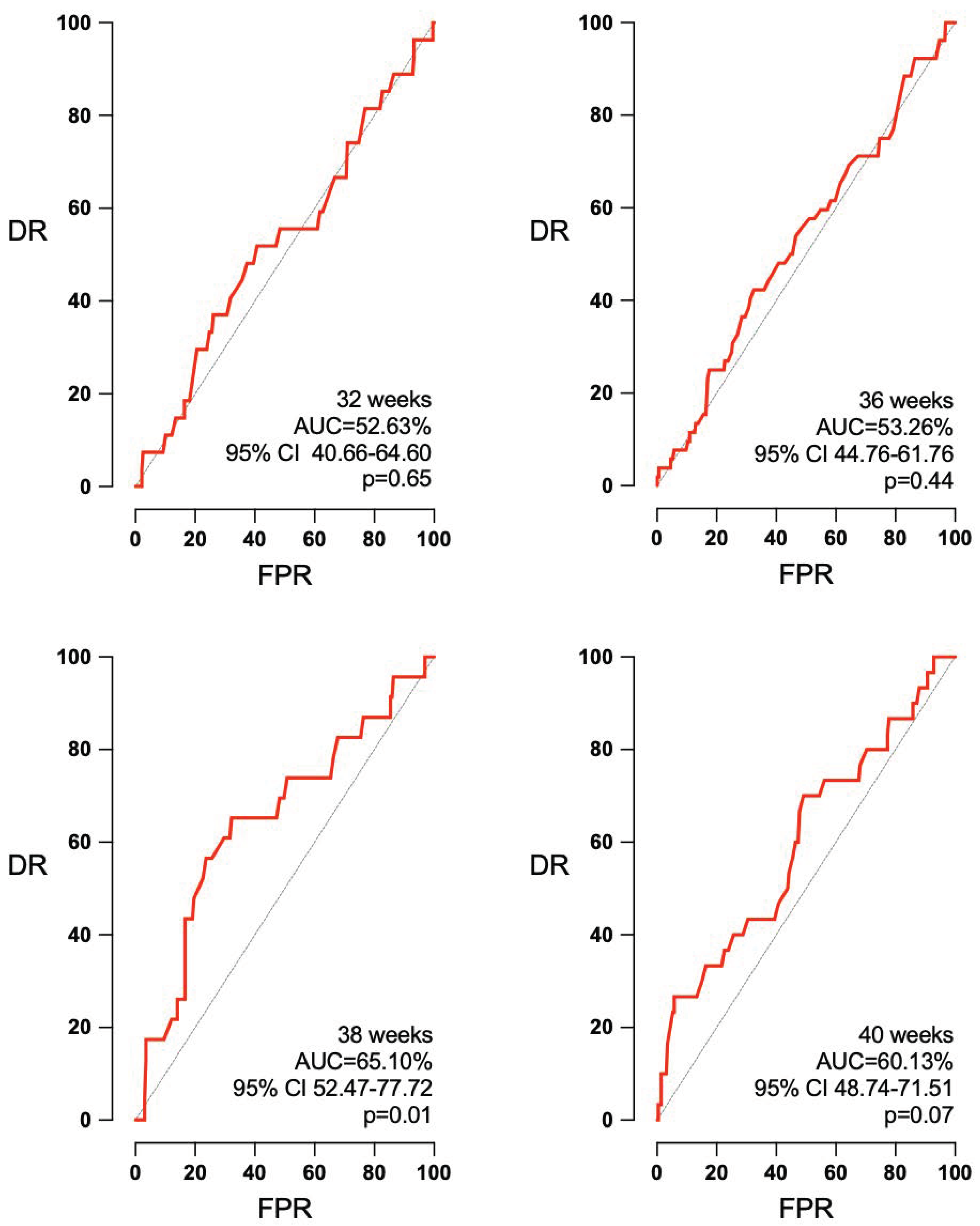
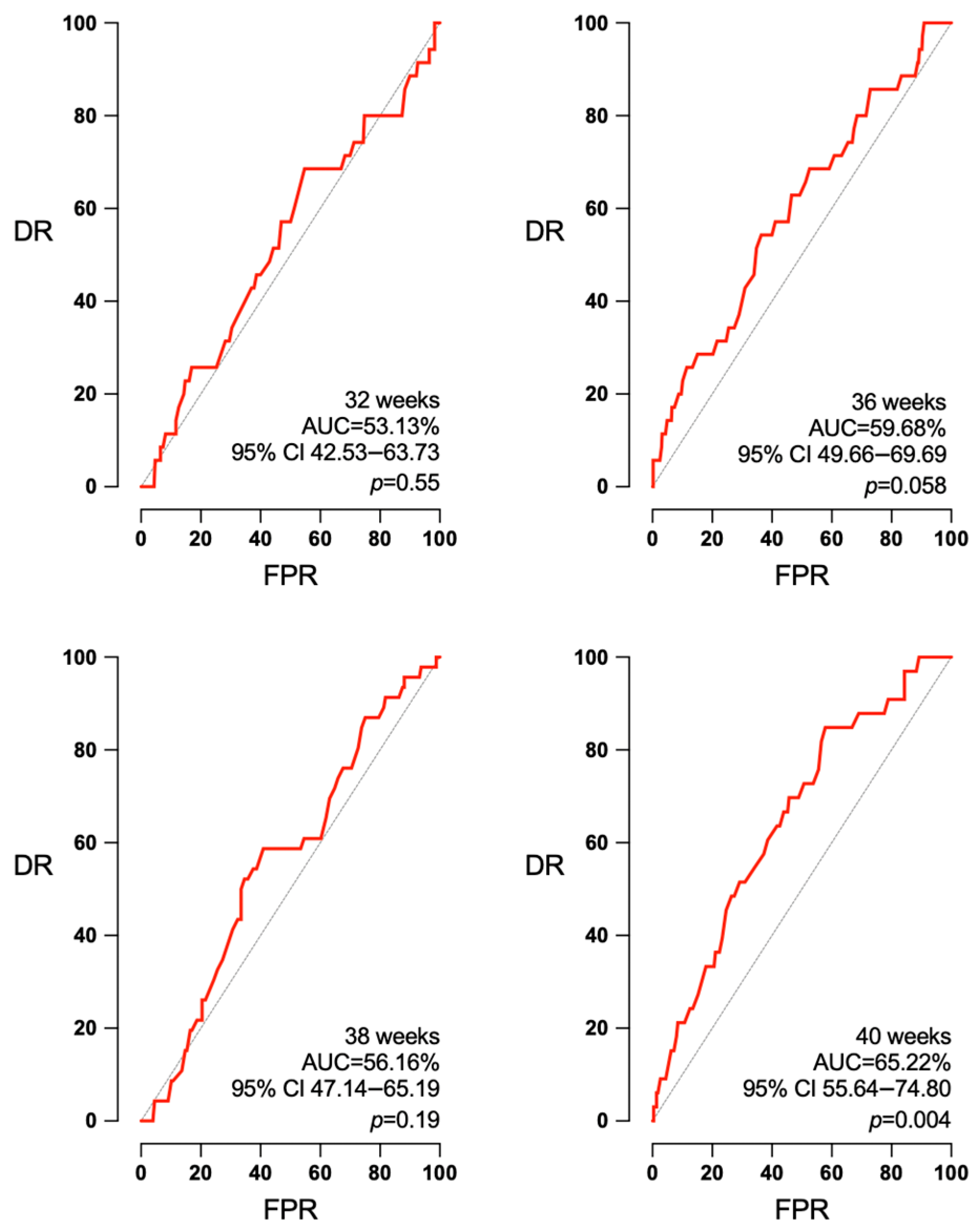
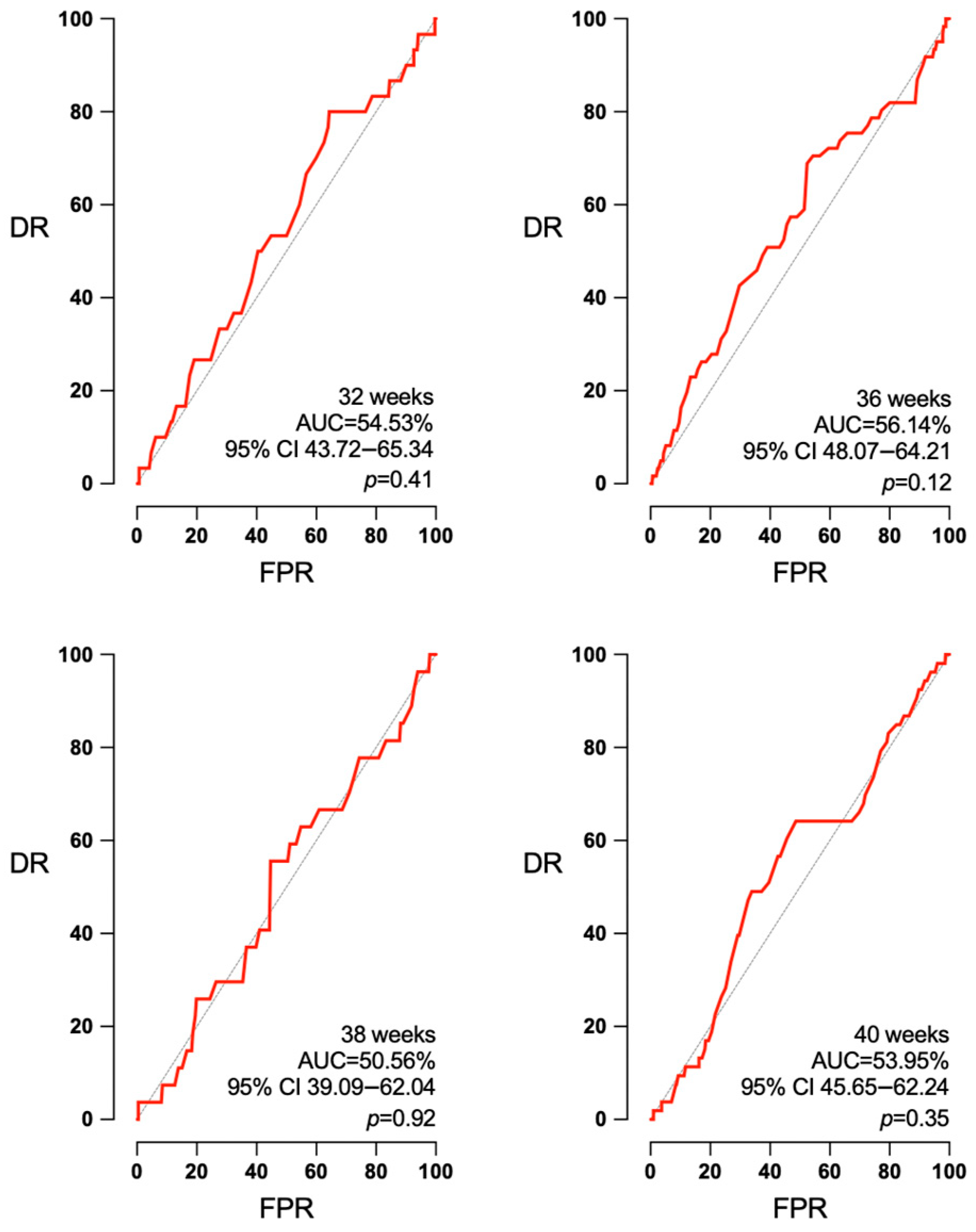
3.3. Accuracy of EFW and Doppler According to the Interval to Labor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baer, R.J.; Rogers, E.E.; Partridge, J.C.; Anderson, J.G.; Morris, M.; Kuppermann, M.; Franck, L.S.; Rand, L.; Jelliffe-Pawlowski, L.L. Population-based risks of mortality and preterm morbidity by gestational age and birth weight. J. Perinatol. 2016, 36, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Caradeux, J.; Crispi, F.; Eixarch, E.; Peguero, A.; Gratacos, E. Diagnosis and surveillance of late-onset fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S790–S802.e1. [Google Scholar] [CrossRef] [PubMed]
- Bricker, L.; Medley, N.; Pratt, J.J. Routine ultrasound in late pregnancy (after 24 weeks’ gestation). Cochrane Database Syst. Rev. 2015, 2015, CD001451. Available online: https://doi.wiley.com/10.1002/14651858.CD001451.pub4 (accessed on 1 June 2024). [CrossRef]
- Sokol Karadjole, V.; Agarwal, U.; Berberovic, E.; Poljak, B.; Alfirevic, Z. Does serial 3rd trimester ultrasound improve detection of small for gestational age babies: Comparison of screening policies in 2 European maternity units. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 45–49. [Google Scholar] [CrossRef]
- Callec, R.; Lamy, C.; Perdriolle-Galet, E.; Patte, C.; Heude, B.; Morel, O.; the EDEN Mother–Child Cohort Study Group. Impact on obstetric outcome of third-trimester screening for small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 2015, 46, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haroush, A.; Yogev, Y.; Hod, M.; Bar, J. Predictive value of a single early fetal weight estimate in normal pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 130, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sovio, U.; White, I.R.; Dacey, A.; Pasupathy, D.; Smith, G.C.S. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: A prospective cohort study. Lancet 2015, 386, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.G.; Molin, J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet. Gynecol. 2005, 25, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Roma, E.; Arnau, A.; Berdala, R.; Bergos, C.; Montesinos, J.; Figueras, F. Ultrasound screening for fetal growth restriction at 36 vs. 32 weeks’ gestation: A randomized trial (ROUTE). Ultrasound Obstet. Gynecol. 2015, 46, 391–397. [Google Scholar] [CrossRef]
- Ciobanu, A.; Khan, N.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Routine ultrasound at 32 vs. 36 weeks’ gestation: Prediction of small-for-gestational-age neonates. Ultrasound Obstet. Gynecol. 2019, 53, 761–768. [Google Scholar] [CrossRef]
- Caradeux, J.; Martinez-Portilla, R.J.; Peguero, A.; Sotiriadis, A.; Figueras, F. Diagnostic performance of third-trimester ultrasound for the prediction of late-onset fetal growth restriction: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 220, 449–459.e19. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Rice, M.M.; Grobman, W.A.M.; Bailit, J.; Reddy, U.M.; Wapner, R.J.; Varner, M.W.; Thorp, J.M.J.; Leveno, K.J.; Caritis, S.N.; et al. Neonatal Morbidity of Small- and Large-for-Gestational-Age Neonates Born at Term in Uncomplicated Pregnancies. Obstet. Gynecol. 2017, 130, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.Y.; Tan, M.Y.; Yerlikaya, G.; Syngelaki, A.; Nicolaides, K.H. Birth weight in live births and stillbirths. Ultrasound Obstet. Gynecol. 2016, 48, 602–606. [Google Scholar] [CrossRef]
- Rial-Crestelo, M.; Lubusky, M.; Parra-Cordero, M.; Krofta, L.; Kajdy, A.; Zohav, E.; Ferriols-Perez, E.; Cruz-Martinez, R.; Kacerovsky, M.; Scazzocchio, E.; et al. Term planned delivery based on fetal growth assessment with or without the cerebroplacental ratio in low-risk pregnancies (RATIO37): An international, multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 545–553. [Google Scholar] [CrossRef]
- Figueras, F.; Meler, E.; Iraola, A.; Eixarch, E.; Coll, O.; Figueras, J.; Francis, A.; Gratacos, E.; Gardosi, J. Customized birthweight standards for a Spanish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Morales-Roselló, J.; Khalil, A.; Morlando, M.; Hervás-Marín, D.; Perales-Marín, A. Doppler reference values of the fetal vertebral and middle cerebral arteries, at 19–41 weeks gestation. J. Matern.-Fetal Neonatal Med. 2015, 28, 338–343. [Google Scholar] [CrossRef]
- Acharya, G.; Wilsgaard, T.; Berntsen, G.K.R.; Maltau, J.M.; Kiserud, T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am. J. Obstet. Gynecol. 2005, 192, 937–944. [Google Scholar] [CrossRef]
- Ayres-de-Campos, D.; Spong, C.Y.; Chandraharan, E. FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int. J. Gynecol. Obstet. 2015, 131, 13–24. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.A.; Da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynecol. Obs. 2021, 152 (Suppl. S1), 3–57. [Google Scholar] [CrossRef]
- Blair, E.M.; Nelson, K.B. Fetal growth restriction and risk of cerebral palsy in singletons born after at least 35 weeks’ gestation. Am. J. Obstet. Gynecol. 2015, 212, 520.e1–520.e7. [Google Scholar] [CrossRef]
- Mendez-Figueroa, H.; Truong, V.T.T.; Pedroza, C.; Khan, A.M.; Chauhan, S.P. Small-for-gestational-age infants among uncomplicated pregnancies at term: A secondary analysis of 9 Maternal-Fetal Medicine Units Network studies. Am. J. Obstet. Gynecol. 2016, 215, 628.e1–628.e7. [Google Scholar] [CrossRef]
- Hertting, E.; Herling, L.; Lindqvist, P.G.; Wiberg-Itzel, E. Importance of antenatal identification of small for gestational age fetuses on perinatal and childhood outcomes: A register-based cohort study. Acta Obs. Gynecol. Scand 2024, 103, 42–50. [Google Scholar] [CrossRef]
- Trudell, A.S.; Cahill, A.G.; Tuuli, M.G.; Macones, G.A.; Odibo, A.O. Risk of stillbirth after 37 weeks in pregnancies complicated by small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 2013, 208, 376.e1–376.e7. [Google Scholar] [CrossRef]
- Souka, A.P.; Papastefanou, I.; Pilalis, A.; Michalitsi, V.; Panagopoulos, P.; Kassanos, D. Performance of the ultrasound examination in the early and late third trimester for the prediction of birth weight deviations: Prediction of birth weight deviations by ultrasound. Prenat. Diagn. 2013, 33, 915–920. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Villar, J.; Kennedy, S.H.; Papageorghiou, A.T. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 52, 430–441. [Google Scholar] [CrossRef]
- Vollgraff Heidweiller-Schreurs, C.A.; De Boer, M.A.; Heymans, M.W.; Schoonmade, L.J.; Bossuyt, P.M.M.; Mol, B.W.J.; De Groot, C.J.M.; Bax, C.J. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 313–322. [Google Scholar] [CrossRef]
- Morales-Roselló, J.; Loscalzo, G.; Gallego, A.; Jakaitė, V.; Perales-Marín, A. Which is the best ultrasound parameter for the prediction of adverse perinatal outcome within 1 day of delivery? J. Matern.-Fetal Neonatal Med. 2022, 35, 8571–8579. [Google Scholar] [CrossRef]
- Stampalija, T.; Thornton, J.; Marlow, N.; Napolitano, R.; Bhide, A.; Pickles, T.; Bilardo, C.M.; Gordijn, S.J.; Gyselaers, W.; Valensise, H.; et al. Fetal cerebral Doppler changes and outcome in late preterm fetal growth restriction: Prospective cohort study. Ultrasound Obstet. Gynecol. 2020, 56, 173–181. [Google Scholar] [CrossRef]
- Moreta, D.; Vo, S.; Eslick, G.D.; Benzie, R. Re-evaluating the role of cerebroplacental ratio in predicting adverse perinatal outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 242, 17–28. [Google Scholar] [CrossRef]



| Parameter (Continuous Data) | Mean (SD) | Median (Q1–Q3) |
|---|---|---|
| Maternal age (years) | 31.61 (5.20) | 32 (28–36) |
| Gravidity | 2.04 (1.18) | 2 (1–3) |
| Parity | 0.67 (0.82) | 1 (0–1) |
| Maternal pre-pregnancy weight (Kg) | 63.68 (11.5) | 62 (55–70) |
| Maternal height (cm) | 163 (6.18) | 163 (159–167) |
| Maternal Body Mass Index | 23.94 (3.95) | 23.15 (21.08–25.89) |
| Gestational age at delivery (weeks) | 39.76 (1.16) | 39.86 (39.14–40.71) |
| Birth weight (g) | 3309 (427.5) | 3320 (3030–3600) |
| Birth weight centile | 47.6 (29.8) | 46 (21–73) |
| Parameter (categorical data) | n (%) | |
| Nulliparity | 498 (49.26) | |
| Smoking | 128 (12.66) | |
| Male sex | 516 (51.04) | |
| Caucasian ethnicity | 964 (95.35) | |
| Type of labor onset | ||
| Spontaneous onset of labor | 579 (57.27) | |
| Induction of labor | 373 (36.89) | |
| Elective cesarean section | 59 (5.83) | |
| Via of delivery | ||
| Spontaneous vaginal delivery | 615 (60.83) | |
| Assisted vaginal delivery | 198 (19.58) | |
| Cesarean section (elective) | 69 (6.83) | |
| Cesarean section (failure to progress) | 72 (7.12) | |
| Cesarean section (abnormal CTG) | 57 (5.64) | |
| Apgar < 7 at 5 min | 3 (0.3) | |
| Arterial pH < 7.10 | 27 (2.67) | |
| Neonate destiny | ||
| Maternal ward | 961 (94.06) | |
| Neonates ward | 49 (4.85) | |
| Pediatric Intensive care unit | 1 (0.1) | |
| Adverse perinatal outcome | 113 (11.17) | |
| Abnormal CTG | 57 (5.64) | |
| Small for gestational age fetuses (<10th centile) | 119 (11.77) |
| P2 32/38 Week Protocol (n = 528) | P1 36 Week Protocol (n = 483) | p-Value | |
|---|---|---|---|
| Mean (SD); Median (Q1–Q3) | Mean (SD); Median (Q1–Q3) | ||
| Maternal age (years) | 31.80 (5.10); 32 (29–35.75) | 31.41 (5.3); 32 (28–36) | 0.25 |
| Gravidity | 1.99 (1.17); 2 (1–2) | 2.09 (1.19); 2 (1–3) | 0.08 |
| Parity | 0.66 (0.79); 1 (0–1) | 0.68 (0.86); 1 (0–1) | 0.96 |
| Maternal pre-pregnancy weight (Kg) | 63.80 (11.21); 62 (56–70) | 63.55 (11.88); 61 (55–70) | 0.47 |
| Maternal height (cm) | 163.1 (6.28); 163 (159–181) | 162.9 (6.07); 163 (159–167) | 0.49 |
| Maternal Body Mass Index | 23.96 (3.92); 23.07 (21.22–26.16) | 23.9 (3.98); 23.34 (20.9–25.8) | 0.75 |
| Gestational age at delivery (weeks) | 39.73 (1.23); 39.86 (39.14–40.71) | 39.8 (1.07); 40 (39.14–40.71) | 0.65 |
| Birth weight (g) | 3284 (448.4); 3300 (3000–3580) | 3336 (402.1); 3350 (3075–3640) | 0.06 |
| Birth weight centile | 46.15 (30.39); 45 (17–72) | 49.18 (29.09); 47 (24–75) | 0.09 |
| n (%) | n (%) | ||
| Nulliparity | 257 (48.67) | 241 (49.9) | 0.71 |
| Smoking | 72 (13.64) | 56 (11.59) | 0.34 |
| Male sex | 258 (48.86) | 258 (53.42) | 0.16 |
| Caucasian ethnicity | 514 (97.35) | 450 (93.17) | <0.0024 |
| Type of labour onset | |||
| Spontaneous onset of labor | 319 (60.42) | 260 (53.83) | 0.03 |
| Induction of labor | 173 (32.77) | 200 (41.41) | <0.005 |
| Elective cesarean section | 36 (6.82) | 23 (4.76) | 0.18 |
| Via of delivery | |||
| Spontaneous vaginal delivery | 316 (59.85) | 299 (61.9) | 0.52 |
| Assisted vaginal delivery | 106 (20.08) | 92 (19.05) | 0.69 |
| Cesarean section (elective) | 41 (7.77) | 28 (5.8) | 0.26 |
| Cesarean section (failure to progress) | 39 (7.39) | 33 (6.83) | 0.81 |
| Cesarean section (abnormal CTG) | 26 (4.92) | 31 (6.42) | 0.34 |
| Apgar < 7 at 5 min | 1 (0.19) | 2 (0.41) | 0.61 |
| Arterial pH < 7.10 | 8 (1.52) | 19 (3.93) | <0.019 |
| Neonate destiny | |||
| Maternal ward | 500 (94.70) | 461 (95.44) | 0.66 |
| Neonates ward | 27 (5.11) | 22 (4.55) | 0.80 |
| Pediatric Intensive care unit | 1 (0.19) | 0 | >0.99 |
| Adverse perinatal outcome | 51 (9.66) | 62 (12.84) | 0.11 |
| Abnormal CTG | 26 (4.92) | 31 (6.42) | 0.34 |
| Small for gestational age fetuses (<10th centile) | 71 (13.45) | 48 (9.94) | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto-Tous, M.; Novillo-Del Álamo, B.; Martínez-Varea, A.; Satorres-Pérez, E.; Morales-Roselló, J. Comparison of a Two (32/38 Weeks) versus One (36 Weeks) Ultrasound Protocol for the Detection of Decreased Fetal Growth and Adverse Perinatal Outcome. J. Pers. Med. 2024, 14, 709. https://doi.org/10.3390/jpm14070709
Nieto-Tous M, Novillo-Del Álamo B, Martínez-Varea A, Satorres-Pérez E, Morales-Roselló J. Comparison of a Two (32/38 Weeks) versus One (36 Weeks) Ultrasound Protocol for the Detection of Decreased Fetal Growth and Adverse Perinatal Outcome. Journal of Personalized Medicine. 2024; 14(7):709. https://doi.org/10.3390/jpm14070709
Chicago/Turabian StyleNieto-Tous, Mar, Blanca Novillo-Del Álamo, Alicia Martínez-Varea, Elena Satorres-Pérez, and José Morales-Roselló. 2024. "Comparison of a Two (32/38 Weeks) versus One (36 Weeks) Ultrasound Protocol for the Detection of Decreased Fetal Growth and Adverse Perinatal Outcome" Journal of Personalized Medicine 14, no. 7: 709. https://doi.org/10.3390/jpm14070709
APA StyleNieto-Tous, M., Novillo-Del Álamo, B., Martínez-Varea, A., Satorres-Pérez, E., & Morales-Roselló, J. (2024). Comparison of a Two (32/38 Weeks) versus One (36 Weeks) Ultrasound Protocol for the Detection of Decreased Fetal Growth and Adverse Perinatal Outcome. Journal of Personalized Medicine, 14(7), 709. https://doi.org/10.3390/jpm14070709






