Local Control, Survival, and Toxicity Outcomes with High-Dose-Rate Peri-Operative Interventional Radiotherapy (Brachytherapy) in Head and Neck Cancers: A Systematic Review
Abstract
:1. Introduction
2. Methods and Materials
2.1. Eligibility
- Study design: Clinical trials, prospective/retrospective cohorts, and case-control studies were included.
- Population: Studies that included patients with primary or recurrent HNC, of any histology without distant metastasis, with or without prior irradiation, and treated with surgical resection and POIRT were eligible. Studies with the following co-interventions were allowed: reconstruction, external radiotherapy, and chemotherapy.
- Outcomes: Studies that reported on any of the following outcomes were eligible: survival (recurrence-free survival, RFS; overall survival, OS), radiation toxicity (acute or late toxicity), peri-operative complications, and quality of life (QOL).
- Setting: Studies that reported on patients treated from 1990 onwards were eligible; this restriction was intended to account for significant changes in diagnostic, medical, and surgical standards.
- Studies with at least six months of follow-up were eligible.
- Language: Only articles reported in the English, French, German, and Italian languages were included, given resource constraints.
- Study design: Case series, case reports, and pre-clinical studies were excluded. Relevant reviews were listed for bibliography scanning. Studies that were available only as an abstract or a conference proceeding were excluded.
- Outcomes: Studies that did not report on the above outcomes of interest, such as feasibility or dosimetric studies) were excluded.
2.2. Information Sources and Search Strategy
- Head and neck cancer [MeSH Major Topic].
- Brachytherapy [MeSH Terms].
- Interventional radiotherapy [Title/Abstract].
- Numbers: 2 OR 3.
- Peri-operative [Title/Abstract].
- Perioperative [Title/Abstract].
- Numbers: 5 OR 6.
- Numbers: 1 AND 4 AND 7.
2.3. Study Records
2.4. Data Items
- Setting: period of treatment, country.
- Study design and size: e.g., clinical trial, prospective cohort, retrospective cohort; number of patients.
- Patient characteristics: median/mean age, performance status, history of irradiation.
- Disease characteristics: histology, site, tumor size, T-stage, N-stage, setting (primary, recurrence, second primary).
- Treatment characteristics: resection status (clear margins, microscopic residual, macroscopic residual), chemotherapy, external radiotherapy, interventional radiotherapy dose and fractionation,
- Dosimetric parameters.
- Outcomes: RFS, OS, incidence of acute and late toxicity, peri-operative complications, QOL.
- Duration of follow-up: median, range.
2.5. Outcomes and Prioritization
2.6. Risk of Bias Assessment
2.7. Data Synthesis
3. Results
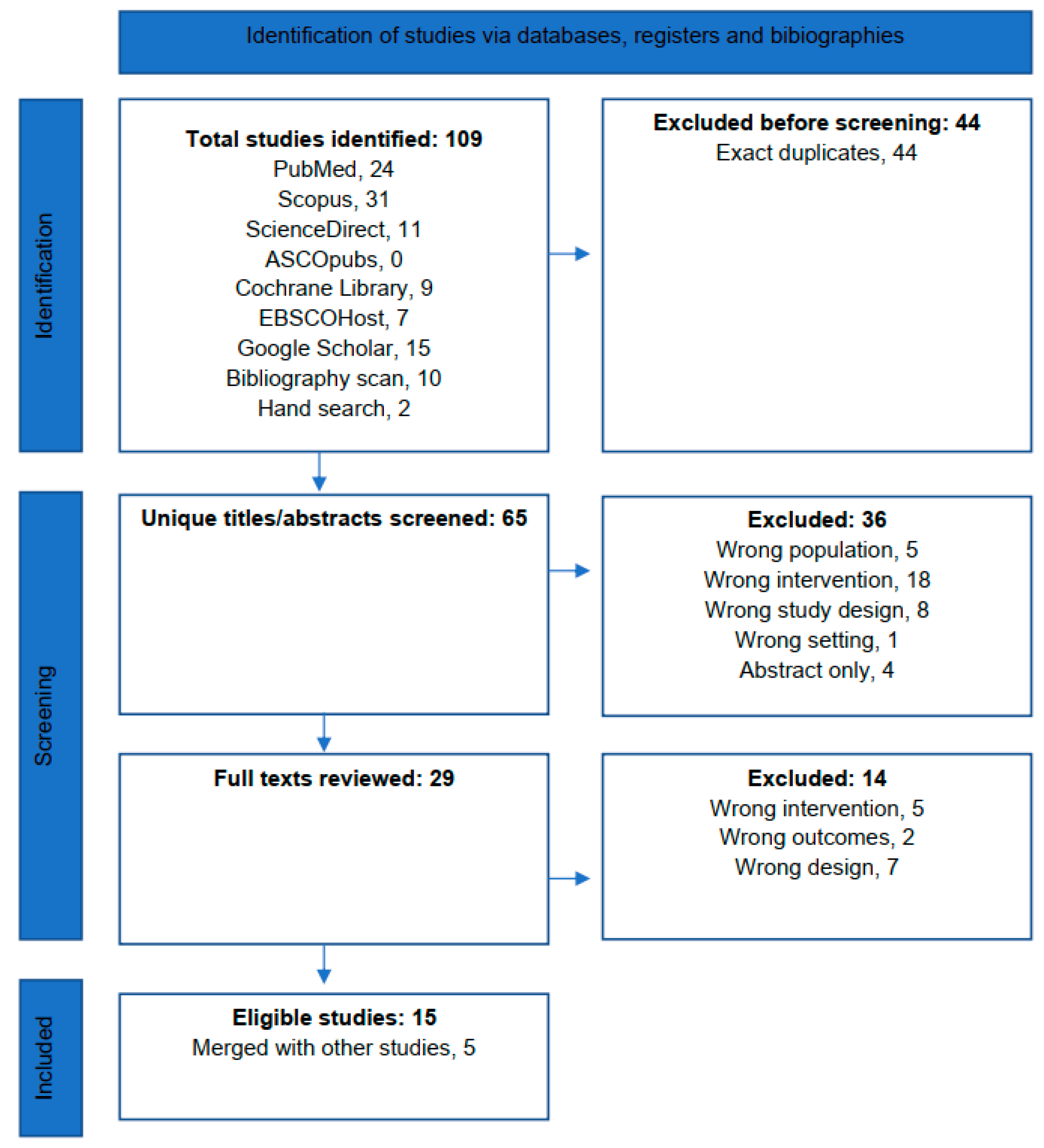
3.1. Search Results
3.2. Screening
4. Critical Appraisal
5. Scope of Extracted Data
6. POIRT in the Primary Setting
7. POIRT in the Re-Irradiation Setting
8. Discussion
8.1. POIRT in the Primary Setting
8.2. POIRT in the Re-Irradiation Setting
8.3. Enhancing the Therapeutic Ratio with POIRT
8.4. Study Limitations and Recommendations
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Comprehensive Cancer Network. Head and Neck Cancers, Version 4. NCCN Clinical Practice Guidelines in Oncology [Internet]. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 11 May 2024).
- Kovács, G.; Martinez-Monge, R.; Budrukkar, A.; Guinot, J.L.; Johansson, B.; Strnad, V.; Skowronek, J.; Rovirosa, A.; Siebert, F.-A. GEC-ESTRO ACROP recommendations for head & neck brachytherapy in squamous cell carcinomas: 1st update—Improvement by cross sectional imaging based treatment planning and stepping source technology. Radiother. Oncol. 2016, 122, 248–254. [Google Scholar]
- Tagliaferri, L.; Fionda, B.; Bacorro, W.; Kovacs, G. Advances in Head-and-Neck Interventional Radiotherapy (Brachytherapy). J. Med. Univ. St. Tomas 2024, 8, 1338–1341. [Google Scholar] [CrossRef]
- Jayalie, V.F.; Johnson, D.; Sudibio, S.; Rudiyo, R.; Jamnasi, J.; Hendriyo, H.; Resubal, J.R.; Manlapaz, D.J.; Cua, M.; Genson, J.M.; et al. Interdisciplinary and Regional Cooperation Towards Head and Neck Cancer Interventional Radiotherapy (Brachytherapy) Implementation in Southeast Asia. J. Med. Univ. St. Tomas 2024, 8, 1381–1389. [Google Scholar] [CrossRef]
- Cua, M.; Jainar, C.; Calapit, J.; Mejia, M.; Bacorro, W. The evolving landscape of head and neck brachytherapy: A scoping review. J. Contemp. Brachytherapy 2024, 16, 225–231. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Bussu, F.; Fionda, B.; Catucci, F.; Rigante, M.; Gambacorta, M.A.; Autorino, R.; Mattiucci, G.C.; Miccichè, F.; Placidi, E.; et al. Perioperative HDR brachytherapy for reirradiation in head and neck recurrences: Single-institution experience and systematic review. Tumori J. 2017, 103, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP Cohort Standard Checklist [Internet]. 2020. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 28 July 2022).
- Bussu, F.; Tagliaferri, L.; Mattiucci, G.; Parrilla, C.; Rizzo, D.; Gambacorta, M.A.; Lancellotta, V.; Autorino, R.; Fonnesu, C.; Kihlgren, C.; et al. HDR interventional radiotherapy (brachytherapy) in the treatment of primary and recurrent head and neck malignancies. Head Neck 2019, 41, 1667–1675. [Google Scholar] [CrossRef]
- Martínez-Monge, R.; Gómez-Iturriaga, A.; Cambeiro, M.; Garrán, C.; Montesdeoca, N.; Aristu, J.J.; Alcalde, J. Phase I-II trial of perioperative high-dose-rate brachytherapy in oral cavity and oropharyngeal cancer. Brachytherapy 2009, 8, 26–33. [Google Scholar] [CrossRef]
- Martínez-Monge, R.; Alcalde, J.; Concejo, C.; Cambeiro, M.; Garrán, C. Perioperative high-dose-rate brachytherapy (PHDRB) in previously irradiated head and neck cancer: Initial results of a Phase I/II reirradiation study. Brachytherapy 2006, 5, 32–40. [Google Scholar] [CrossRef]
- Martínez-Monge, R.; Divassón, M.P.; Cambeiro, M.; Gaztañaga, M.; Moreno, M.; Arbea, L.; Montesdeoca, N.; Alcalde, J. Determinants of Complications and Outcome in High-Risk Squamous Cell Head-and-Neck Cancer Treated With Perioperative High–Dose Rate Brachytherapy (PHDRB). Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e245–e254. [Google Scholar] [CrossRef]
- Potharaju, M.; Raj, H.; Muthukumaran, M.; Venkataraman, M.; Ilangovan, B.; Kuppusamy, S. Long-term outcome of high-dose-rate brachytherapy and perioperative brachytherapy in early mobile tongue cancer. J. Contemp. Brachytherapy 2018, 10, 64–72. [Google Scholar] [CrossRef]
- Teudt, I.U.; Meyer, J.E.; Ritter, M.; Wollenberg, B.; Kolb, T.; Maune, S.; Kovàcs, G. Perioperative image-adapted brachytherapy for the treatment of paranasal sinus and nasal cavity malignancies. Brachytherapy 2014, 13, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, M.I.; Alcalde, J.; Cambeiro, M.; Peydró, G.V.; Martínez-Monge, R. Perioperative high dose rate brachytherapy (PHDRB) in previously irradiated head and neck cancer: Results of a phase I/II reirradiation study. Radiother. Oncol. 2017, 122, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Bussu, F.; Fionda, B.; Rigante, M.; Rizzo, D.; Loperfido, A.; Gallus, R.; De Luca, L.M.; Corbisiero, M.F.; Lancellotta, V.; Tondo, A.; et al. Interventional radiotherapy (brachytherapy) for re-irradiation of recurrent head and neck malignancies: Oncologic outcomes and morbidity. Acta Otorhinolaryngol. Ital. 2024, 44 (Suppl. S1), S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Soror, T.; Paul, J.; Melchert, C.; Idel, C.; Rades, D.; Bruchhage, K.-L.; Kovács, G.; Leichtle, A. Salvage High-Dose-Rate Interventional Radiotherapy (Brachytherapy) Combined with Surgery for Regionally Relapsed Head and Neck Cancers. Cancers 2023, 15, 4549. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.; Teudt, I.U.; Meyer, J.E.; Schröder, U.; Kovács, G.; Wollenberg, B. Second-line treatment of recurrent HNSCC: Tumor debulking in combination with high-dose-rate brachytherapy and a simultaneous cetuximab-paclitaxel protocol. Radiat. Oncol. 2016, 11, 6. [Google Scholar] [CrossRef]
- Rudzianskas, V.; Inciura, A.; Juozaityte, E.; Rudzianskiene, M.; Kubilius, R.; Vaitkus, S.; Kaseta, M.; Adliene, D. Reirradiation of recurrent head and neck cancer using high-dose-rate brachytherapy. Acta Otorhinolaryngol. Ital. 2012, 32, 297–303. [Google Scholar] [PubMed]
- Pellizzon, A.C.; Salvajoli, J.V.; Kowalski, L.P.; Carvalho, A.L. Salvage for cervical recurrences of head and neck cancer with dissection and interstitial high dose rate brachytherapy. Radiat. Oncol. 2006, 1, 27. [Google Scholar] [CrossRef]
- Ianovski, I.; Mlynarek, A.M.; Black, M.J.; Bahoric, B.; Sultanem, K.; Hier, M.P. The role of brachytherapy for margin control in oral tongue squamous cell carcinoma. J. Otolaryngol. Head Neck Surg. 2020, 49, 74. [Google Scholar] [CrossRef]
- Gaztañaga, M.; Pagola, M.; Cambeiro, M.; Ruiz, M.E.R.; Aristu, J.; Montesdeoca, N.; Alcalde, J.; Martínez-Monge, R. Comparison of limited-volume perioperative high-dose-rate brachytherapy and wide-field external irradiation in resected head and neck cancer. Head Neck 2012, 34, 1081–1088. [Google Scholar] [CrossRef]
- de Almeida-Silva, L.A.; dos Santos Lupp, J.; Sobral-Silva, L.A.; Dos Santos, L.A.R.; Marques, T.O.; da Silva, D.B.R.; Caneppele, T.M.F.; Bianchi-de-Moraes, M. The incidence of osteoradionecrosis of the jaws in oral cavity cancer patients treated with intensity-modulated radiotherapy: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2024, 138, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.W.; Yu, T.P.; Kao, Y.S. A systematic review and meta-analysis of osteoradionecrosis following proton therapy in patients with head and neck cancer. Oral Oncol. 2024, 148, 106649. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, L.; Budrukkar, A.; Lenkowicz, J.; Cambeiro, M.; Bussu, F.; Guinot, J.L.; Hildebrandt, G.; Johansson, B.; Meyer, J.E.; Niehoff, P.; et al. ENT COBRA ONTOLOGY: The covariates classification system proposed by the Head & Neck and Skin GEC-ESTRO Working Group for interdisciplinary standardized data collection in head and neck patient cohorts treated with interventional radiotherapy (brachytherapy). J. Contemp. Brachytherapy 2018, 10, 260–266. [Google Scholar]
| Risk of Bias Assessment (CASP Checklist for Cohort Studies) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Research Question | Selection Bias | Measurement Bias (Exposure) | Measurement Bias (Outcomes) | Confounding Factors | Follow-Up | Magnitude of Effect | Precision of Estimate | Credibility | Empiric Congruence | Applicability | Implications to Practice |
| Peri-operative interventional radiotherapy in the primary setting | ||||||||||||
| Non-controlled clinical trial | ||||||||||||
| Ianovski 2020 [21] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Gaztañaga 2012 [22] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | High risk | Low risk | Low risk |
| Retrospective cohort | ||||||||||||
| Potharaju 2018 [13] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Teudt 2014 [14] | Low risk | Low risk | High risk | Low risk | High risk | Low risk | Uncertain risk | High risk | Low risk | Low risk | High risk | Low risk |
| Peri-operative interventional radiotherapy in the re-irradiation setting | ||||||||||||
| Non-controlled clinical trial | ||||||||||||
| Martínez-Fernández 2017 [15] | Low risk | Low risk | High risk | Low risk | High risk | Low risk | Low risk | High risk | Low risk | Low risk | High risk | Low risk |
| Retrospective cohort | ||||||||||||
| Bussu 2024 [16] | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | High risk | High risk | Low risk | Low risk | High risk | Low risk |
| Soror 2023 [17] | Low risk | High risk | High risk | Low risk | High risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk |
| Ritter 2016 [18] | Low risk | High risk | High risk | Low risk | Low risk | High risk | Low risk | Low risk | Uncertain risk | Uncertain risk | High risk | Low risk |
| Teudt 2014 [14] | Low risk | Uncertain risk | High risk | Low risk | High risk | Low risk | Uncertain risk | High risk | Uncertain risk | Uncertain risk | High risk | Low risk |
| Rudzianskas, 2012 [19] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | Uncertain risk | Low risk | Low risk |
| Pellizzon, 2006 [20] | Low risk | High risk | High risk | High risk | Low risk | High risk | Low risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Study ID (References) | Country | Study Period | n (%) a | M/F | Mdn Age (Range) | Site, % | Histology, % | Stage, % | Resection/ Margin Status, % /Reconstruction, % | EBRT, % /Dosing | BRT Dosing | Implant Technique and CTV | Dosimetry CTV | Start of BRT (Day Post-op) | Chemo, %/Regimen, % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-controlled clinical trials | |||||||||||||||||
| Ianovski, 2020 [21] | Canada | Sep 2009 to Apr 2017 | 55 (75) | 0.90 b | 62 b (24–92) | OT, 100 b | SCC, 100 b | pT1, 49 b pT2, 47 pT3, 4 pT4, 0 pN0, 65 b pN1, 15 pN2, 20 | R0, 0 Close (2.1–5 mm), 58 R1, 42 Recon, 100 | 39 Involved neck, 55 Gy/25 F Uninvolved neck, 50 Gy/25 F | Close margins 34 Gy/10 F, 63 R1 40.8 Gy/12 F, 37 | ISIRT CTV: Tumor bed | CTV: 5 mm around catheters | D3–5 | 34 If ENE, EBRT + concurrent weekly carboplatin 100 mg/m2 + taxol 40 mg/m2 | ||
| Gaztañaga, 2012 [10,12,22] | Spain | Oct 2000 to Oct 2008 | 57 (70) | 2.17 | 59 b (25–85) | OC, 52 b OPx, 21 HPx, 7 Neck, 18 | SCC, 100 | cN0, 21 cN1-2, 79 pN0, 30 pN+, 63 pNx, 7 | R0 (10 mm), 12 b Close (Mdn 3.0 mm), 35 b R1, 53 b | 100 b 45 Gy/25 F | R0 32 Gy/8 F BID 6 h apart R1 40 Gy/10 F BID 6 h apart | ISIRT CTV: Tumor bed and all surgical bed considered recurrence risk category 2 (≥2 nodes or ENE) or 3 (R1) | CTV: Tumor bed and high-risk volumes | D2–3 | 63 Cisplatin–paclitaxel, 60 Cisplatin–other, 4 | ||
| Retrospective cohort | |||||||||||||||||
| Potharaju, 2018 [13] | India | Jan 2000 to Sep 2010 | 73 (36) | 2.25 b | 52 b | OT, 100 b | SCC, 100 b | T1, 14 b T2, 12 N0, 100 b | <5 mm, x ≥5–10 mm, x Recon, 0 | None | 40 Gy/10 F BID 6 h apart | ISIRT, single-plane CTV: Tumor bed | CTV: 5 mm around catheters | D5–7 | None | ||
| Teudt, 2014 [14] | Germany | Jan 2006 to Jan 2013 | 35 (63) | 2.89 b | 60 b | NC, 46 b PNS, 54 | SCC, 63 b Adeno, 20 Other, 17 | I, 17 b II, 20 III, 11 IV, 51 | R0, 54 b R1, 31 R2, 3 Rx, 11 Osteosynthesis plates as needed | 57 b Mdn 50.4 Gy (40–63 Gy) | Mdn 20 Gy (10–35 Gy)/2.5 Gy-F BID 6 h apartb | ISIRT Intensity-modulation by variable catheter spacing (5–12 mm) | CTV: Maximum 10 mm around catheters | Mdn D7 (D2–14) | 31 b (chemo given only for SCC) Cisplatin, 26 Taxane, 9 Etoposide, 3 | ||
| Adeno, adenocarcinoma; BID, twice daily; c, clinical; CTV, clinical target volume; D, day; EBRT, external beam radiotherapy; ENE, extranodal extension; F, fraction; Gy, Gray; h, hour; HPx, hypopharynx; IRT, interventional radiotherapy; ISIRT, interstitial interventional radiotherapy; Mdn, median; N, nodal stage; NC, nasal cavity; OC, oral cavity; OPx, oropharynx; OT, oral tongue; PNS, paranasal sinus; p, pathologic; R, resection status; SCC, squamous cell carcinoma; T, primary tumor stage; x, unknown | |||||||||||||||||
| a. Percentage comprising the population and intervention of interest, if from a mixed cohort. b. Separate numbers not derivable for the population or intervention of interest, numbers reported for the entire cohort. | |||||||||||||||||
| Peri-operative interventional radiotherapy in the re-irradiation setting | |||||||||||||||||
| Study Information | Patient Characteristics | Intervention Characteristics | |||||||||||||||
| Study ID (References) | Country | Study Period | n (%) a | M/F | Mdn Age (Range) | Site, % | Histology, % | Setting and Stage, % | Prior RT, % /Setting, Dosing/Chemo/Time to ReRT | Resection/Margin Status, % /Reconstruction, % | EBRT, % /Dosing | BRT Dosing | Implant Technique and CTV | Dosimetry CTV | Start of BRT (day post-op) | Chemo, % /Regimen, % | |
| Non-controlled clinical trial | |||||||||||||||||
| Martínez-Fernández, 2017 [11,12,15,22] | Spain | Feb 2001 to Nov 2015 | 63 (100) | 2.7 | 63 (26–82) | Neck, 32 OT, 24 OPx, 21 Other, 23 | SCC, 95 Adeno, 2 Other, 4 | Second primary, 24 T1-2N0, 18 T3/N+, 6 Recurrence 76 pN0, 38 pN+, 38 pNx, 24 ECE, 67 | 100 EBRT, 98 IRT, 14 Prior surgery, 64 Chemo, 32 | R0 (10 mm), 11 Close (Mdn 3.0 mm), 35 R1, 54 | None | ≤32 Gy, 29 40 Gy, 71 R0: 32 Gy/8 F BID 6 h apart R1: 40 Gy/10 F BID 6 h apart | ISBT CTV: Tumor bed and all surgical bed considered recurrence risk category 2 (≥2 nodes or ENE) or 3 (positive margins) | CTV: Tumor bed and high-risk volumes | Mdn D4 (D0-D10) | None | |
| Retrospective cohort | |||||||||||||||||
| Bussu, 2024 [6,9,16] | Italy | Dec 2010 to Jun 2023 | 34 (85) | 2.6 | Mean 64.5 | ICIRT group NPx, 64 Ethmoid, 21 NC, 14 ISIRT group OC, 27 Lx, 20 HPx, 13 OPx, 13 Other, 27 | ICIRT group SCC, 72 Adeno, 14 Other, 14 ISIRT group SCC, 87 Other, 13 | ICIRT group LR, 100 (Second reRT, 3 Third reRT, 3) ISIRT group LR, x% RR, x% | ICIRT group Definitive, 64 Adjuvant, 36 ISIRT group Definitive, 33 Adjuvant, 67 >65 Gy, 100% | GTR, 100 Recon ICBT, 7 ISBT, 87 | None | 30 Gy/12 F BID 6 h apart | ISIRT, ICIRT CTV: Tumor bed and high-risk volumes | CTV: Tumor bed and high-risk volumes | D3–5 | ICBT, 21 ISBT, 0 | |
| Soror, 2023 [17] | Germany | Jan 2016 to Dec 2020 | 60 (70) | 3.29 b | 65.6 b (15.4–92.7) | OPx, 25 b Neck, 23 OC, 23 Other, 26 | SCC, 90 b Adeno, 8 Other, 2 | LR, 68 b RR, 23 Second primary, 8. | 70 Mdn 60 Gy (32–70) Chemo, 45 | R0, 32 b Close margin (<5 mm), 5 R1, 18 R2, 45 Recon Pedicled or free flap, as indicated, x% | 12 b 30–50 Gy | Mdn 30 Gy (12–40)/3 Gy-F BID 6 h apart b | ISIRT CTV: Tumor bed + 15–20 mm and high-risk volumes 8–12 mm spacing | CTV: Tumor bed + 15–20 mm and high-risk volumes | D2–5 | None | |
| Ritter, 2016 [18] | Germany | Jan 2006 to May 2013 | 94 (71) | Not reported | <60, 38 b ≥60, 62 | OPx/NPx, 28 b OC, 26 Neck, 8 HPx/Lx, 6 Other 32 | SCC, 80 b Other, 44 | I-II, 33 b III-IV, 67 T1-2, 40 T3-4, 48 Tx, 10 N0, 71 N1-2, 22 N3, 3 | 67 Mdn 64.2 Gy (33–105) Chemo, 26 Time to first recurrence Mdn 24 mo (10–73) <3 mo, 10 ≤ 3 mo, 84 | R0, 39 R1, 34 R2, 12 Rx, 6 No resection, 8 Recon Pedicled, microvascular or random pattern flap, as indicated, x% | 26 Mdn 48.7 Gy (30–60) | Mdn 25.9 Gy (10–35)/2.5 Gy (2.5–4.5) F BID 6 h apart | ISIRT | Intensity-modulation allowed for up to 200% within macroscopic tumor OAR doses less than the reference isodose | 16 b Platinum, 5 Cetuximab–taxane, 19 | ||
| Teudt, 2014 [14] | Germany | Jan 2006 to Jan 2013 | 35 (47) | 2.89 b | 60 b | NC, 46 b PNS, 54 | SCC, 63 b Adeno, 20 Other, 17 | I, 17 b II, 20 III, 11 IV, 51 | Not reported | R0, 54 b R1, 31 R2, 3 Rx, 11 Recon Osteosynthesis plates as needed | 57 b Mdn 50.4 Gy (40–63 Gy) | Mdn 20 Gy (10–35 Gy)/2.5 Gy-F BID 6 h apart | ISIRT CTV: Tumor bed Intensity-modulation by variable catheter spacing (5–12 mm) | CTV: Maximum 10 mm around catheters | Mdn D7 (D2–14) | 31 (chemo given only for SCC) b Cisplatin, 26 Taxane, 9 Etoposide, 3 | |
| Rudzianskas, 2012 [19] | Lithuania | Dec 2008 to Mar 2010 | 30 (43) | 2.33 b | 59 b (41–79) | OC, 27 b NC/PNS, 13 Parotid, 3 OPx, 13 Neck, 44 | SCC, 100 b | LR, 57 b RR, 43 | 100 Definitive, 33 Adjuvant, 67 Mdn 66 Gy (50–72) Chemo, 30 Time to first recurrence Mdn 12 mo (3–19) | Not reported | None | 30 Gy/12 F BID 6 h apart | ISIRT Catheter spacing 10–15 mm | 3 D: CTV D90 isodose | |||
| Pellizzon, 2006 [20] | Brazil | Oct 1994 to Jun 2004 | 21 (71) | 3.2 b | 53.5 b (31–73) | Pharynx 48 b OC, 29 Skin, 19 Neck, 5 | SCC, 100 b | RR, 100 b | 71 Mdn 52 Gy (30–66 Gy) Chemo, 5 Time to salvage therapy Mdn 32 mo (14–86) | GTR, 100 Recon As needed, x% | 100 ReRT subset Mdn 30 Gy (25–50) | ReRT subset Mdn 24 Gy | ISIRT CTV: Tumor bed + 15–20 mm margins Single plane, 90.5% Double plane, 9.5% | CTV: Tumor bed + 5 mm | D5 (D4-D12) | 3 b Platinum | |
| Adeno, adenocarcinoma; BID, twice daily; c, clinical; CTV, clinical target volume; D, day; D90, dose received by 90% of the volume; EBRT, external beam radiotherapy; ENE, extranodal extension; F, fraction; GTR, gross total resection; Gy, Gray; h, hour; HPx, hypopharynx; ICIRT, intracavitary interventional radiotherapy; IRT, interventional radiotherapy; ISIRT, interstitial interventional radiotherapy; LR, local recurrence; Lx, larynx; Mdn, median; mo, month; N, nodal stage; NC, nasal cavity; NPx, nasopharynx; OAR, organ at risk; OC, oral cavity; OPx, oropharynx; OT, oral tongue; PNS, paranasal sinus; p, pathologic; R, resection status; reRT, reirradiation; RR, regional recurrence; RT, radiotherapy; SCC, squamous cell carcinoma; T, primary tumor stage; x, unknown; 3D, three-dimensional | |||||||||||||||||
| a. Percentage comprising the population and intervention of interest, if from a mixed cohort. b. Separate numbers not derivable for the population or intervention of interest, numbers reported for the entire cohort. | |||||||||||||||||
| Peri-Operative Interventional Radiotherapy in the Primary Setting | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Characteristics | Intervention | Survival Outcomes | ||||||||||||||||
| Study ID | n, % a | Mdn Age | Site % | SCC % | T1-2 % | N0 % | GTR % | EBRT % | Mdn EBRT Dose (Gy) | Mdn POIRT Dose (Gy) | Mdn FU (mo) | 3y RFS % | 3y OS % | 5y RFS % | 5y OS % | |||
| Non-controlled clinical trials | ||||||||||||||||||
| Ianovski, 2020 [21] | 55, 75 | 62 b | OT, 100 b | 100 b | 96 b | 65 b | 100 b | 39 | 50–55 c | 34 | 25 | 74 b | 76 b | 69 b,d | 59 b | |||
| Gaztañaga, 2012 [10,12,22] | 57, 70 | 59 b | OT, 35 OPx, 21 FOM, 11 Other, 33 | 100 | -- | 30 | 100 | 100 | 45 | 40 | 52 b | -- | -- | 52 (9y) | 55 (9y) | |||
| Retrospective cohort | ||||||||||||||||||
| Potharaju, 2018 [13] | 73, 36 | 52 b | OT, 100 b | 100 b | 100 | 100 | 100 | 0 | 0 | 40 | 74 b | -- | -- | 92 d (6y) | 92 (6y) | |||
| Teudt, 2014 [14] | 35, 63 | 60 b | PNS, 54 b NC, 46 b | 63 b | -- | 37 b | 85 b | 57 b | 50.4 | 20 | 28 b | 83 d | 72 b | -- | -- | |||
| a percentage of the cohort that received POIRT in the primary setting; b for the entire cohort (n); c non-overlapping with POIRT; d DFS | ||||||||||||||||||
| DFS, disease-free survival; EBRT, external beam radiotherapy; FOM, floor of mouth; FU, follow up; Gy, Gray; GTR, gross total resection; Mdn, median; mo, month; N, nodal stage; NC, nasal cavity; OPx, oropharynx; OS, overall survival; OT, oral tongue; PNS, paranasal sinus; POIRT, peri-operative interventional radiotherapy; RFS, recurrence-free survival; SCC, squamous cell carcinoma; T, primary tumor stage; y, year | ||||||||||||||||||
| Peri-operative interventional radiotherapy in the re-irradiation setting | ||||||||||||||||||
| Baseline Characteristics | Intervention | Survival Outcomes | ||||||||||||||||
| Study ID | n, % a | Mdn Age | Site % | SCC % | T1-2 % | N0 % | Rec %; Sec % | Mdn Prior EBRT dose (Gy) | Mdn Time to ReRT (mo) | GTR % | EBRT % | Mdn EBRT Dose (Gy) | Mdn POIRT Dose (Gy) | Mdn FU (mo) | 3y RFS % | 3y OS % | 5y RFS % | 5y OS % |
| Non-controlled clinical trials | ||||||||||||||||||
| Martínez-Fernández, 2017 [11,12,15,22] | 63, 100 | 63 | Neck, 32 OT, 24 BOT, 13 OPx, 8 Other, 23 | 95 | -- | 38 | 76; 24 | -- | -- | 100 | 0 | 0 | 40 | 82 | -- | -- | 55 | 36 |
| Retrospective cohort | ||||||||||||||||||
| Bussu, 2024 [6,9,16] | 34, 85 | 65 | NPx, 31 c OC, 14 Ethmoid, 10 c Lx, 10 Other, 35 | 79 | -- | -- | 100; 0 | >65 | -- | 100 | 0 | 0 | 30 | 25 | 29 (2y) | 46 (2y) | -- | -- |
| Soror, 2023 [17] | 60, 70 | 66 b | OPx, 25 b OC, 23 Neck, 23 Other, 29 | 90 b | -- | -- | 92 b; 8 | 60 b | -- | 55 b | 12 b | (30–50) | 30 | 22 | 88 b,d | 39 b | 37 b,d | 17 b |
| Ritter, 2016 [18] | 94, ~67 | ≥60 | OPx/NPx, 28 b OC, 26 Neck, 9 HPx/Lx, 9 Other, 32 | 80 b | 40 b | 71 b | 100 b; 0 | 64 | 24 | 73 b | 26 b | 49 b | 26 b | 13 b | -- | -- | -- | -- |
| Teudt, 2014 [14] | 35, ~37 | 60 b | PNS, 54 b NC, 46 b | 63 b | -- | 37 b | -- | -- | -- | 85 b | 55 e | 28 b | 20 | 28 b | 34 f | 72 b | -- | -- |
| Rudzianskas, 2012 [19] | 30, 43 | 59 b | Neck, 44 b OC, 27 OPx, 13 Other, 16 | 100 b | -- | -- | 100 b; 0 | 66 b | ~12 g | -- | 0 | 0 | 30 b | 16 b | 53 b,f (2y) | 62 (2y) | -- | -- |
| Pellizzon, 2006 [20] | 21, 71 | 54 b | Pharynx, 47 b OC, 29 Other, 24 | 100 b | (rT0) | 0 b | 100 b; 0 | 52 | ~32 h | 100 | 100 b | 30 | 24 | 36 b | -- | -- | 43 b,d | 50 b |
| a percentage of the cohort (N) that received POIRT in the re-irradiation setting; b for the entire cohort (n); c endocavitary; d local RFS; e including pre-op or post-op EBRT; f DFS; g time to recurrence; h time to salvage therapy | ||||||||||||||||||
| BOT, base of tongue; EBRT, external beam radiotherapy; FU, follow up; Gy, Gray; GTR, gross total resection; HPx, hypopharynx; Lx, larynx; Mdn, median; mo, month; N, nodal stage; NC, nasal cavity; NPx, nasopharynx; OC, oral cavity; OPx, oropharynx; OS, overall survival; OT, oral tongue; PNS, paranasal sinus; POIRT, peri-operative interventional radiotherapy; Rec, recurrence; ReRT, reirradiation; RFS, recurrence-free survival; SCC, squamous cell carcinoma; Sec, secondary primary; T, primary tumor stage; y, year | ||||||||||||||||||
| Peri-Operative Interventional Radiotherapy in the Primary Setting | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Characteristics | Intervention | Toxicity Outcomes | |||||||||||||||||
| Study ID | n, % a | Mdn Age | Site % | GTR % | Recon % | EBRT % | Mdn EBRT Dose (Gy) | Mdn POIRT Dose (Gy) | Dosimetry Constraints | POIRT Start (Day PO) | Mdn FU (mo) | Acute | Late | ||||||
| Grade 1–2 % | Grade 3–4 % | Grade 5 % | Grade 1–2 % | Grade 3–4 % | Grade 5 % | ||||||||||||||
| Non-controlled clinical trials | |||||||||||||||||||
| Ianovski, 2020 [21] | 55, 75 | 62 b | OT, 100 b | 100 b | 100 b | 39 | 50–55 c | 34 | -- | 3–5 | 25 | Glossitis, 100 | Bleeding, 2 | 0 | Local pain, 7 | 0 | 0 | ||
| Gaztañaga, 2012 [10,12,22] | 57, 70 | 59 b | OT, 35 OPx, 21 FOM, 11 Other, 33 | 100 | -- | 100 | 45 | 40 | DHI ≥ 0.6 | 2–3 | 52 b | -- | Fistula, 5 b Bleeding, 2 b Graft failure, 2 b Wound complication, 2 b | Bleeding, 2 b | -- | Fibrosis, 5 b STN, 5 b Bleeding, 2 b Fistula, 2 b Nerve damage, 2 b Wound complication, 2 b ORN, 0 b | Bleeding, 4 b | ||
| Retrospective cohort | |||||||||||||||||||
| Potharaju, 2018 [13] | 73, 36 | 52 b | OT, 100 b | 100 | 0 | 0 | 0 | 40 | -- | 5–7 | 74 b | -- | -- | 0 | -- | STN, 0 ORN, 0 | 0 | ||
| Teudt, 2014 [14] | 35, 63 | 60 b | PNS, 54 b NC, 46 b | 85 b | -- | 57 b | 50.4 | 20 | -- | 2–14 | 28 b | Mucosal crusting, 11 b Peri-orbital edema, 9 b Allodynia, 6 b Wound complication, 6 b Alopecia, 3 b Dysesthesia, 3 b Epiphora, 3 b Fatigue, 3 b Flushing, 3 b | Wound complication, 3 b | 0 b | Mucosal crusting, 17 b Wound complication, 14 b Dysgeusia due to hyposmia, 14 b Allodynia, 6 b Epiphora, 6 b Peri-orbital Edema, 6 b Eustachian tube dysfunction, 3 b | 0 b | 0 b | ||
| a percentage of the cohort that received POIRT in the primary setting; b for the entire cohort (n); c non-overlapping with POIRT | |||||||||||||||||||
| DHI, dose homogeneity index; EBRT, external beam radiotherapy; FOM, floor of mouth; FU, follow up; GTR, gross total resection; Gy, Gray; Mdn, median; N, nodal stage; NC, nasal cavity; OPx, oropharynx; ORN, osteoradionecrosis; OT, oral tongue; PNS, paranasal sinus; PO, post-op; POIRT, peri-operative interventional radiotherapy; STN, soft tissue necrosis; T, primary tumor stage | |||||||||||||||||||
| Peri-operative interventional radiotherapy in the re-irradiation setting | |||||||||||||||||||
| Baseline Characteristics | Intervention | Toxicity Outcomes | |||||||||||||||||
| Study ID | n, % a | Mdn Age | Site % | Mdn Prior EBRT Dose (Gy) | Mdn Time to ReRT (mo) | GTR % | Recon % | EBRT % | Mdn EBRT Dose (Gy) | Mdn POIRT Dose (Gy) | Dosimetry Constraints | POIRT Start (Day PO) | Mdn FU (mo) | Acute | Late | ||||
| Grade 1–2 % | Grade 3–4 % | Grade 5 % | Grade 1–2 % | Grade 3–4 % | Grade 5 % | ||||||||||||||
| Non-controlled clinical trials | |||||||||||||||||||
| Martínez-Fernández, 2017 [11,12,15,22] | 63, 100 | 63 | Neck, 32 OT, 24 BOT, 13 OPx, 8 Other, 23 | -- | -- | 100 | -- | 0 | 0 | 40 | V150 (6 Gy) <13 cc Mandibular/vascular D10 cc <4 Gy | 0–10 | 82 | -- | Wound dehiscence, 8 b Graft failure, 6 b Bleeding, 5 b | Delayed bleeding, 3 b Post-op bleeding, 2 b Post-op mortality before BRT completion, 2 b | -- | Fistula, 19 b ORN, 5 b STN, 3 b Dysphagia, 3 b Fibrosis, 3 b Nerve damage, 3 b | Fistula, 2 b STN, 2 b |
| Retrospective cohort | |||||||||||||||||||
| Bussu, 2024 [6,9,16] | 34, 85 | 65 | NPx, 31 c OC, 14 Ethmoid, 10 c Lx, 10 Other, 35 | >65 | -- | 100 | 94 | 0 | 0 | 30 | QUANTEC | 3–5 | 25 | Cranial neuropathy, 3 Graft failure, 3 | 0 | 0 | 0 | 0 | 0 |
| Soror, 2023 [17] | 60, 70 | 66 b | OPx, 25 b OC, 23 Neck, 23 Other, 29 | 60 b | -- | 55 b | -- | 12 b | (30–50) | 30 | -- | 2–5 | 22 | Pain, 25 b Mucositis, 22 b Xerostomia, 15 b Dysphagia, 13 b Hypogeusia, 8 b Hyposmia, 3 b Bleeding, 3 b | Dysphagia, 20 b Pain, 17 b Xerostomia, 10 b Hyposmia, 3 b Local infection, 3 b Respiratory infection, 3 b Hypogeusia, 2 b Mucositis, 2 b | 0 | Xerostomia, 32 b Pain, 18 b Dysphagia, 17 b Hypogeusia, 15 b Mucositis, 10 b Hyposmia, 3 b | Xerostomia, 13 b Dysphagia, 10 b Pain, 8 b Hyposmia, 5 b Mucositis, 5 b Hypogeusia, 3 b ORN, 2 b STN, 2 b | 0 |
| Ritter, 2016 [18] | 94, ~67 | ≥60 | OPx/NPx, 28 b OC, 26 Neck, 9 HPx/Lx, 9 Other, 32 | 64 | 24 | 73 b | -- | 26 b | 49 b | 26 b | GTV boost up to 200% allowed OAR doses less than reference isodose | -- | 13 b | --e | --e | 0 | STN, 0 ORN, 0 | STN, 0 ORN, 0 | 0 |
| Teudt, 2014 [14] | 35, ~37 | 60 b | PNS, 54 b NC, 46 b | -- | -- | 50.4 | -- | 20 | 28 b | 20 | -- | 2–14 | 28 b | Mucosal crusting, 11 b Peri-orbital edema, 9 b Allodynia, 6 b Wound complication, 6 b Alopecia, 3 b Dysesthesia, 3 b Epiphora, 3 b Fatigue, 3 b Flushing, 3 b | Wound complication, 3 b | 0 b | Mucosal crusting, 17 b Dysgeusia due to Hyposmia, 14 b Wound complication, 14 b Allodynia, 6 b Epiphora, 6 b Peri-orbital edema, 6 b Eustachian tube dysfunction, 3 b | 0 b | 0 b |
| Rudzianskas, 2012 [19] | 30, 43 | 59 b | Neck, 44 b OC, 27 OPx, 13 Other, 16 | 66 b | ~12 c | -- | -- | 0 | 0 | 30 b | -- | -- | 16 b | Fibrosis, 6 b | Wound complication, 3 b Bleeding, 0 b | 0 b | Dysphagia, 3 b Hoarseness, 3 b | ORN, 3 b | 0 b |
| Pellizzon, 2006 [20] | 21, 71 | 54 b | Pharynx, 47 b OC, 29 Other, 24 | 52 | ~32 d | 100 | -- | 100 b | 30 | 24 | Dmax ≤135% Skin dose <60% | 4–12 | 36 b | -- | Wound dehiscence, 14 b Subcutaneous infection, 5 b | 0 b | -- | Local ulcer, 14 b Neck fibrosis, 5 b STN, 0 b ORN, 0 b | 0 b |
| a percentage of the cohort (N) that received POIRT in the re-irradiation setting; b for the entire cohort (n); c time to recurrence; d time to salvage therapy; e reported overall grade 1–2 and grade 3 toxicity rates of 17% and 10%, chronicity not specified | |||||||||||||||||||
| BOT, base of tongue; cc, cubic centimeter; Dmax, maximum dose; EBRT, external beam radiotherapy; FOM, floor of mouth; FU, follow up; GTR, gross total resection; GTV, gross tumor volume; Gy, Gray; Lx, larynx; Mdn, median; N, nodal stage; NC, nasal cavity; NPx, nasopharynx; OC, oral cavity; OAR, organ at risk; OPx, oropharynx; ORN, osteoradionecrosis; OS, overall survival; OT, oral tongue; PNS, paranasal sinus; PO, post-op; POIRT, peri-operative interventional radiotherapy; QUANTEC, Quantitative Analysis of Normal Tissue Effects in the Clinic; ReRT, re-irradiation; RFS, recurrence-free survival; SCC, squamous cell carcinoma; STN, soft tissue necrosis; T, primary tumor stage; Vn, volume receiving n% of the prescribed dose | |||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacorro, W.; Fionda, B.; Soror, T.; Bussu, F.; Kovács, G.; Tagliaferri, L. Local Control, Survival, and Toxicity Outcomes with High-Dose-Rate Peri-Operative Interventional Radiotherapy (Brachytherapy) in Head and Neck Cancers: A Systematic Review. J. Pers. Med. 2024, 14, 853. https://doi.org/10.3390/jpm14080853
Bacorro W, Fionda B, Soror T, Bussu F, Kovács G, Tagliaferri L. Local Control, Survival, and Toxicity Outcomes with High-Dose-Rate Peri-Operative Interventional Radiotherapy (Brachytherapy) in Head and Neck Cancers: A Systematic Review. Journal of Personalized Medicine. 2024; 14(8):853. https://doi.org/10.3390/jpm14080853
Chicago/Turabian StyleBacorro, Warren, Bruno Fionda, Tamer Soror, Francesco Bussu, György Kovács, and Luca Tagliaferri. 2024. "Local Control, Survival, and Toxicity Outcomes with High-Dose-Rate Peri-Operative Interventional Radiotherapy (Brachytherapy) in Head and Neck Cancers: A Systematic Review" Journal of Personalized Medicine 14, no. 8: 853. https://doi.org/10.3390/jpm14080853
APA StyleBacorro, W., Fionda, B., Soror, T., Bussu, F., Kovács, G., & Tagliaferri, L. (2024). Local Control, Survival, and Toxicity Outcomes with High-Dose-Rate Peri-Operative Interventional Radiotherapy (Brachytherapy) in Head and Neck Cancers: A Systematic Review. Journal of Personalized Medicine, 14(8), 853. https://doi.org/10.3390/jpm14080853






