Targeted Screening for Cancer: Learnings and Applicability to Melanoma: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Selection
- Participants from the adult general population;
- Studies on personalized cancer-screening programs, where screening was tailored according to personal risk (beyond age or sex alone);
- Focus on cancer(s);
- January 2013 to May 2023, to focus on contemporary screening programs;
- Research in humans.
- Written in a language other than English;
- General screening (not risk-based) studies;
- Reviews, letters, editorials;
- Evaluations of hypothetical personalized cancer screening.
2.3. Data Extraction
2.4. Quality Assessment
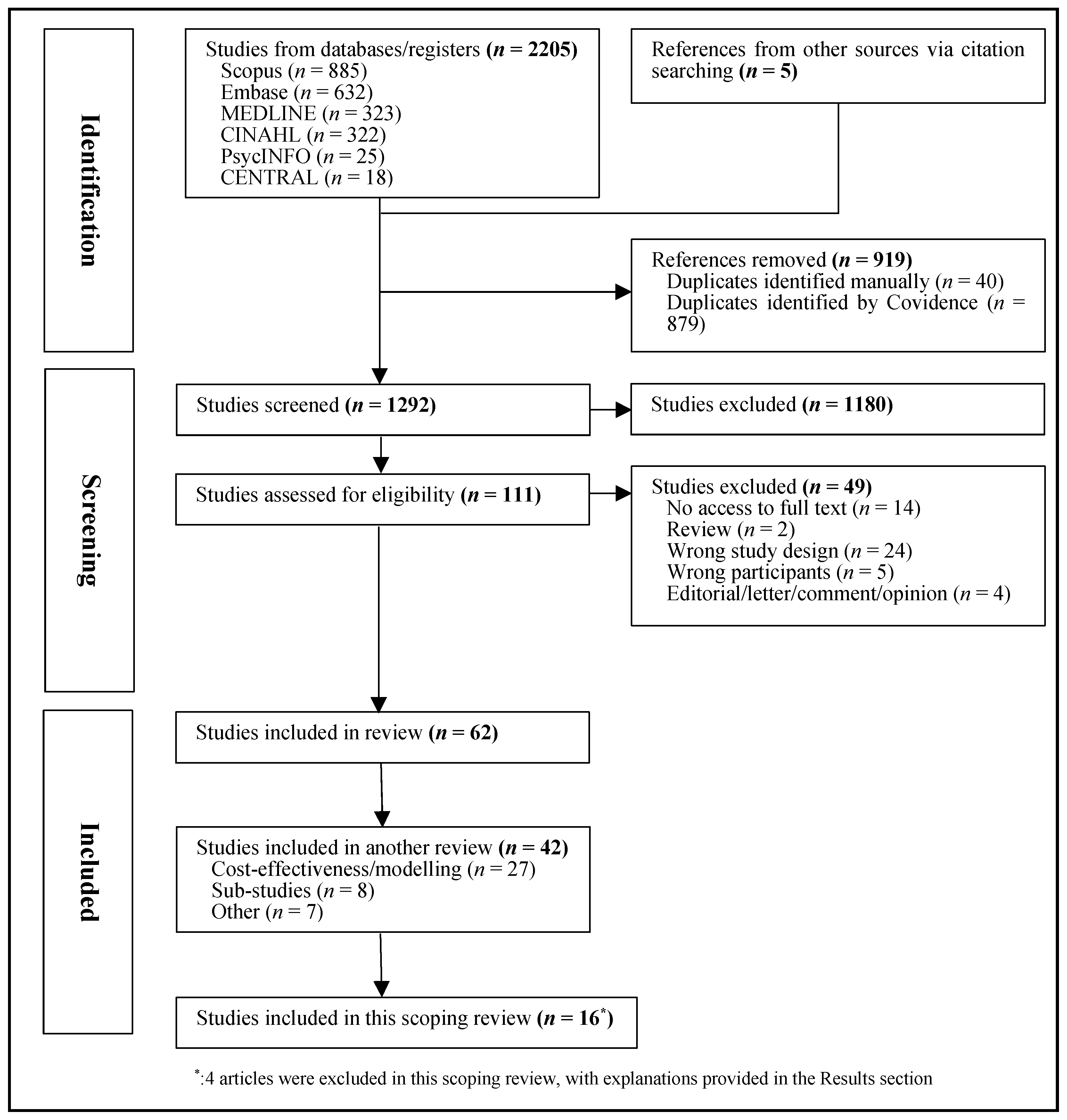
3. Results
3.1. Key Characteristics of Eligible Studies
3.2. Cancer Types
3.3. Study Locations
3.4. Participants
3.5. Risk Assessments
3.6. Risk Thresholds and Proposed Pathways
3.7. Key Components of Eligible Studies
3.7.1. Screening Process and Evaluation
3.7.2. Main Outcomes of Screening
3.7.3. Results by Cancer Types
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Learoyd, D.L. Estimating the magnitude of cancer overdiagnosis in Australia. Med. J. Aust. 2020, 213, 189. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.-B.; Feletto, E.; Wade, S.; Caruana, M.; Kang, Y.-J.; Nickson, C.; Simms, K.; Procopio, P.; Taylor, N.; Worthington, J.; et al. Benefits, harms and cost-effectiveness of cancer screening in Australia: An overview of modelling estimates. Public Health Res. Pract. 2019, 29, 29121913. [Google Scholar] [CrossRef]
- Marcus, P.M.; Prorok, P.C.; Miller, A.B.; DeVoto, E.J.; Kramer, B.S. Conceptualizing overdiagnosis in cancer screening. JNCI J. Natl. Cancer Inst. 2015, 107, djv014. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.C.; Hutchinson, A.; Law, K.; Shah, V.; Usher-Smith, J.A.; Dennison, R.A. Acceptability of risk stratification within population-based cancer screening from the perspective of the general public: A mixed-methods systematic review. Health Expect. 2023, 26, 989–1008. [Google Scholar] [CrossRef]
- Taylor, L.C.; Law, K.; Hutchinson, A.; Dennison, R.A.; Usher-Smith, J.A. Acceptability of risk stratification within population-based cancer screening from the perspective of healthcare professionals: A mixed methods systematic review and recommendations to support implementation. PLoS ONE 2023, 18, e0279201. [Google Scholar] [CrossRef]
- Clift, A.K.; Dodwell, D.; Lord, S.; Petrou, S.; Brady, S.M.; Collins, G.S.; Hippisley-Cox, J. The current status of risk-stratified breast screening. Br. J. Cancer 2022, 126, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Rees, C.J.; Sharp, L.; Koo, S. A risk-stratified approach to colorectal cancer prevention and diagnosis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 773–780. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Emery, J.D.; A Jenkins, M.; Saya, S.; Chondros, P.; Oberoi, J.; Milton, S.; Novy, K.; Habgood, E.; Karnchanachari, N.; Pirotta, M.; et al. The Colorectal cancer RISk Prediction (CRISP) trial: A randomised controlled trial of a decision support tool for risk-stratified colorectal cancer screening. Br. J. Gen. Pract. 2023, 73, e556–e565. [Google Scholar] [CrossRef]
- Lansdorp-Vogelaar, I.; Meester, R.; de Jonge, L.; Buron, A.; Haug, U.; Senore, C. Risk-stratified strategies in population screening for colorectal cancer. Int. J. Cancer 2022, 150, 397–405. [Google Scholar] [CrossRef]
- World Health Organization. Existence of National Screening Program for Breast Cancer. 2022. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/existence-of-national-screening-program-for-breast-cancer (accessed on 23 April 2024).
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health 2022, 10, e1115–e1127. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; E Schoen, R.; Sung, J.J.Y.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Breitbart, E.W.; Waldmann, A.; Nolte, S.; Capellaro, M.; Greinert, R.; Volkmer, B.; Katalinic, A. Systematic skin cancer screening in Northern Germany. J. Am. Acad. Dermatol. 2012, 66, 201–211. [Google Scholar] [CrossRef]
- Rat, C.; Blachier, L.; Hild, S.; Molinie, F.; Gaultier, A.; Dreno, B.; Nguyen, J.-M. Targeted screening for melanoma after a 5-year follow-up: Comparison of melanoma incidence and lesion thickness at diagnosis in screened (versus unscreened) patients. La Presse Médicale Open 2021, 2, 100013. [Google Scholar] [CrossRef]
- Dunlop, K.L.A.; Singh, N.; Robbins, H.A.; Zahed, H.; Johansson, M.; Rankin, N.M.; Cust, A.E. Implementation considerations for risk-tailored cancer screening in the population: A scoping review. Prev. Med. 2024, 181, 107897. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Ivlev, I.; Blasi, P.R.; Nguyen, M.B.; Senger, C.A.; Perdue, L.A.; Lin, J.S. Skin Cancer Screening: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 329, 1296–1307. [Google Scholar] [CrossRef]
- Bhave, P.; Wong, J.; McInerney-Leo, A.; E Cust, A.; Lawn, C.; Janda, M.; Mar, V.J. Management of cutaneous melanoma in Australia: A narrative review. Med. J. Aust. 2023, 218, 426–431. [Google Scholar] [CrossRef]
- Reyes-Marcelino, G.; Tabbakh, T.; Espinoza, D.; Sinclair, C.; Kang, Y.-J.; McLoughlin, K.; Caruana, M.; Fernández-Peñas, P.; Guitera, P.; Aitken, J.F.; et al. Prevalence of skin examination behaviours among Australians over time. Cancer Epidemiol. 2021, 70, 101874. [Google Scholar] [CrossRef]
- Aitken, J.F.; Elwood, M.; Baade, P.D.; Youl, P.; English, D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int. J. Cancer 2010, 126, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.C.; Elwood, M.; Swetter, S.M.; Brooks, D.R.; Aitken, J.; Youl, P.H.; Demierre, M.; Baade, P.D. Factors related to the presentation of thin and thick nodular melanoma from a population-based cancer registry in Queensland Australia. Cancer 2009, 115, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.G.; Shih, S.; Watts, C.; Goldsbury, D.; Green, A.C. The economics of skin cancer prevention with implications for Australia and New Zealand: Where are we now? Public Health Res. Pract. 2022, 32, 31502119. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; O’connell, D.L.; Yu, X.Q.; Kahn, C.; Caruana, M.; Pesola, F.; Sasieni, P.; Grogan, P.B.; Aranda, S.; Cabasag, C.J.; et al. Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: A statistical modelling study. Lancet Public Health 2022, 7, e537–e548. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, S.; Lin, G.; Kerr, P.; Grant-Kels, J.M. Evidence concerning the accusation that melanoma is overdiagnosed. J. Am. Acad. Dermatol. 2021, 85, 841–846. [Google Scholar] [CrossRef]
- Watts, C.; Dieng, M.; Morton, R.; Mann, G.; Menzies, S.; Cust, A. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: A systematic review. Br. J. Dermatol. 2015, 172, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, O.A.; Dekio, I.; Layton, A.M.; Li, H.; Hughes, H.; Morris, T.; Zouboulis, C.C.; Patrick, S. Why we continue to use the name Propionibacterium acnes. Br. J. Dermatol. 2018, 179, 1227. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Cust, A.E.; Neale, R.E.; Aitken, J.F.; Baade, P.D.; Green, A.C.; Khosrotehrani, K.; Mar, V.; Soyer, H.P.; Whiteman, D.C. Early detection of melanoma: A consensus report from the Australian Skin and Skin Cancer Research Centre Melanoma Screening Summit. Aust. N. Z. J. Public Health 2020, 44, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.G.; Jungner, G.; World Health Organization. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Joanna Briggs Institute. Scoping Reviews. 2023. Available online: https://guides.library.unisa.edu.au/ScopingReviews (accessed on 23 May 2023).
- Cochrane Methods. Risk of Bias 2 (RoB 2) Tool. 2021. Available online: https://methods.cochrane.org/risk-bias-2 (accessed on 23 May 2023).
- Cochrane Methods. ROBINS-I Tool. 2017. Available online: https://methods.cochrane.org/robins-I (accessed on 23 May 2023).
- Chen, H.; Shi, J.; Lu, M.; Li, Y.; Du, L.; Liao, X.; Wei, D.; Dong, D.; Gao, Y.; Zhu, C.; et al. Comparison of Colonoscopy, Fecal Immunochemical Test, and Risk-Adapted Approach in a Colorectal Cancer Screening Trial (TARGET-C). Clin. Gastroenterol. Hepatol. 2023, 21, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Schroy, P.C., 3rd; Duhovic, E.; Chen, C.A.; Heeren, T.C.; Lopez, W.; Apodaca, D.L.; Wong, J.B. Risk Stratification and Shared Decision Making for Colorectal Cancer Screening: A Randomized Controlled Trial. Med. Decis. Mak. 2016, 36, 526–535. [Google Scholar] [CrossRef]
- Fredsøe, J.; Koetsenruyter, J.; Vedsted, P.; Kirkegaard, P.; Væth, M.; Edwards, A.; Ørntoft, T.F.; Sørensen, K.D.; Bro, F. The effect of assessing genetic risk of prostate cancer on the use of PSA tests in primary care: A cluster randomized controlled trial. PLoS Med. 2020, 17, e1003033. [Google Scholar] [CrossRef]
- Field, J.K.; Duffy, S.W.; Baldwin, D.R.; Whynes, D.K.; Devaraj, A.; Brain, K.E.; Eisen, T.; Gosney, J.; A Green, B.; A Holemans, J.; et al. UK Lung Cancer RCT Pilot Screening Trial: Baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016, 71, 161–170. [Google Scholar] [CrossRef]
- Esserman, L.J.; Study, W.; Athena, I. The WISDOM Study: Breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 2017, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Trevena, L.J.; Meiser, B.; Mills, L.; Dobbins, T.; Mazza, D.; Emery, J.D.; Kirk, J.; Goodwin, A.; Barlow-Stewart, K.; Naicker, S. Which Test Is Best? A Cluster-Randomized Controlled Trial of a Risk Calculator and Recommendations on Colorectal Cancer Screening Behaviour in General Practice. Public Health Genom. 2022, 25, 193–208. [Google Scholar] [CrossRef]
- Saya, S.; Boyd, L.; Chondros, P.; McNamara, M.; King, M.; Milton, S.; Lourenco, R.D.A.; Clark, M.; Fishman, G.; Marker, J.; et al. The SCRIPT trial: Study protocol for a randomised controlled trial of a polygenic risk score to tailor colorectal cancer screening in primary care. Trials 2022, 23, 810. [Google Scholar] [CrossRef]
- Liu, J.; Ho, P.J.; Tan, T.H.L.; Yeoh, Y.S.; Chew, Y.J.; Riza, N.K.M.; Khng, A.J.; Goh, S.-A.; Wang, Y.; Oh, H.B.; et al. BREAst screening Tailored for HEr (BREATHE)—A study protocol on personalised risk-based breast cancer screening programme. PLoS ONE 2022, 17, e0265965. [Google Scholar] [CrossRef]
- Yen, A.M.; Tsau, H.-S.; Fann, J.C.-Y.; Chen, S.L.-S.; Chiu, S.Y.-H.; Lee, Y.-C.; Pan, S.-L.; Chiu, H.-M.; Kuo, W.-H.; Chang, K.-J.; et al. Population-Based Breast Cancer Screening with Risk-Based and Universal Mammography Screening Compared with Clinical Breast Examination: A Propensity Score Analysis of 1 429 890 Taiwanese Women. JAMA Oncol. 2016, 2, 915–921. [Google Scholar] [CrossRef]
- Evans, D.G.; McWilliams, L.; Astley, S.; Brentnall, A.R.; Cuzick, J.; Dobrashian, R.; Duffy, S.W.; Gorman, L.S.; Harkness, E.F.; Harrison, F.; et al. Quantifying the effects of risk-stratified breast cancer screening when delivered in real time as routine practice versus usual screening: The BC-Predict non-randomised controlled study (NCT04359420). Br. J. Cancer 2023, 128, 2063–2071. [Google Scholar] [CrossRef]
- Gaba, F.; Blyuss, O.; Liu, X.; Goyal, S.; Lahoti, N.; Chandrasekaran, D.; Kurzer, M.; Kalsi, J.; Sanderson, S.; Lanceley, A.; et al. Population Study of Ovarian Cancer Risk Prediction for Targeted Screening and Prevention. Cancers 2020, 12, 1241. [Google Scholar] [CrossRef]
- Laza-Vásquez, C.; Martínez-Alonso, M.; Forné-Izquierdo, C.; Vilaplana-Mayoral, J.; Cruz-Esteve, I.; Sánchez-López, I.; Reñé-Reñé, M.; Cazorla-Sánchez, C.; Hernández-Andreu, M.; Galindo-Ortego, G.; et al. Feasibility and Acceptability of Personalized Breast Cancer Screening (DECIDO Study): A Single-Arm Proof-of-Concept Trial. Int. J. Environ. Res. Public Health 2022, 19, 10426. [Google Scholar] [CrossRef]
- Brooks, J.D.; Nabi, H.H.; Andrulis, I.L.; Antoniou, A.C.; Chiquette, J.; Després, P.; Devilee, P.; Dorval, M.; Droit, A.; Easton, D.F.; et al. Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I). J. Pers. Med. 2021, 11, 511. [Google Scholar] [CrossRef]
- Shah, A.; Polascik, T.J.; George, D.J.; Anderson, J.; Hyslop, T.; Ellis, A.M.; Armstrong, A.J.; Ferrandino, M.; Preminger, G.M.; Gupta, R.T.; et al. Implementation and Impact of a Risk-Stratified Prostate Cancer Screening Algorithm as a Clinical Decision Support Tool in a Primary Care Network. J. Gen. Intern. Med. 2021, 36, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rainey, L.; van der Waal, D.; Donnelly, L.S.; Southworth, J.; French, D.P.; Evans, D.G.; Broeders, M.J.M. Women’s health behaviour change after receiving breast cancer risk estimates with tailored screening and prevention recommendations. BMC Cancer 2022, 22, 69. [Google Scholar] [CrossRef]
- Nash, Z.; Menon, U. Ovarian cancer screening: Current status and future directions. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 32–45. [Google Scholar] [CrossRef]
- QIMR-Berghofer. QSkin2. 2023. Available online: https://publications.qimrberghofer.edu.au/Custom/QSkinMelanomaRisk (accessed on 30 August 2023).
- Vuong, K.; Armstrong, B.K.; Weiderpass, E.; Lund, E.; Adami, H.-O.; Veierod, M.B.; Barrett, J.H.; Davies, J.R.; Bishop, D.T.; Whiteman, D.C.; et al. Development and External Validation of a Melanoma Risk Prediction Model Based on Self-assessed Risk Factors. JAMA Dermatol. 2016, 152, 889–896. [Google Scholar] [CrossRef]
- Kaiser, I.; Pfahlberg, A.B.; Uter, W.; Heppt, M.V.; Veierød, M.B.; Gefeller, O. Risk Prediction Models for Melanoma: A Systematic Review on the Heterogeneity in Model Development and Validation. Int. J. Environ. Res. Public Health 2020, 17, 7919. [Google Scholar] [CrossRef] [PubMed]
- Usher-Smith, J.A.; Emery, J.; Kassianos, A.P.; Walter, F.M. Risk prediction models for melanoma: A systematic review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Kashani-Sabet, M.; Leachman, S.A.; Stein, J.A.; Arbiser, J.L.; Berry, E.G.; Celebi, J.T.; Curiel-Lewandrowski, C.; Ferris, L.K.; Grant-Kels, J.M.; Grossman, D.; et al. Early Detection and Prognostic Assessment of Cutaneous Melanoma: Consensus on Optimal Practice and the Role of Gene Expression Profile Testing. JAMA Dermatol. 2023, 159, 545–553. [Google Scholar] [CrossRef]
- Wong, C.K.; Dite, G.S.; Spaeth, E.; Murphy, N.M.; Allman, R. Melanoma risk prediction based on a polygenic risk score and clinical risk factors. Melanoma Res. 2023, 33, 293–299. [Google Scholar] [CrossRef]
- E Johns, L.; A Coleman, D.; Swerdlow, A.J.; Moss, S.M. Effect of population breast screening on breast cancer mortality up to 2005 in England and Wales: An individual-level cohort study. Br. J. Cancer 2016, 116, 246–252. [Google Scholar] [CrossRef]
- Katalinic, A.; Eisemann, N.; Kraywinkel, K.; Noftz, M.R.; Hübner, J. Breast cancer incidence and mortality before and after implementation of the German mammography screening program. Int. J. Cancer 2020, 147, 709–718. [Google Scholar] [CrossRef]
- Morrell, S.; Taylor, R.; Roder, D.; Robson, B.; Gregory, M.; Craig, K. Mammography service screening and breast cancer mortality in New Zealand: A National Cohort Study 1999–2011. Br. J. Cancer 2017, 116, 828–839. [Google Scholar] [CrossRef]
- Sankatsing, V.D.; van Ravesteyn, N.T.; Heijnsdijk, E.A.; Looman, C.W.; van Luijt, P.A.; Fracheboud, J.; Heeten, G.J.D.; Broeders, M.J.; de Koning, H.J. The effect of population-based mammography screening in Dutch municipalities on breast cancer mortality: 20 years of follow-up. Int. J. Cancer 2017, 141, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Van Ourti, T.; O’Donnell, O.; Koç, H.; Fracheboud, J.; de Koning, H.J. Effect of screening mammography on breast cancer mortality: Quasi-experimental evidence from rollout of the Dutch population-based program with 17-year follow-up of a cohort. Int. J. Cancer 2020, 146, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, R.; Duggan, C.; Anderson, B.O. Demographic changes in breast cancer incidence, stage at diagnosis and age associated with population-based mammographic screening. J. Surg. Oncol. 2017, 115, 517–522. [Google Scholar]
- Welch, H.G.; Mazer, B.L.; Adamson, A.S. The Rapid Rise in Cutaneous Melanoma Diagnoses. N. Engl. J. Med. 2021, 341, 72–79. [Google Scholar] [CrossRef]
- Adamson, A.S.; Naik, G.; A Jones, M.; Bell, K.J. Ecological study estimating melanoma overdiagnosis in the USA using the lifetime risk method. BMJ Evid.-Based Med. 2024, 29, 156–161. [Google Scholar] [CrossRef]
- Cust, A.E.; Aitken, J.F.; Baade, P.D.; Whiteman, D.C.; Soyer, H.P.; Janda, M. Why a randomized melanoma screening trial may be a good idea. Br. J. Dermatol. 2018, 179, 1227. [Google Scholar] [CrossRef]
- Halvorsen, J.; Løberg, M.; Gjersvik, P.; Roscher, I.; Veierød, M.; Robsahm, T.; Nilsen, L.; Kalager, M.; Bretthauer, M. Why a randomized melanoma screening trial is not a good idea. Br. J. Dermatol. 2018, 179, 532–533. [Google Scholar] [CrossRef]
| Cancer(s) | Author (Year) | Location | Period | Study Design | Participants | Risk Thresholds | Screening Pathways | |
|---|---|---|---|---|---|---|---|---|
| Sample | Size | |||||||
| Breast | Brooks (2021) [46] | Canada | 2021–2022 | Mixed-methods | Unaffected women; 40–69 years | 5000 | 10-year risk corresponding to remaining lifetime risk: Average: <15%; Higher than average: 15–25%; High: ≥25% | Average: 40–49 years: no regular screening with mammogram; 50–69 years: screen with a mammogram/2 years. Higher than average: - Ontario 40–49 years: screen with mammogram/year; 50–69 years: screen with mammogram/year; - Quebec Screen with mammogram/1–2 years, ultrasound considered if breast density is >75%; High: 40–69 years: screen with mammogram and † MRI/year. |
| Esserman (2017) [38] | United States | 2016–2021 | ** RCT | Unaffected women; 40–74 years | 100,000 | 5-y absolute risk: <1.3%; 1.3–6%; ≥6% | 50 years: Digital mammography/2 years; 40–49 years: Screening is recommended when their 5-year risk equals or exceeds that of the average woman aged 50 years. Screen/2 year, except women with extremely dense breasts, who will be offered annual screening. | |
| Evans (2023) [43] | United Kingdom | 2019–2021 | Non-RCT | Biologically female reach 60 years; | 2472 | Below average risk: 10 years < 2%; Average risk: 2% ≤ 10 years < 5%; Moderate risk: 5% ≤ 10 years < 8%; High risk: 10-years ≥ 8% | No screening intervals specified; participants were followed at baseline, 3 months, and 6 months | |
| Laza-Vasquez (2022) [45] | Spain | 2019–2021 | Single-arm proof-of-concept study | Women; 40–50 years; | 387 | Absolute risk of breast cancer at 5 years: <0.99%; 0.99–1.16%; >1.16% | 40–44 years: <0.99%—watch and wait; 0.99–1.16%—biennial; >1.16%—annual. 45–48 years: <0.99%—watch and wait; 0.99–1.19%—biennial; >1.19%—annual. 49–50 years: <0.8%—triennial; 0.8–1.19%—biennial; >1.19%—annual. 40–50 years: >6%: referral to hospital breast unit and or genetic counseling | |
| Liu (2022) [41] | Singapore | 2021 | Cohort study | Women; 35–59 years; No cancer history | 3500 | 5-years absolute risk above 3% as a threshold | Current national guidelines: 35–39 years: no recommendation; 40–49 years: mammography screening/year; 50–59 years: mammography screening/2 years; In this study, women identified to be above average in breast cancer risk are referred to breast specialists at designated study sites, in addition to prevailing guidelines. | |
| Rainey (2022) [48] | United Kingdom | 2014–2019 | Focus group | Unaffected women; 50–70 years; | 325 | 10-year risk: Low risk: ≤1.5%; Average risk: 1.5–4.99%; Moderate risk: 5–7.99%; High risk: ≥8% | Low risk: screening/3 years; Average risk: screening/3 years; Moderate risk: screening/3 years; High risk: screening/18 m | |
| Yen (2016) [42] | Taiwan | 1999–2009 | Cohort study | Asymptomatic women; 40–69 years | 1,429,890 | High risk: risk scores > median value of the underlying population | Single screening method: 1 clinical breast examination (CBE); 1 risk-based mammography; 1 universal mammography; 2 screening methods: Risk-based and universal mammography; CBE and universal mammography; CBE and risk-based mammography; 3 screening methods: Risk-based + universal mammography + CBE. | |
| Colorectal | Chen (2023) [34] | China | 2018–2021 | ** RCT | Unaffected; 50–74 years; | 19,373 | 5-year risk: Low-risk: APCS < 4; High-risk: APCS ≥ 4 | Arm 1 (n = 3883): 1-time colonoscopy; Arm 2 (n = 7793): annual †† FIT; Arm 3 (n = 7697):
|
| Emery (2023) [11] | Australia | 2017–2018 | ** RCT | 50–74 years; | 734 | Intervention group determined by CRISP-calculated 5-year CRC risk: 2.5% as threshold from biennial FOBT testing to 5-yearly colonoscopy; Control group determined by family history in accordance with the NHMRC-endorsed guidelines | At baseline, 1, 6, and 12 months post-randomization. | |
| Saya (2022) [40] | Australia | 2022 | ** RCT | 45–70 years | 274 | 10-years risk: <1%; 1–4%; ≥4% | Risk <1%: no screening recommended; Risk 1–4%: iFOBT Risk ≥4%: colonoscopy. | |
| Schroy (2016) [35] | United States | 2012–2014 | ** RCT | 50–75 years; due for CRC screening in an urban safety net Hhealthcare setting | 352 | Low risk: cumulative scores <5; Intermediate/high risk: cumulative scores: 6–12; | No screening intervals specified. | |
| Trevena (2022) [39] | Australia | 2012–2014 | Cluster RCT | 25–74 years | 1495 | Lifetime risk: At or slightly above average risk; Moderately increased risk; Potentially high risk. | At or slightly above average risk: ≥50 years—FOBT/2 years; Moderately increased risk: 1 colonoscopy in past 5 years if >50 years old, or if older than 10 years, earlier than youngest bowel cancer diagnosis in first-degree relatives. Potentially high risk: assessment by familial cancer service (or similar) OR colonoscopy at a frequency consistent with family history (as assessed by a panel of clinicians). | |
| Prostate | Fredsøe (2020) [36] | Denmark | 2013–2014 | Cluster RCT | Men; 18–80 years; Normal PSA test result (normal PSA: <3.0 ng/mL for men below age 60; <4.0 ng/mL for men aged 60–70; <5.0 ng/mL for men aged ≥70) | 5000 (actual number registered at clinicaltrials.gov) | Lifetime risk: High risk: ≥30% Normal risk: <30% Unknown risk: cannot be estimated due to missing information for family history | No screening intervals specified; participants were followed at 2 years. |
| Shah (2021) [47] | United States | 2016–2018 | Pre-post study | Men; 40–75 years | Pre: 49,053; Post: 49,980 | High risk: African-Americans, with family history of prostate cancer/abnormal genetic evaluation | 40–49 years: PSA ≥ 1.5 ng/mL—refer to multidisciplinary prostate screening clinic; PSA < 1.5 ng/mL and high risk—screen/2 years; PSA < 1.5 ng/mL and average risk—resume screening at age 50; 50–69 years: PSA < 3 ng/mL—screen/2 years; PSA ≥ 3 ng/mL—refer to multidisciplinary prostate screening clinic; 70–75 years: PSA ≤ 6.5 ng/mL—refer to multidisciplinary prostate screening clinic; PSA > 6.5 ng/mL—screen/2 years. | |
| Lung | Field (2015) [37] | United Kingdom | 2011 | ** RCT | 50–75 years; 5-year lung cancer risk of ≥5% based on the LLPv2 risk prediction model | 4055 | LLPv2 risk prediction model selects subjects with ≥5% risk of developing lung cancer in 5 years |
|
| Ovary | Gaba (2020) [44] | United Kingdom | 2017 | Feasibility | Women; ≥18 years; No ovarian/tubal/primary peritoneal cancer or ovarian cancer susceptibility genes | 123 | Lifetime risk: Low risk: <5%; Intermediate risk: 5–10%; High risk: ≥10% | No screening intervals specified; |
| Cancer (s) | Author (Year) | Questionnaire | Imaging, Blood, or Saliva Test | Polygenic Score | Model | Combinations |
|---|---|---|---|---|---|---|
| Breast | Brooks (2021) [46] | ✓ | ✓ (Mammographic density) | ✓ Single-nucleotide polymorphisms | ✓ BOADICEA model | ✓ |
| Esserman (2017) [38] | ✓ | ✓ | ✓ | ✓ Breast Cancer Surveillance Consortium (BCSC) model | ✓ | |
| Evans (2023) [43] | ✓ | ✓ (Mammographic density) | ✓ Single-nucleotide polymorphisms | ✓ Tyrer–Cuzick risk model | ✓ | |
| Laza-Vasquez (2022) [45] | ✓ | ✓ (Mammographic density) | ✓ Single-nucleotide polymorphisms | ✓ Breast Cancer Surveillance Consortium (BCSC) model V2.0 | ✓ | |
| Liu (2022) [41] | ✓ | ✓ (Mammographic density) | ✓ Single-nucleotide polymorphisms | ✓ BOADICEA model Gail model | ✓ | |
| Rainey (2022) [48] | ✓ | ✓ (Mammographic density) | ✓ Single-nucleotide polymorphisms | ✓ Tyrer– Cuzick (TC) risk prediction model | ✓ | |
| Yen (2016) [42] | ✓ | X | X | X | X | |
| Colorectal | Chen (2023) [34] | ✓ [Asia-Pacific Colorectal Screening (APCS) score] | X | X | X | X |
| Emery (2023) [11] | ✓ [Colorectal cancer RISk Prediction (CRISP) risk tool] | X | X | X | X | |
| Saya (2022) [40] | ✓ | X | ✓ Single-nucleotide polymorphisms | X | ✓ | |
| Schroy (2016) [35] | ✓ (6-item risk assessment tool) | X | X | X | X | |
| Trevena (2022) [39] | ✓ [Colorectal cancer (CRC risk calculator)] | X | X | X | X | |
| Prostate | Fredsøe (2020) [36] | ✓) | ✓ (PSA test) | ✓ Single-nucleotide polymorphisms | X | ✓ |
| Shah (2021) [47] | ✓ | ✓ (PSA test) | X | X | ✓ | |
| Lung | Field (2015) [37] | ✓ | ✓ [Computed tomography (CT) scan] | X | ✓ LLPv2 risk prediction model | ✓ |
| Ovary | Gaba (2020) [44] | ✓ | X | ✓ Single-nucleotide polymorphisms | ✓ (Epidemiological/hormonal/reproductive data combined with genetic information) | ✓ |
| Cancer (s) | Author (Year) | Risk Thresholds | Screening Pathway |
|---|---|---|---|
| Breast | Brooks (2021) [46] | 3 risk categories based on 10-year/remaining lifetime risk | 6 pathways based on 2 age categories and 3 risk levels |
| Esserman (2017) [38] | 3 risk categories based on 5-year risk | 3 pathways based on age, risk levels and breast density | |
| Evans (2023) [43] | 4 risk categories | No screening intervals specified; participants were followed at baseline, 3 months, and 6 months | |
| Laza-Vasquez (2022) [45] | 3 risk categories | 8 groups based on age and risk levels | |
| Liu (2022) [41] | 2 risk categories | 3 groups based on age and risk threshold levels | |
| Rainey (2022) [48] | 4 risk categories | 4 groups based on age and risk threshold levels | |
| Yen (2016) [42] | 2 risk categories | 3 groups based on risk threshold levels | |
| Colorectal | Chen (2023) [34] | 2 risk categories | 2 groups based on risk threshold levels |
| Emery (2023) [11] | 2 risk categories | 2 groups based on risk threshold levels | |
| Saya (2022) [40] | 3 risk categories | 2 groups based on risk threshold levels | |
| Schroy (2016) [35] | 2 risk categories | No screening intervals specified | |
| Trevena (2022) [39] | 3 risk categories | 3 groups based on age, family history of cancer, and risk threshold levels | |
| Prostate | Fredsøe (2020) [36] | 3 risk categories | No screening intervals specified; participants were followed at 2-year |
| Shah (2021) [47] | 2 risk categories | 7 groups based on age, PSA levels, and risk threshold levels | |
| Lung | Field (2015) [37] | 2 risk categories | 5 groups based on nodule classifications |
| Ovary | Gaba (2020) [44] | 3 risk categories | No screening intervals specified |
| Study Name (Registration No.) | Primary Outcome | Measurements | Main Results | |
|---|---|---|---|---|
| Breast | PERSPECTIVE I&I 1 (Not found) [46] | Acceptability and feasibility of risk-based screening, uptake of genetic testing for risk assessment and screening behaviors. | Identification of new predisposition genes; Assessment of different recruitment and data-collection strategies using questionnaires; Assessment of acceptability and healthcare system readiness through survey and online forums; Economic analysis using administrative data. | Not yet available. |
| WISDOM (NCT02620852) [38] | Safety (non-inferiority). | Rate of stage IIB cancers or higher diagnosed in annual vs. risk-based screening arms. | Not yet available (estimated completed date: 1 December 2024). | |
| Morbidity. | Rate of recall and breast biopsy between arms. | |||
| BC-Predict (NCT04359420) [43] | Feasibility. | Screening attendance at or within 180 days of the initial screening appointment; Uptake to BC-Predict of those attending screening; Time to provision of risk feedback letter and proportion over 8-week threshold; Subsequent consultation in clinics (telephone, as face-to-face not possible); Subsequent enrolment for more frequent mammography (NICE approved through clinics to age 60 or self-funded outside); Subsequent prescription of breast-cancer-preventive-medication. | Overall uptake of BC-Predict in screening attendees was 16.9%; 76.8% of those received risk feedback within the 8-week timeframe. | |
| DECIDO (NCT03791008) [45] | Attitude towards personalized breast cancer screening. | Attitude was measured with a three-item scale, each item ranging from 1 to 5, with higher scores indicating more positive attitudes. A “positive attitude” was defined as a total score greater than or equal to 12. | High positive attitude score towards personalized screening, with a median of 12. 2 out of 3 women scored > 12 indicting positive attitudes towards personalized screening; The intention to participate in personalized breast screening rated as “definitely will” or “likely to” by 9 out of 10 women; 97% of the women declared satisfied/very satisfied with personalized breast screening; 2.6% were not sure; 1 was very unsatisfied. | |
| Intention to participate in personalized breast cancer screening. | Intention to participate was measured with a 5-point Likert scale from definitely will (1) to definitely will not (5). | |||
| Satisfaction with personalized breast cancer screening. | Satisfaction was measured on a 5-point Likert scale from very unsatisfied to very satisfied. | |||
| BREATHE (Not found) 2 [41] | Acceptability, willingness, and cost-effectiveness. | Satisfaction survey | Not yet available. | |
| PROCAS (ISRCTN91372184) [48] | Key factors in participants’ adoption of screening and prevention recommendations after cancer risk communication. | Early detection behaviors:
| Predictors of intent to request supplemental mammography outside the national screening program includes the following:
| |
| Not found 3 [42] | Effectiveness | Detection (diagnosis) rates; Stage II+ disease incidence; Mortality from breast cancer; Overdiagnosis | Cancer detection rates were highest for universal biennial mammography (4.9% and 3.0%, respectively), followed by risk-based mammography (2.8% and 2.8%, respectively), and lowest for annual CBE (0.97% and 0.70%, respectively). Universal biennial mammography screening, compared with annual CBE, was associated with a 41% mortality reduction (*** RR = 0.59; 95%CI, 0.48–0.73) and a 30% reduction in stage II+ breast cancer (*** RR = 0.70; 95%CI, 0.66–0.74). Risk-based mammography screening was associated with an 8%reduction in stage II+ breast cancer (*** RR = 0.92; 95%CI, 0.86–0.99) but was not associated with a statistically significant mortality reduction (*** RR = 0.86; 95%CI, 0.73–1.02). Estimates of overdiagnosis were no different from CBE for risk-based screening and 13% higher than CBE for universal mammography. | |
| Colorectal | TARGET-C (ChiCTR1800015506) [34] | Detection rate of advanced colorectal neoplasms (CRC and advanced precancerous lesions) | Advanced adenoma was defined as adenoma with at least 1 of the following features:
Both advanced adenoma and advanced serrated lesions were regarded as advanced precancerous lesions. | Colonoscopy (Arm 1): 2.76% * FIT (Arm 2): 2.17% Risk-adapted (Arm 3): 2.35% ** OR colonoscopy vs. FIT = 1.27 (95% CI: 0.99–1.63) ** OR colonoscopy vs. risk-adapted = 1.17 (95% CI:0.91–1.49) ** OR risk-adapted vs. FIT = 1.09 (95% CI:0.88–1.35) Numbers of colonoscopies to detect 1 advanced neoplasm: 15.4, 7.8, and 10.2, respectively |
| CRISP (ACTRN12616001573448) [11] | Proportion of participants who completed risk-appropriate CRC screening at 12-month follow-up | Screening participation rate was obtained from: Self-report; GP record audit; Medicare Benefits Schedule; NBCSP; Victorian Admitted Episodes Dataset (VAED). | Intervention vs. Control: 6.5% absolute increase (95%CI: –0.28–13.2); 20.3% increase in those due CRC screening during follow-up (95%CI: 10.3–30.4) | |
| SCRIPT (ACTRN12621000092897p) [40] | Impact of the SCRIPT intervention on risk-appropriate CRC screening after 12 months. | Difference between intervention and control arms in the proportion of participants who have had risk appropriate CRC screening at 12 months. follow-up. | Not available | |
| NCT01596582 [35] | Concordance between patient preference and test ordered. | Tracked using BMC’s electronic medical record ordering system. | No significant differences in concordance were observed. | |
| ACTRN12611000534987 [39] | Risk Appropriate Screening (RAS) and colorectal cancer screening uptake. | Through online CRC patient survey (24 screening and 9 patient demographic items). | The intervention significantly increased RAS in high-risk participants compared with UCG (80.0% vs. 64.0%, respectively; OR = 3.14, 95% CI: 1.25–7.96) but not in average-risk (44.9% vs. 49.5%, respectively; OR = 0.97, 95% CI: 0.99–1.12) or moderate-risk individuals (67.9% vs. 81.1%, respectively; OR = 0.40, 95% CI: 0.12–1.33). | |
| Prostate | ProCaRis (NCT01739062) [36] | Proportion of men having a repeated PSA test within 2 years. | Proportion of men having a repeated PSA test within 2 years. | At 2 years after inclusion, a total of 1218 men (34.2%) in the intervention practices and 1628 (38.4%) men in the control practices had a PSA test (OR = 0.95, 95% CI 0.78–1.14, p = 0.56); Men of high genetic risk had a higher propensity for repeated PSA testing within 2 years than men of normal genetic risk (** OR = 8.94, p < 0.01). |
| Not found 4 [47] | Evaluate the implementation of a risk-based prostate-cancer-screening algorithm. | Percent of men who met screening algorithm criteria; Percent of men with a PSA result. | Percent of men who met screening algorithm criteria: 49.3% (pre-implementation) vs. 68.0% (post-implementation) (p < 0.001); Total number of men with abnormal PSA: Pre-implementation n = 366, 2.7%; Post-implementation n = 583, 4.9%. | |
| Lung | UKLS (ISRCTN 78513845) [37] | Effectiveness of risk prediction modeling; Evaluation of volumetric analysis in the management of CT-detected nodules; Cost-effectiveness. | Population-based recruitment based on risk stratification; Study management through a web-based database; Define optimal characteristics of CT readers (radiologists vs. radiographers); Characterization of CT-detected nodules utilizing volumetric analysis; Prevalence of lung cancer at baseline; Socio-demographic factors affecting participation; Psychosocial measures; Cost-effectiveness modeling. | In total, 42 participants (2.1%) had confirmed lung cancer, 34 (1.7%) at baseline and 8 (0.4%) at the 12-month scan;
|
| Ovary | PROMISE-FS (ISRCTN54246466) [44] | Acceptability. | Responses to the decision aid questions and overall score. | Decision aid satisfaction: 92.2%; Telephone helpline use rate:13% Questionnaire response rate at six months: 75%. |
| Uptake of the study. | Number of individuals who express interest in participating in the study (by post/email/telephone). |
| Cancer (s) | Study Name/No. | Benefits | Harms | Benefit-Harm Assessment |
|---|---|---|---|---|
| Breast | PERSPECTIVE I&I 1 [46] | Not available | Not available | Not available |
| WISDOM [38] | Not available (estimated primary completed date: 1 December 2024) | Not available (estimated primary completed date: 1 December 2024) | Not available (estimated completed date: 1 December 2024) | |
| BC-Predict [43] | Risk stratification can be performed as part of routine NHS Breast Screening Programme delivery and supports the uptake of preventive medicines for women at high risk. | No evidence of adverse effects on anxiety beyond transient cancer worry; The practice of delivering risk-based screening was much less burdensome than healthcare professionals anticipated prior to Delivery. | Measures to increase uptake should be addressed, especially among women from lower socioeconomic and ethnic minority backgrounds. | |
| DECIDO [45] | Positive attitude towards personalized breast screening; expressed strong intention to participate; and very satisfied with having participated in the study. | Knowledge of the benefits and harms of breast screening was low, especially with regard to false positives and overdiagnosis. | Demonstrates the need to create tools and strategies for developing interventions that focus on raising awareness about personalized screening and increasing literacy in risk measurement. | |
| BREATHE 2 [41] | Only the study protocol is available. | Only the study protocol is available. | Only the study protocol is available. | |
| PROCAS [48] | Breast cancer risk communication predicts the uptake of key tailored primary and secondary preventive Behaviors. | Not reported | Effective communication of breast cancer risk information is essential to optimize the population wide impact of tailored screening. | |
| Not found 3 [42] | Compared with population-based screening for breast cancer with annual CBE, universal biennial mammography resulted in a substantial reduction in breast cancer deaths, whereas risk-based biennial mammography resulted in only a modest benefit. | Compared with annual CBE, risk-based and universal mammography screening did not result in significant overdiagnosis of breast cancer. | The findings should be informative to health policymakers seeking to determine if and how they might initiate breast-cancer-screening programs. | |
| Colorectal | TARGET-C [34] | The risk-adapted approach saved 33% of endoscopy resources required for detecting 1 advanced neoplasm compared with the 1-time colonoscopy screening. | Costs for detecting 1 advanced neoplasm from the societal perspective were higher in the risk-adapted screening compared with the 1-time colonoscopy screening. | The risk-adapted approach is a feasible and cost-effective strategy for population-based CRC screening; The need for additional screening after 3 rounds or an expansion the screening interval should be addressed in future studies. |
| CRISP [11] | A risk assessment and decision support tool increase risk-appropriate CRC screening in those due screening. | There were no differences between groups at any timepoint on general or cancer-specific anxiety or absolute risk perception. | Not reported | |
| SCRIPT [40] | Only the study protocol is available. | Only the study protocol is available. | Only the study protocol is available. | |
| NCT01596582 [35] | Concordance was positively associated with satisfaction with the decision-making process, screening intentions, and test completion rates. | Not specified | Providers perceived risk stratification to be useful in their decision-making but often failed to comply with patient preferences for tests other than colonoscopy. | |
| ACTRN12611000534987 [39] | Online CRC risk calculator increased risk appropriate screening in high-risk participants and improved screening uptake overall within a 12 m follow-up period. | Harms and costs of more invasive screening tests such as colonoscopy in lower risk individuals. | Online CRC risk calculator may be useful for facilitating the uptake of risk-based screening guidelines. | |
| Prostate | ProCaRis [36] | Among participants who had a genetic test, knowledge of genetic risk significantly influenced future PSA testing. | Genetic test to assess lifetime risk of prostate cancer did not reduce the overall number of future PSA test. | The follow-up period of 2 years was not long enough to draw final conclusions about the effect of this intervention on the diagnosis or mortality rate of prostate cancer. |
| Not found 4 [47] | The adjusted odds of meeting algorithm-based screening was 6.5 times higher in the post-implementation period than in the pre-implementation period. | Not reported | An increase in screening in higher-risk groups balanced with a decrease in screening in low-risk groups. | |
| Lung | UKLS [37] | It is possible to detect lung cancer at an early stage and deliver potentially curative treatment in over 80% of cases; Health economic analysis suggests that the intervention would be cost effective. | False-positive rate: 3.6% Interval imaging rate: 23.2% | CT screening in the United Kingdom is possible using a risk prediction model that avoids the selection of people at very low risk who are unlikely to benefit, and a nodule management algorithm that effectively manages indeterminate CT findings, yet detects a high number of early-stage lung cancers. |
| Ovary | PROMISE-FS [44] | 85.5–98.7% were satisfied with their decisions; Ovarian cancer related worry (p = 0.021) and general cancer risk perception (p = 0.015) decreased over 6 months. | There were no significant effects on overall depression (p = 0.30), anxiety (p = 0.10), quality of life (p = 0.99), or distress level (p = 0.26). | Population-based personalized ovarian cancer risk stratification is feasible and acceptable, has high satisfaction, reduces cancer worry/risk perception, and does not negatively impact psychological health/quality of life. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Smit, A.K.; Cust, A.E.; Janda, M. Targeted Screening for Cancer: Learnings and Applicability to Melanoma: A Scoping Review. J. Pers. Med. 2024, 14, 863. https://doi.org/10.3390/jpm14080863
Zheng L, Smit AK, Cust AE, Janda M. Targeted Screening for Cancer: Learnings and Applicability to Melanoma: A Scoping Review. Journal of Personalized Medicine. 2024; 14(8):863. https://doi.org/10.3390/jpm14080863
Chicago/Turabian StyleZheng, Lejie, Amelia K. Smit, Anne E. Cust, and Monika Janda. 2024. "Targeted Screening for Cancer: Learnings and Applicability to Melanoma: A Scoping Review" Journal of Personalized Medicine 14, no. 8: 863. https://doi.org/10.3390/jpm14080863
APA StyleZheng, L., Smit, A. K., Cust, A. E., & Janda, M. (2024). Targeted Screening for Cancer: Learnings and Applicability to Melanoma: A Scoping Review. Journal of Personalized Medicine, 14(8), 863. https://doi.org/10.3390/jpm14080863





