Coffee Consumption and CYP1A2 Polymorphism Involvement in Type 2 Diabetes in a Romanian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. SNP Selection and Genotyping
2.3. Data Analysis
2.3.1. Binomial Logistic Regression
2.3.2. OR (Odds Ratio)
3. Results
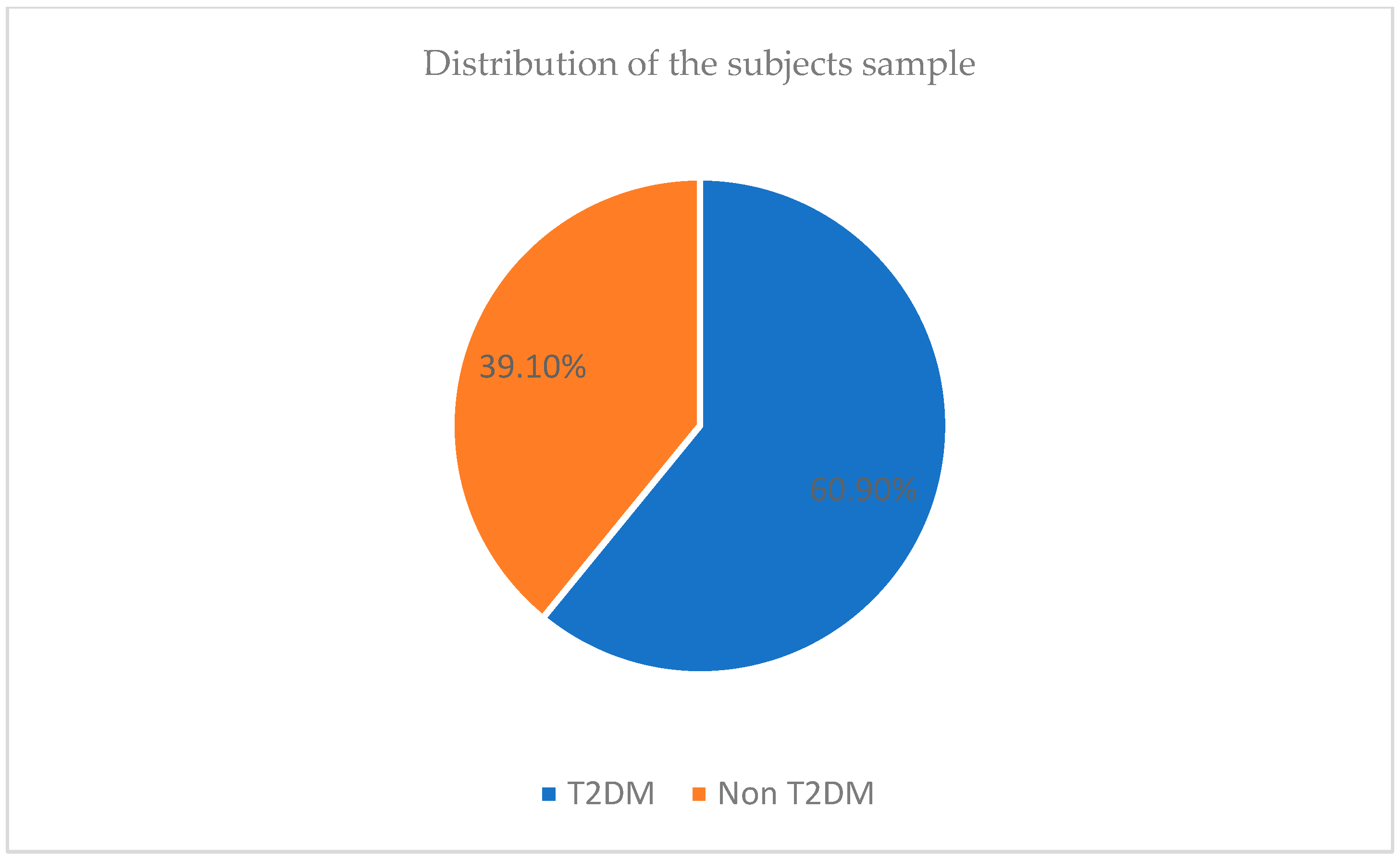
3.1. Description of the Sample Size
3.1.1. Logistic Regression
3.1.2. Logistic Regression Regarding the Genotype and Coffee Relation
3.1.3. Genotype and the Presence of Diabetes
3.1.4. Diabetes and Coffee Intake
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlström, M.; Larsson, S.C. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018, 76, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and Decaffeinated Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review and a Dose-Response Meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P. Coffee consumption and risk of type 2 diabetes. Diabetologia 2015, 58, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Pan, A.; Malik, V.S.; Manson, J.E.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 155–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Dam, R.M.; Willett, W.C.; Manson, J.E.; Hu, F.B. Coffee, caffeine, and risk of type 2 diabetes: A prospective cohort study in younger and middle-aged U.S. women. Diabetes Care 2006, 29, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, P. Association of CYP19A1 and CYP1A2 genetic polymorphisms with type 2 diabetes mellitus risk in the Chinese Han population. Lipids Health Dis. 2020, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513253/ (accessed on 23 June 2023).
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global etiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Nikitin, A.G.; Potapov, V.Y.; Brovkina, O.I.; Koksharova, E.O.; Khodyrev, D.S.; Philippov, Y.I.; Michurova, M.S.; Shamkhalova, M.S.; Vikulova, O.K.; Smetanina, S.A.; et al. Association of polymorphic markers of genes FTO, KCNJ11, CDKAL1, SLC30A8, and CDKN2B with type 2 diabetes mellitus in the Russian population. Peer J. 2017, 5, e3414. [Google Scholar] [CrossRef]
- Bao, X.Y.; Peng, B.; Yang, M.S. Replication study of novel risk variants in six genes with type 2 diabetes and related quantitative traits in the Han Chinese lean individuals. Mol. Biol. Rep. 2012, 39, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.V.H.; West, S.B. Multiplicity of mammalian cytochrome P-450. Pharnw. Col. Rev. 1980, 31, 277–295. [Google Scholar]
- Guengerich, F.P. Reactions and significance of cytochrome P-450 enzymes. J. Biol. Chem. 1991, 266, 10019–10022. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Gonzalez, F.J.; Kalow, W.; Tang, B.K. Biotransformation of caffeine, paraxanthine, theobromine, and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenetics 1992, 2, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Adehin, A.; Bolaji, O.O. Polymorphisms of CYP1A2 and CYP2A6 activity: Phenotypes and the effect of age and sex in a Nigerian population. Drug Metab. Pers. Ther. 2015, 30, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.Z.; Zhang, Z.Y.; Wang, X.; Yin, S.J.; Lou, Y.Q.; Zhang, G.L. Functional allele and genotype frequencies of CYP1A2, CYP2B6, and iNOS among mainland Chinese Tibetan, Mongolian, Uygur, and Han populations. J. Clin. Pharm. Ther. 2016, 41, 84–91. [Google Scholar] [CrossRef]
- Faber, M.S.; Jetter, A.; Fuhr, U. Assessment of CYP1A2 activity in clinical practice: Why, how, and when? Basic Clin. Pharmacol. Toxicol. 2005, 97, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Han, X.M.; Ou-Yang, D.S.; Lu, P.X.; Jiang, C.H.; Shu, Y.; Chen, X.P.; Tan, Z.R.; Zhou, H.H. Plasma caffeine metabolite ratio (17X/137X) in vivo associated with G-2964A and C734 polymorphisms of human CYP1A2. Pharmacogenetics 2001, 11, 429–435. [Google Scholar] [CrossRef]
- Urry, E.; Jetter, A.; Holst, S.C.; Berger, W.; Spinas, G.A.; Langhans, W.; Landolt, H.P. Relationships among type 2 diabetes, sleepiness, and habitual caffeine intake: A case-control field study. J. Psychopharmacol. 2016, in press. [Google Scholar] [CrossRef]
- Abalo, R. Coffee and Caffeine Consumption for Human Health. Nutrients 2021, 13, 2918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alshawi, A.H. The Effect of Coffee Consumption on Blood Glucose: A Review. Pak. J. Nutr. 2020, 19, 420–429. [Google Scholar] [CrossRef]
- Alperet, D.J.; Rebello, S.A.; Khoo, E.Y.; Tay, Z.; Seah, S.S.; Tai, B.C.; Tai, E.S.; Emady-Azar, S.; Chou, C.J.; Darimont, C.; et al. The effect of coffee consumption on insulin sensitivity and other biological risk factors for type 2 diabetes: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2020, 111, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Martin, S.; Kempf, K. Coffee and Lower Risk of Type 2 Diabetes: Arguments for a Causal Relationship. Nutrients 2021, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yusni, Y.; Yusuf, H. The acute effects of coffee consumption on blood glucose and its relationship with serum cortisol and insulin in females. Pharmacia 2022, 69, 903–910. [Google Scholar] [CrossRef]
- Dam, R.M.; van Hu, F.B.; Willett, W.C. Coffee, caffeine, and health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Woolf, B.; Gill, D. Appraisal of the causal effect of plasma caffeine on adiposity, type 2 diabetes, and cardiovascular disease: Two sample mendelian randomisation study. BMJ Med. 2023, 2, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; El Ghoch, M.; Castellucci, B.; Chapela, S.P.; Carignano, M.L.A.; Laudisio, D.; Savastano, S.; Colao, A.; et al. Coffee consumption, health benefits and side effects: A narrative review and update for dietitians and nutritionists. Crit. Rev. Food Sci. Nutr. 2023, 63, 1238–1261. [Google Scholar] [CrossRef] [PubMed]
- Nugrahini, A.D.; Ishida, M.; Nakagawa, T.; Nishi, K.; Sugahara, T. Trigonelline: An alkaloid with anti-degranulation properties. Mol. Immunol. 2020, 118, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://insp.gov.ro/download/cnepss/stare-de-sanatate/boli_netransmisibile/diabet/Analiza-de-situatie-Diabet-2018 (accessed on 18 January 2023).
- World Health Organization. Available online: https://www.who.int/publications/i/item/classification-of-diabetes-mellitus (accessed on 24 January 2023).
- Conway, J.M.; Ingwersen, L.A.; Moshfegh, A.J. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: An observational validation study. J. Am. Diet. Assoc. 2004, 104, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Morgovan, C.; Cosma, S.A.; Valeanu, M.; Juncan, A.M.; Rus, L.L.; Gligor, F.G.; Butuca, A.; Tit, D.M.; Bungau, S.; Ghibu, S. An Exploratory Research of 18 Years on the Economic Burden of Diabetes for the Romanian National Health Insurance System. Int. J. Environ. Res. Public Health 2020, 17, 4456. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. The Global Picture in IDF Diabetes Atlas Eight Edition. Diabetes Res. Clin. Pract. 2017, 40, 65. Available online: https://diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf (accessed on 12 February 2023).
- Jiang, X.; Zhang, D.; Jiang, W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: A meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.M.; Clifford, M.N.; Penson, S.; Williams, P.; Robertson, M.D. Postprandial glycaemic and lipaemic responses to chronic coffee consumption may be modulated by CYP1A2 polymorphisms. Br. J. Nutr. 2018, 119, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Gkouskou, K.G.; Georgiopoulos, G.; Vlastos, I.; Lazou, E.; Chaniotis, D.; Papaioannou, T.G.; Mantzoros, C.S.; Sanoudou, D.; Eliopoulos, A.G. CYP1A2 polymorphisms modify the association of habitual coffee consumption with appetite, macronutrient intake, and body mass index: Results from an observational cohort and a cross-over randomized study. Int. J. Obes. 2022, 46, 162–168. [Google Scholar] [CrossRef]
- Greenberg, J.; Axen, K.; Schnoll, R.; Boozer, C.N. Coffee, tea, and diabetes: The role of weight loss and caffeine. Int. J. Obes. 2005, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Hammar, N.; Grill, V.; Kaprio, J. Coffee consumption and risk of type 2 diabetes in Finnish twins. Int. J. Epidemiol. 2004, 33, 616–617. [Google Scholar] [CrossRef]
- Hamer, M.; Witte, D.; Mosdøl, A.; Marmot, M.; Brunner, E. Prospective study of coffee and tea consumption about risk of type 2 diabetes mellitus among men and women: The Whitehall II study. Br. J. Nutr. 2008, 100, 1046–1053. [Google Scholar] [CrossRef]
- Kohno, M. Cytochrome P450 1A2 Polymorphisms, Coffee Consumption and Impaired Glucose Metabolism in Japanese Men. Endocrinol. Metab. Syndr. 2013, 2, 3. [Google Scholar] [CrossRef]
- Platt, D.E.; Ghassibe-Sabbagh, M.; Salameh, P.; Salloum, A.K.; Haber, M.; Mouzaya, F.; Gauguier, D.; Al-Sarraj, Y.; El-Shanti, H.; Zalloua, P.A.; et al. A CYP1A2 Variant modulates Caffeine’s Impact on Metabolic Syndrome Components. Ann. Nutr. Metab. 2016, 68, 1–11. [Google Scholar] [CrossRef]
- Çelik, F.N.; Deniz, E.; Pektaş, E.; Özcan, Ö.Ö.; Polat, T.; Doğan, C.S.; Karahan, M. Determination of Allele Distribution of CYP1A2 Gene rs762551 Polymorphism on Caffeine Metabolism in Healthy Individuals. Asian J. Biotechnol. Genet. Eng. 2021, 4, 1–8. [Google Scholar]
- Denden, S.; Bouden, B.; Khelil Haj Ben Chibani, J.; Hamdaoui, M.H. Gender and ethnicity modify the association between the CYP1A2 rs762551 polymorphism and habitual coffee intake: Evidence from a meta-analysis. Genet. Mol. Res. 2016, 15, gmr7487. [Google Scholar] [CrossRef] [PubMed]
| Sample | p | |||
|---|---|---|---|---|
| Parameters | Whole (n = 358) Sample | Diabetes (n = 218) | Control (n = 140) | |
| Gender—n (%) | ||||
| Males | 179 (50.0%) | 99 (45.4%) | 80 (57.1%) | 0.030 |
| Females | 179 (50.0%) | 119 (54.6%) | 60 (42.9%) | |
| Age—mean (±SD) | 53.19 (±13.15) | 54.65 (±11.00) | 50.92 (±13.15) | 0.004 |
| Coffee (medium amount)—mean (±SD) | 129.18 (±117.04) | 134.70 (±130.96) | 120.58 (±91.01) | 0.266 |
| Coffee cups—n (%) | ||||
| - 1 cup | 106 (29.6%) | 68 (31.2%) | 38 (27.1%) | 0.161 |
| - 1–3 cups | 199 (55.6%) | 113 (51.8%) | 86 (61.4%) | |
| - >3 cups | 53 (14.8%) | 37 (17.0%) | 16 (11.4%) | |
| Caffeine—mean (±SD) | 99.52 (±115.37) | 100.16 (±110.97) | 98.52 (±122.30) | 0.896 |
| Genotype CYP1A2 rs762551—n (%) | ||||
| - AA | 65 (18.2%) | 43 (19.7%) | 22 (15.7%) | 0.583 |
| - AC | 163 (45.5%) | 99 (45.4%) | 64 (45.7%) | |
| - CC | 130 (36.3%) | 76 (34.9%) | 54 (38.6%) | |
| Glucose—mean (±SD) | 159.49 (±67.95) | 170.74 (±63.50) | 141.97 (±71.12) | <0.001 |
| Obesity—n (%) | ||||
| - no | 74 (20.7%) | 28 (12.8%) | 46 (32.9%) | <0.001 |
| - yes | 284 (79.3%) | 190 (87.2%) | 94 (67.1%) | |
| Cholesterol—mean (±SD) | 313.15 (±186.11) | 305.58 (±169.92) | 325.12 (±209.24) | 0.335 |
| Genotype | p | |||
|---|---|---|---|---|
| Parameters | AA (n = 65) | AC (n = 163) | CC (n = 130) | |
| Gender—n (%) | ||||
| Male | 33 (50.8%) | 83 (50.9%) | 63 (48.5%) | 0.908 |
| Female | 32 (49.2%) | 80 (49.1%) | 67 (51.5%) | |
| Age—mean (±SD) | 50.18 (±13.86) | 53.61 (±11.55) | 54.16 (±11.42) | 0.077 |
| Coffee (medium amount)—mean (±SD) | 151.33 (±142.75) | 132.91 (±117.18) | 113.42 (±100.19) | 0.088 |
| Caffeine—mean (±SD) | 106.06 (±119.56) | 102.33 (±127.12) | 92.62 (±96.60) | 0.684 |
| Glucose—mean (±SD) | 167.08 (±92.76) | 158.39 (±66.28) | 157.07 (±54.38) | 0.602 |
| Cholesterol—mean (±SD) | 348.49 (±229.90) | 290.90 (±167.27) | 323.74 (±182.39) | 0.080 |
| Type 2 Diabetes Subjects | Genotype | p | ||
|---|---|---|---|---|
| Parameters | AA (n = 43) | AC (n = 99) | CC (n = 76) | |
| Gender—n (%) | ||||
| Male | 19 (44.2%) | 48 (48.5%) | 32 (42.1%) | 0.691 |
| Female | 24 (55.8%) | 51 (51.5%) | 44 (57.9%) | |
| Age—mean (±SD) | 52.51 (±12.97) | 55.52 (±10.06) | 54.72 (±10.96) | 0.328 |
| Coffee (medium amount)—mean (±SD) | 155.00 (±164.26) | 143.24 (±127.18) | 112.08 (111.92) | 0.156 |
| Caffeine—mean (±SD) | 110.70 (±137.15) | 99.15 (±114.51) | 95.43 (±88.46) | 0.768 |
| Glucose—mean (±SD) | 169.02 (±67.95) | 174.10 (±69.42) | 167.33 (±63.50) | 0.770 |
| Cholesterol—mean (±SD) | 333.06 (±196.99) | 283.13 (±161.04) | 319.26 (±163.19) | 0.188 |
| Obesity—n (%) | ||||
| No | 7 (16.3%) | 13 (13.1%) | 8 (10.5%) | 0.662 |
| Yes | 36 (83.7%) | 86 (89.5%) | 68 (89.5%) | |
| Groups | Sample Size | Sum | Mean | Variance | ||
|---|---|---|---|---|---|---|
| Glucose (mg/dL) | 218 | 37,221 | 170.738532 | 4032.44284 | ||
| Coffee amount (mL) | 218 | 29,365.35 | 134.70344 | 17,151.6284 | ||
| Variance analysis | ||||||
| Sources of variations | Sum of squares | Degree of liberty | Mean of squares | F | p-value | Critical value of F |
| Between groups | 141,539.534 | 1 | 141,539.534 | 13.3628265 | 0.00028812 | 3.8629743 |
| Within groups | 4,596,943.45 | 434 | 10,592.0356 | |||
| Total | 4,738,482.99 | 435 | ||||
| Control Sample | Genotype | p | ||
|---|---|---|---|---|
| Parameters | AA (n = 22) | AC (n = 64) | CC (n = 54) | |
| Gender—n (%) | ||||
| Male | 14 (63.6%) | 35 (54.7%) | 31 (57.4%) | 0.764 |
| Female | 8 (36.4%) | 29 (45.3%) | 23 (42.6%) | |
| Age—mean (±SD) | 45.64 (±14.70) | 50.67 (±13.07) | 53.37 (±12.11) | 0.065 |
| Coffee (medium amount)—mean (±SD) | 144.17 (±89.78) | 116.93 (±98.59) | 115.30 (±81.85) | 0.417 |
| Caffeine—mean (±SD) | 97.00 (±76.23) | 107.21 (±145.15) | 88.66 (±107.83) | 0.718 |
| Glucose—mean (±SD) | 163.27 (±130.25) | 134.09 (±53.04) | 142.63 (±54.18) | 0.253 |
| Cholesterol—mean (±SD) | 380.08 (±288.85) | 302.92 (±177.08) | 330.15 (±208.35) | 0.335 |
| Obesity—n (%) | ||||
| No | 8 (36.4%) | 20 (31.3%) | 18 (33.3%) | 0.903 |
| Yes | 14 (63.6%) | 44 (68.8%) | 36 (66.7%) | |
| Groups | Sample Size | Sum | Mean | Variance | ||
|---|---|---|---|---|---|---|
| Glucose (mg/dL) | 140 | 19,876 | 141.971429 | 5058.3445 | ||
| Mean amount (mL) | 140 | 16,882.1 | 120.586429 | 8283.36179 | ||
| Variance analysis | ||||||
| Sources of variations | Sum of squares | Degree of liberty | Mean of squares | F | p-value | Critical value of F |
| Between groups | 32,012.2758 | 1 | 32,012.2758 | 4.79882783 | 0.02930949 | 3.87512601 |
| Within groups | 1,854,497.17 | 278 | 6670.85315 | |||
| Total | 1,886,509.45 | 279 | ||||
| Genotype | |||
|---|---|---|---|
| Parameters | AA + AC (n = 228) | AA + CC (n = 195) | AC + CC (n = 293) |
| Coffee (medium amount)—mean (±SD) | 138.16 (±124.96) | 126.06 (±117.12) | 124.26 (±110.21) |
| Caffeine—mean (±SD) | 103.40 (±124.75) | 97.15 (±104.77) | 98.05 (±114.57) |
| Glucose—mean (±SD) | 160.87 (±74.68) | 160.41 (±69.48) | 157.81 (±61.19) |
| Cholesterol—mean (±SD) | 307.14 (±188.32) | 331.94 (±199.14) | 305.41 (±174.57) |
| Diabetes—n (%) | |||
| No | 86 (37.7%) | 76 (39.0%) | 118 (40.3%) |
| Yes | 142 (62.3%) | 119 (61.0%) | 175 (59.7%) |
| Model Fitting Information | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Model Fitting Criteria | Likelihood Ratio Tests | |||||||
| −2 Log Likelihood | Chi-Square | df | Sig. | ||||||
| Intercept Only | 23.960 | ||||||||
| Final | 19.621 | 4.339 | 2 | 0.114 | |||||
| Pseudo R-Square | |||||||||
| Cox and Snell | 0.012 | ||||||||
| Nagelkerke | 0.014 | ||||||||
| McFadden | 0.006 | ||||||||
| Likelihood Ratio Tests | |||||||||
| Effect | Model Fitting Criteria | Likelihood Ratio Tests | |||||||
| −2 Log Likelihood of Reduced Model | Chi-Square | df | Sig. | ||||||
| Intercept | 19.621 a | 0.000 | 0 | . | |||||
| Genotype | 23.960 | 4.339 | 2 | 0.114 | |||||
| The chi-square statistic is the difference in −2 log-likelihoods between the final and reduced models. The reduced model is formed by omitting an effect from the final model. The null hypothesis is that all parameters of that effect are 0. | |||||||||
| Parameter Estimates | |||||||||
| Coffee cups | B | Std. Error | Wald | df | Sig. | Exp(B) | 95% Confidence Interval for Exp(B) | ||
| Lower Bound | Upper Bound | ||||||||
| 1–3 cups | Intercept | 0.543 | 0.130 | 17,314 | 1 | 0.000 | |||
| (rs = 0) | 0.556 | 0.346 | 2.586 | 1 | 0.108 | 1.744 | 0.885 | 3.434 | |
| (rs = 1) | 0 b | . | . | 0 | . | . | . | . | |
| >3 cups | Intercept | −0.844 | 0.189 | 19,911 | 1 | 0.000 | |||
| (rs = 0) | 0.844 | 0.435 | 3.755 | 1 | 0.053 | 2.325 | 0.990 | 5.458 | |
| (rs = 1) | 0 b | . | . | 0 | . | . | . | . | |
| The reference category is: 1 cup equals 70 mL. | |||||||||
| Variables Not in the Equation | |||||||
|---|---|---|---|---|---|---|---|
| Score | df | Sig. | |||||
| Step 0 | Variables | Genotype | 0.923 | 1 | 0.337 | ||
| Overall Statistics | 0.923 | 1 | 0.337 | ||||
| Omnibus Tests of Model Coefficients | |||||||
| Chi-square | df | Sig. | |||||
| Step 1 | Step | 0.936 | 1 | 0.333 | |||
| Block | 0.936 | 1 | 0.333 | ||||
| Model | 0.936 | 1 | 0.333 | ||||
| Model Summary | |||||||
| Step 1 | −2 Log likelihood | Cox and Snell R Square | Nagelkerke R Square | ||||
| 478,226 a | 0.003 | 0.004 | |||||
| Variables in the Equation | |||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | ||
| Step 1 b | Genotype | −0.276 | 0.288 | 0.919 | 1 | 0.338 | 0.759 |
| Constant | 0.670 | 0.262 | 6.536 | 1 | 0.011 | 1.955 | |
| Variables Not in the Equation | |||||||
|---|---|---|---|---|---|---|---|
| Score | df | Sig. | |||||
| Step 0 | Variables | Coffee cups | 3.654 | 2 | 0.161 | ||
| Coffee cups (1) | 0.671 | 1 | 0.413 | ||||
| Coffee cups (2) | 3.178 | 1 | 0.075 | ||||
| Overall Statistics | 3.654 | 2 | 0.161 | ||||
| Omnibus Tests of Model Coefficients | |||||||
| Chi-square | df | Sig. | |||||
| Step 1 | Step | 3704 | 2 | 0.157 | |||
| Block | 3704 | 2 | 0.157 | ||||
| Model | 3704 | 2 | 0.157 | ||||
| Model Summary | |||||||
| Step | −2 Log likelihood | Cox and Snell R Square | Nagelkerke R Square | ||||
| 1 | 475,458 a | 0.010 | 0.014 | ||||
| Variables in the Equation | |||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | ||
| Step 1 b | Coffee cups | 3.624 | 2 | 0.163 | |||
| Coffee cups (1) | −0.256 | 0.361 | 0.504 | 1 | 0.478 | 0.774 | |
| Coffee cups (2) | −0.565 | 0.332 | 2.905 | 1 | 0.088 | 0.568 | |
| Constant | 0.838 | 0.299 | 7.850 | 1 | 0.005 | 2.312 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, L.C.; Farcas, S.S.; Andreescu, N.I. Coffee Consumption and CYP1A2 Polymorphism Involvement in Type 2 Diabetes in a Romanian Population. J. Pers. Med. 2024, 14, 717. https://doi.org/10.3390/jpm14070717
Popa LC, Farcas SS, Andreescu NI. Coffee Consumption and CYP1A2 Polymorphism Involvement in Type 2 Diabetes in a Romanian Population. Journal of Personalized Medicine. 2024; 14(7):717. https://doi.org/10.3390/jpm14070717
Chicago/Turabian StylePopa, Laura Claudia, Simona Sorina Farcas, and Nicoleta Ioana Andreescu. 2024. "Coffee Consumption and CYP1A2 Polymorphism Involvement in Type 2 Diabetes in a Romanian Population" Journal of Personalized Medicine 14, no. 7: 717. https://doi.org/10.3390/jpm14070717
APA StylePopa, L. C., Farcas, S. S., & Andreescu, N. I. (2024). Coffee Consumption and CYP1A2 Polymorphism Involvement in Type 2 Diabetes in a Romanian Population. Journal of Personalized Medicine, 14(7), 717. https://doi.org/10.3390/jpm14070717






