Clinicopathological Findings and Comprehensive Review of Buschke–Lowenstein Tumors Based on a Case Study
Abstract
:1. Introduction
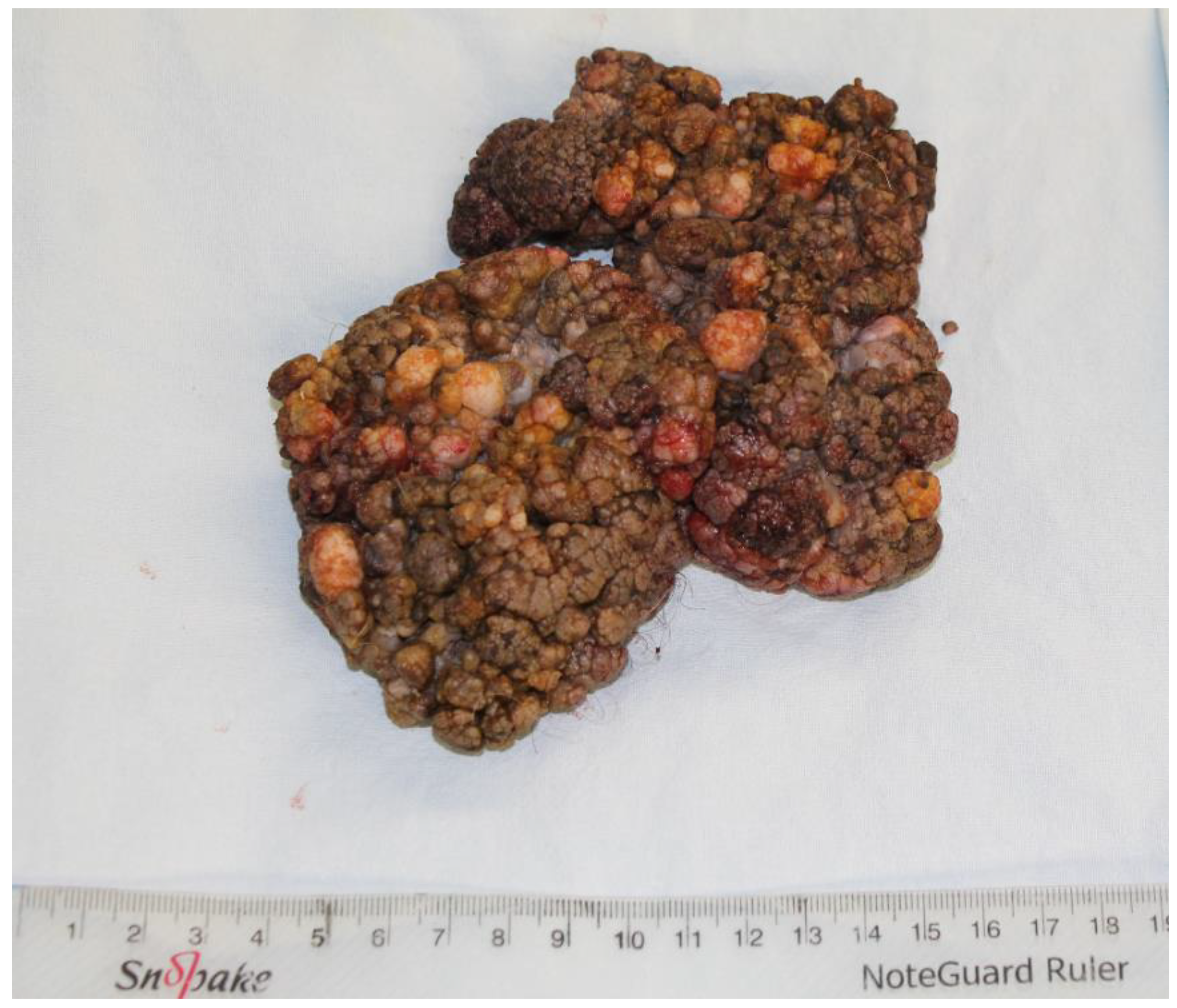
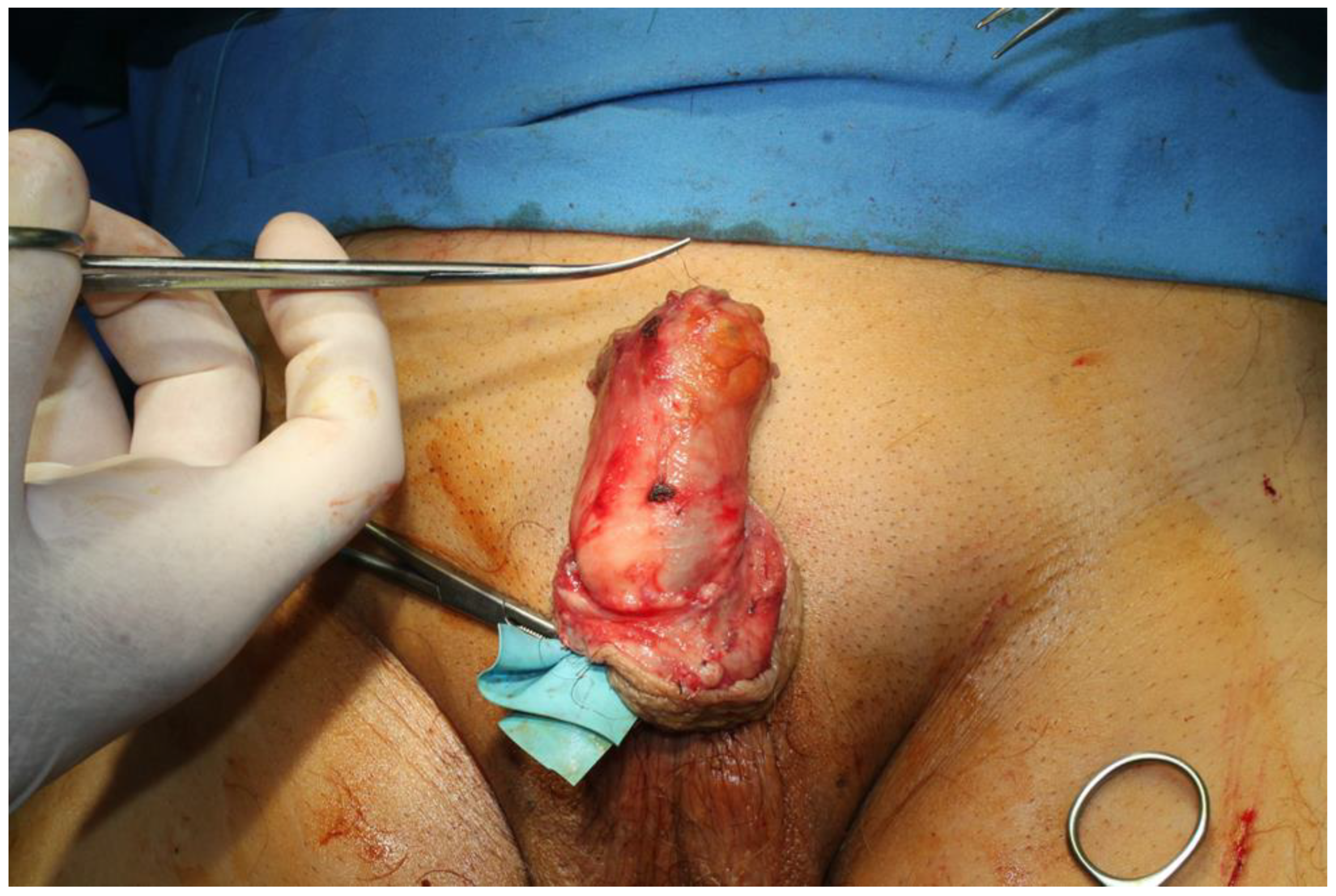
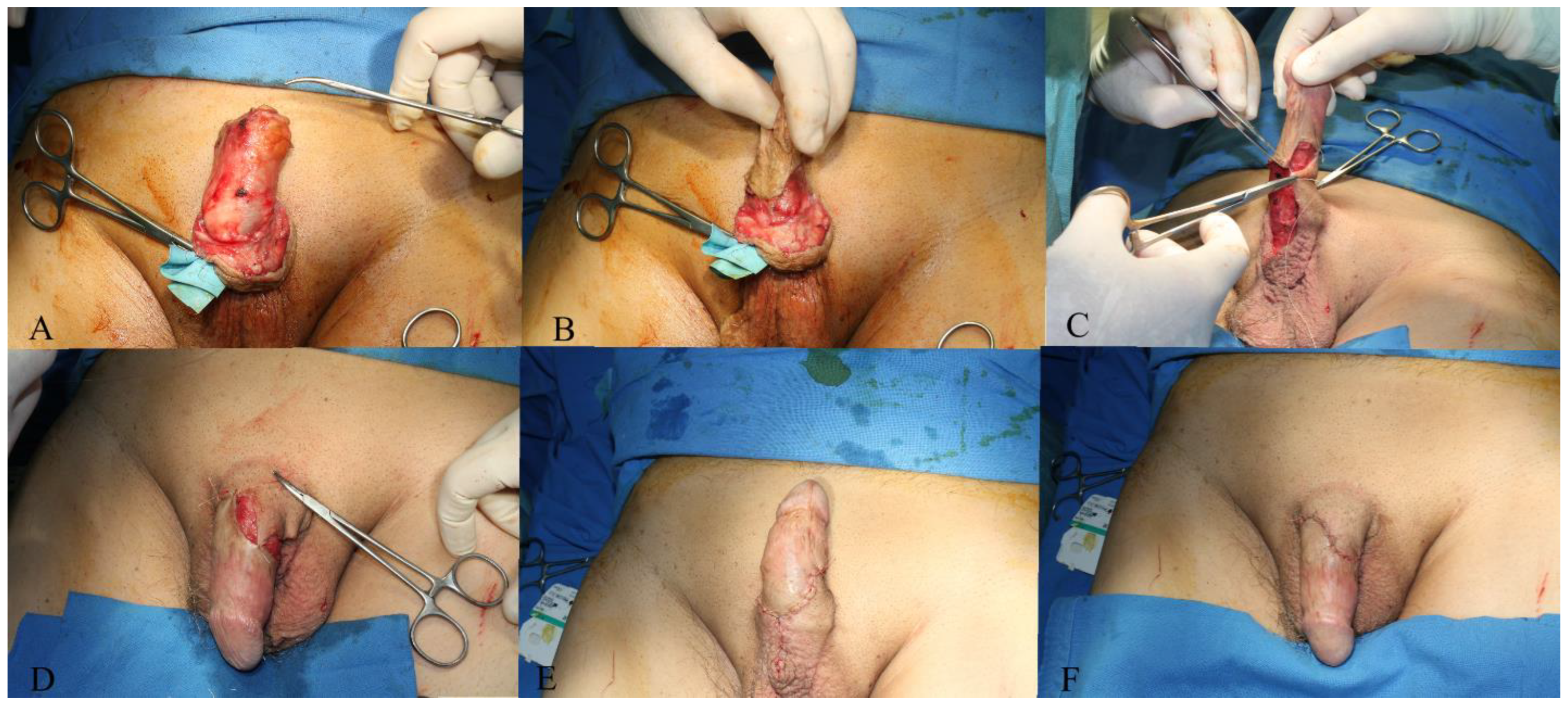
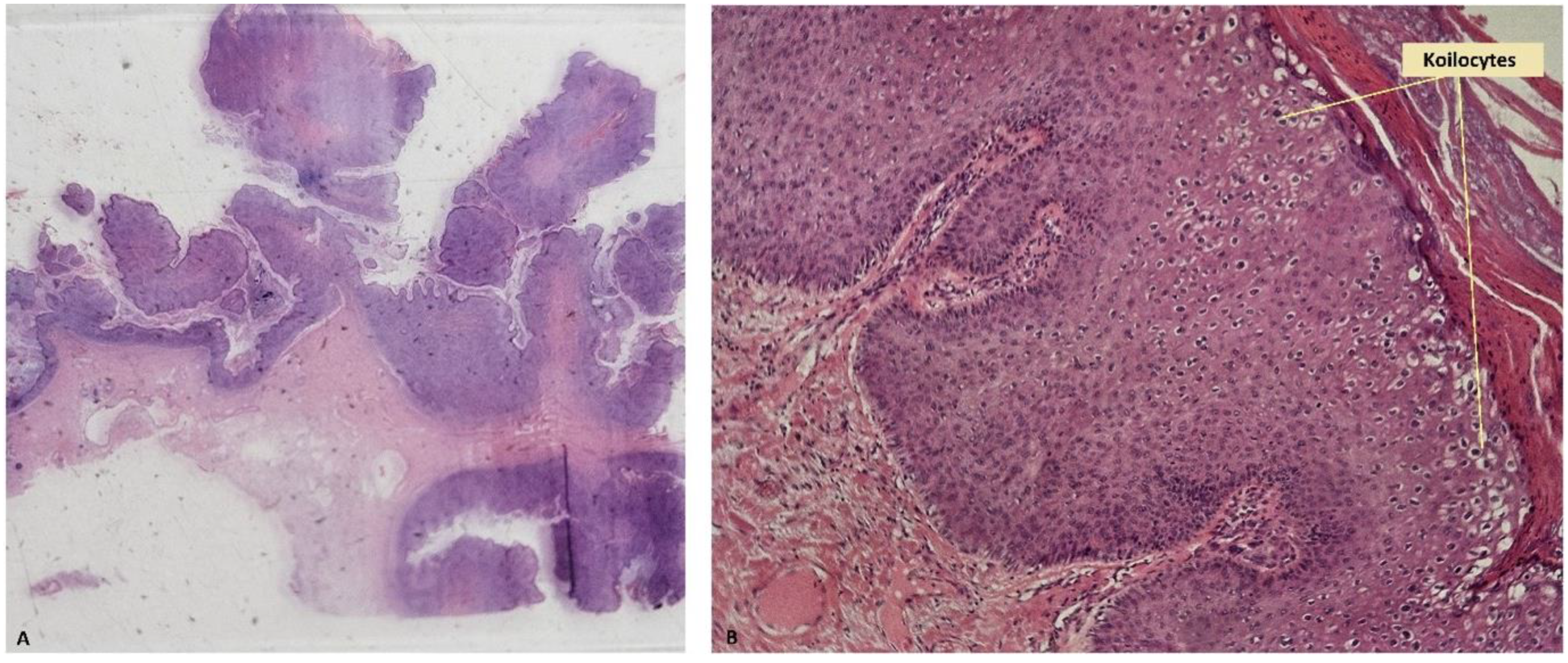
2. Case Presentation
3. Discussion and Literature Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa Almeida, C.E.; Azevedo, J.; Botelho, I.; Vilaça, J. Buschke-Löwenstein tumour: A rare and challenging entity. BMJ Case Rep. 2021, 14, e244192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buschke, A.; Loewenstein, L. Über Carcinomähnliche Condylomata Acuminata des penis. Klin. Wochenschr. 1925, 4, 1726–1728. [Google Scholar] [CrossRef]
- Irshad, U.; Puckett, Y. Giant Condylomata Acuminata of Buschke and Lowenstein. [Updated 14 Apr 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560714/ (accessed on 13 July 2024).
- Nieves-Condoy, J.F.; Acuña-Pinzón, C.L.; Chavarría-Chavira, J.L.; Hinojosa-Ugarte, D.; Zúñiga-Vázquez, L.A. Giant Condyloma Acuminata (Buschke-Lowenstein Tumor): Review of an Unusual Disease and Difficult to Manage. Infect. Dis. Obstet. Gynecol. 2021, 30, 9919446. [Google Scholar] [CrossRef] [PubMed]
- Mihailov, R.; Tatu, A.L.; Niculet, E.; Olaru, I.; Manole, C.; Olaru, F.; Mihailov, O.M.; Guliciuc, M.; Beznea, A.; Bușilă, C.; et al. Surgical Management of Perianal Giant Condyloma Acuminatum of Buschke and Löwenstein: Case Presentation. Life 2023, 13, 1916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ajagbe, O.A.; Ayandipo, O.O.; Afuwape, O.O.; Idowu, O.K.; Adeleye, A.O.; Ogundiran, T.O. Surgical treatment of perineal giant condylomata acuminata (Buschke Lowenstein tumor): Case series from a developing country. Int. J. Surg. Case Rep. 2024, 121, 109994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rydzewska-Rosołowska, A.; Kakareko, K.; Kowalik, M.; Zaręba, K.; Zbroch, E.; Hryszko, T. An unexpected giant problem—Giant condyloma (Buschke-Lowenstein tumor). Int. J. Infect. Dis. 2021, 103, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Purzycka-Bohdan, D.; Nowicki, R.J.; Herms, F.; Casanova, J.L.; Fouéré, S.; Béziat, V. The Pathogenesis of Giant Condyloma Acuminatum (Buschke-Lowenstein Tumor): An Overview. Int. J. Mol. Sci. 2022, 23, 4547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ntanasis-Stathopoulos, I.; Kyriazoglou, A.; Liontos, M.; ADimopoulos, M.; Gavriatopoulou, M. Current trends in the management and prevention of human papillomavirus (HPV) infection. J. BUON 2020, 25, 1281–1285. [Google Scholar] [PubMed]
- Smola, S. Immunopathogenesis of HPV-Associated Cancers and Prospects for Immunotherapy. Viruses 2017, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Mattoscio, D.; Gheit, T.; Strati, K.; Venuti, A. Editorial: HPV and Host Interaction. Front. Cell Infect. Microbiol. 2021, 11, 638005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Templeton, C.W.; Laimins, L.A. p53-dependent R-loop formation and HPV pathogenesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2305907120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussain, S.S.; Lundine, D.; Leeman, J.E.; Higginson, D.S. Genomic Signatures in HPV-Associated Tumors. Viruses 2021, 13, 1998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mlynarczyk-Bonikowska, B.; Rudnicka, L. HPV Infections-Classification, Pathogenesis, and Potential New Therapies. Int. J. Mol. Sci. 2024, 25, 7616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, S.; Nirwal, G.K.; Singh, H. Buschke-Lowenstein tumour of glans penis. Int. J. Surg. Case Rep. 2014, 5, 215–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pineda-Murillo, J.; Lugo-García, J.A.; Martínez-Carrillo, G.; Torres-Aguilar, J.; Viveros-Contreras, C.; Schettino-Peredo, M.V. Buschke–Löwenstein tumor of the penis. Afr. J. Urol. 2019, 25, 9. [Google Scholar] [CrossRef]
- Balla, D.; Ba, P.A.; Diouf, A.; NDiaye, B.; Sarre, S.M.; Sylla, C. Peri-Anal and Genital Localization of Giant Condyloma Acuminatum or Buschke-Lowenstein’s Tumor: A Report of Two Cases. J. West Afr. Coll. Surg. 2020, 10, 42–46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Gutiérrez, R.; Rodarte-Shade, M.; González-Saldivar, G.; González-González, J.G. Buschke-Lowenstein tumor. Am. J. Med. Sci. 2015, 349, e1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gonzalez, R.S.; Feely, M.; Umrau, K.; Lee, H.; Allende, D.S.; Karamchandani, D.M.; Zaleski, M.; Lin, J.; Westerhoff, M.; et al. Clinicopathologic features of Buschke-Löwenstein tumor: A multi-institutional analysis of 38 cases. Virchows Arch. 2020, 476, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Balogh, N.; Kolozsi, P.; Tóth, D. A rare malignancy: A case report of early progression of anal Buschke-Löwenstein tumor into squamous cell carcinoma in an immunocompetent patient. Int. J. Surg. Case Rep. 2024, 119, 109715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boda, D.; Cutoiu, A.; Bratu, D.; Bejinariu, N.; Crutescu, R. Buschke-Löwenstein tumors: A series of 7 case reports. Exp. Ther. Med. 2022, 23, 393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pennycook, K.B.; McCready, T.A. Condyloma Acuminata. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547667/ (accessed on 10 July 2024).
- El Khoury, A.; Jensen, J.C.; Pacioles, T. Neoadjuvant chemotherapy and penile conservation in the management of Buschke—Lowenstein tumor, a case report. Urol. Case Rep. 2019, 29, 101111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safi, F.; Bekdache, O.; Al-Salam, S.; Alashari, M.; Mazen, T.; El-Salhat, H. Management of peri-anal giant condyloma acuminatum—A case report and literature review. Asian J. Surg. 2013, 36, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Leventoglu, S.; Yildiz, A.; Inan, A.; Mentes, B.B. Radical surgical management of perianal giant condyloma acuminatum of Buschke and Löwenstein: Long-term results of 11 cases. Ann. Coloproctol. 2023, 39, 204–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kosmidis, C.; Sevva, C.; Magra, V.; Varsamis, N.; Koulouris, C.; Charalampous, I.; Papadopoulos, K.; Roulia, P.; Dagher, M.; Theodorou, V.; et al. HPV-Induced Anal and Peri-Anal Neoplasia, a Surgeon’s Experience: 5-Year Case Series. Diagnostics 2023, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Kowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef] [PubMed Central]
- Kowo, M.; Nzoume Nsope Mengang, J.M.; Simeni Njonnou, S.R.; Kouotou, E.A.; Atangana, P.J.A.; Ankouane Andoulo, F. Giant anogenital tumor of Buschke-Löwenstein in a patient living with human immunodeficiency virus/acquired immunodeficiency syndrome: A case report. J. Med. Case Rep. 2022, 16, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dos Santos, L.M.; de Souza, J.D.; Mbakwa, H.A.; Nobre, A.F.S.; Vieira, R.C.; Ferrari, S.F.; Rodrigues, A.R.; Ishikawa, E.A.Y.; Guerreiro, J.F.; de Sousa, M.S. High prevalence of sexual infection by human papillomavirus and Chlamydia trachomatis in sexually-active women from a large city in the Amazon region of Brazil. PLoS ONE 2022, 17, e0270874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldstone, S.; Palefsky, J.M.; Giuliano, A.R.; Moreira, E.D., Jr.; Aranda, C.; Jessen, H.; Hillman, R.J.; Ferris, D.G.; Coutlee, F.; Liaw, K.-L.; et al. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J. Infect. Dis. 2011, 203, 66–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wulandari, D.; Meidyandra, R.W.; Andrijono. Genotype profiles of high-risk human papillomavirus in women of reproductive age: A community-based study. PLoS ONE 2023, 18, e0287399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hewavisenti, R.V.; Arena, J.; Ahlenstiel, C.L.; Sasson, S.C. Human papillomavirus in the setting of immunodeficiency: Pathogenesis and the emergence of next-generation therapies to reduce the high associated cancer risk. Front. Immunol. 2023, 14, 1112513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ates, M.; Akbulut, S.; Tuncer, A.; Sahin, E.; Karabulut, E.; Sarici, K.B. Squamous Cell Carcinoma Arising from Perianal Buschke-Lowenstein Tumor (Giant Condyloma Acuminatum): Comprehensive Literature Review. J. Gastrointest. Cancer 2022, 53, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Achdiat, P.A.; Maharani, R.H.; Hamada, N.; Dwiyana, R.F.; Tsaqilah, L.; Usman, H.A.; Zulfan. Vulvar squamous cell carcinoma due to human papillomavirus type 11. Ski. Res. Technol. 2023, 29, e13436. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.L.D.; Teles, A.M.; Santos, J.M.O.; Souza de Andrade, M.; Medeiros, R.; Faustino-Rocha, A.I.; Oliveira, P.A.; Dos Santos, A.P.A.; Ferreira Lopes, F.; Braz, G.; et al. Malignancy Associated with Low-Risk HPV6 and HPV11: A Systematic Review and Implications for Cancer Prevention. Cancers 2023, 15, 4068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kidd, L.C.; Chaing, S.; Chipollini, J.; Giuliano, A.R.; Spiess, P.E.; Sharma, P. Relationship between human papillomavirus and penile cancer-implications for prevention and treatment. Transl. Androl. Urol. 2017, 6, 791–802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jorgaqi, E.; Jafferany, M. Giant condyloma acuminatum (Buschke-Lowenstein tumor): Combined treatment with surgery and chemotherapy. Dermatol. Ther. 2020, 33, e13193. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, Y.; Wang, H.; Chen, B.O.; Wang, Z.; Zhao, Y.; Zhu, S.; Chen, G. Diagnosis and treatment of penile verrucous carcinoma. Oncol. Lett. 2015, 9, 1687–1690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lau, W.D.; Ong, C.H.; Lim, T.P.; Teo, C. Penile cancer: A local case series and literature review. Singap. Med. J. 2015, 56, 637–640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hariga, C.S.; Achim, S.C.; Savu, A.C.; Enache, V.; Jecan, C.R. A case of extrapleural solitary fibrous tumor of the thigh with eight years follow-up. Rom. J. Morphol. Embryol. 2016, 57, 303–306. [Google Scholar] [PubMed]
- Putra, A.A.; Bramono, I.A.; Harahap, E.U.; Santoso, R.B.; Suzanna, E.; Rahman, F. Malignant solitary fibrous tumor of the penis: A case report of rare malignancy. Urol. Case Rep. 2023, 49, 102418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castellani, D.; Sebastiani, G.; Maurelli, S.; Andrisano, A.; Mazzone, L.; Feroce, A.; Primi, F.; Verrico, G.; Rizzotto, A. Solitary fibrous tumor/hemangiopericytoma of the penis: Report of the first case. Urol. J. 2015, 82, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Chokoeva, A.; Tchernev, G.; Cardoso, J.; Patterson, J.; Dechev, I.; Valkanov, S.; Zanardelli, M.; Lotti, T.; Wollina, U. Vulvar sarcomas: Short guideline for histopathological recognition and clinical management. Part 1. Int. J. Immunopathol. Pharmacol. 2015, 28, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Venyo, A.K.G. Kaposi’s sarcoma of the penis: A review of the literature and update. MOJ Tumor. Res. 2018, 1, 187–195. [Google Scholar]
- Hoyos, J.A.; Catano, J.G.; Serrano, J.; Meek, E. Primary Radiation-Induced Sarcoma of the Penis: Case Report and Review of the Literature. Int. Arch. Urol. Complic. 2022, 8, 085. [Google Scholar] [CrossRef]
- Elsayed, A.G.; Sola-Rufai, S.T.; Griswold, D.; Pacioles, T. Verrucous carcinoma arising in a long standing Buschke-Löwenstein tumor. Clin. Case Rep. 2019, 7, 836–838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leone, P.; Carvajal, M. Buschke–Löwenstein Tumor: Presentation of a Case. Rev. Oncol. 2022, 32, 253–265. [Google Scholar] [CrossRef]
- Fischer, A. Fundamentals of Cytological Diagnosis and Its Biological Basis. In Pathobiology of Human Disease [Internet]; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3311–3344. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780123864567065011 (accessed on 9 July 2024).
- Knottenbelt, D.C.; Patterson-Kane, J.C.; Snalune, K.L. Squamous cell carcinoma. In Clinical Equine Oncology [Internet]; Elsevier: Amsterdam, The Netherlands, 2015; pp. 220–236. Available online: https://linkinghub.elsevier.com/retrieve/pii/B978070204266900012X (accessed on 11 July 2024).
- Ledouble, V.; Sclafani, F.; Hendlisz, A.; Gomez Galdon, M.; Liberale, G. Buschke-Löwenstein tumor in a human immunodeficiency virus-positive patient: A case report and short literature review. Acta Gastroenterol. Belg. 2021, 84, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, Z.; Radovanovic, D.; Semnic, R.; Nikin, Z.; Petrovic, T.; Kukic, B. Highly aggressive Buschke-löwenstein tumor of the perineal region with fatal outcome. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 648–650. [Google Scholar] [CrossRef]
- Russano, F.; Russo, I.; Del Fiore, P.; Di Prata, C.; Mocellin, S.; Alaibac, M. Bleomycin-based electrochemotherapy for the treatment of a Buschke-Löwenstein tumor (perianal giant condyloma) in an HIV-positive kidney transplant recipient: A case report. Oncol. Lett. 2022, 24, 466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gholam, P.; Enk, A.; Hartschuh, W. Successful surgical management of giant condyloma acuminatum (Buschke-Löwenstein tumor) in the genitoanal region: A case report and evaluation of current therapies. Dermatology 2009, 218, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Ulas, M.; Bostanci, E.B.; Teke, Z.; Karaman, K.; Ercan, M.; Sakaogullari, Z.; Akoglu, M. Giant anorectal condyloma acuminatum of buschke-lowenstein: Successful plastic reconstruction with bilateral gluteal musculocutaneous v-y advancement flap. Indian J. Surg. 2013, 75 (Suppl. 1), 168–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nthumba, P.M.; Ngure, P.; Nyoro, P. Giant condyloma acuminatum of the scrotum in a man with AIDS: A case report. J. Med. Case Rep. 2011, 5, 272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atkinson, A.L.; Pursell, N.; Sisay, A. The giant condyloma (buschke-löwenstein tumor) in the immunocompromised patient. Case Rep. Obstet. Gynecol. 2014, 2014, 793534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reichert, M.; Eckerth, L.; Fritzenwanker, M.; Imirzalioglu, C.; Amati, A.L.; Askevold, I.; Padberg, W.; Hecker, A.; Liese, J.; Bender, F. New Perianal sis Risk Score Predicts Outcome of Elderly Patients with Perianal Abscesses. J. Clin. Med. 2023, 12, 5219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bender, F.; Eckerth, L.; Fritzenwanker, M.; Liese, J.; Askevold, I.; Imirzalioglu, C.; Padberg, W.; Hecker, A.; Reichert, M. Drug resistant bacteria in perianal abscesses are frequent and relevant. Sci. Rep. 2022, 12, 14866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hodea, F.V.; Lazarescu, A.L.; Grosu-Bularda, A.; Cretu, A.; Teodoreanu, R.N.; Lascar, I.; Hariga, C.S. Antimicrobial resistance of ESKAPE pathogens in major burns patients—One-year retrospective study. Farmacia 2023, 71, 549–555. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Esposito, S.; Bassetti, M.; Concia, E.; De Simone, G.; De Rosa, F.G.; Grossi, P.; Novelli, A.; Menichetti, F.; Petrosillo, N.; Tinelli, M.; et al. Italian Society of Infectious and Tropical Diseases. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: An update. J. Chemother. 2017, 29, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Abu-Baker, A.; Țigăran, A.E.; Peligrad, T.; Ion, D.E.; Gheoca-Mutu, D.E.; Avino, A.; Hariga, C.S.; Moraru, O.E.; Răducu, L.; Jecan, R.C. Exploring an Innovative Approach: Integrating Negative-Pressure Wound Therapy with Silver Nanoparticle Dressings in Skin Graft Procedures. J. Pers. Med. 2024, 14, 206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-González, A.; Cachay, E.; Ocampo, A.; Poveda, E. Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection. Microorganisms 2022, 10, 1047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mukherjee, A.G.; Wanjari, U.R.; Gopalakrishnan, A.V.; Kannampuzha, S.; Murali, R.; Namachivayam, A.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; et al. Exploring the Molecular Pathogenesis, Pathogen Association, and Therapeutic Strategies against HPV Infection. Pathogens 2022, 12, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ardhaputri, K.A.D.L.; Lestari, D.P.O.; Armerinayanti, N.W.; Sari, N.L.P.E.K.; Laksmini, L.Y.; Luhur, I.M.J.; Upadana, I.N.; Dewi, K.P. The Proportion of p16 Expression in Penile Squamous Cell Carcinoma Based on Immunohistochemistry Examination in the Balinese Population. Indones. J. Cancer 2022, 16, 94. [Google Scholar] [CrossRef]
- Zargar-Shoshtari, K.; Sharma, P.; Spiess, P.E. Insight into novel biomarkers in penile cancer: Redefining the present and future treatment paradigm? Urol. Oncol. Semin. Orig. Investig. 2018, 36, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.; Singh, S.; O’Hanlon, C.; Shiatis, V.; Brunckhorst, O.; Muneer, A.; Ahmed, K. A systematic review of non-HPV prognostic biomarkers used in penile squamous cell carcinoma. Turk. J. Urol. 2021, 47, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Mrouj, K.; Andrés-Sánchez, N.; Dubra, G.; Singh, P.; Sobecki, M.; Chahar, D.; Al Ghoul, E.; Aznar, A.B.; Prieto, S.; Pirot, N.; et al. Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2026507118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shetty, M.; Adiga, D.S.A.; Chaithra, G.V. Study of Expression of P16 in Premalignant and Malignant Lesions of Penis and Their Significance. Iran. J. Pathol. 2024, 19, 50–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parza, K.; Mustasam, A.; Ionescu, F.; Paravathaneni, M.; Sandstrom, R.; Safa, H.; Grass, G.D.; Johnstone, P.A.; Eschrich, S.A.; Chadha, J.; et al. The Prognostic Role of Human Papillomavirus and p16 Status in Penile Squamous Cell Carcinoma-A Systematic Review. Cancers 2023, 15, 3713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu-Bularda, A.; Hariga, C.-S.; Dumitru, C.-S.; Calcaianu, N.; Creanga, C.-A.; Enache, V.; Tache, S.-E.; Bordeanu-Diaconescu, E.-M.; Ratoiu, V.-A.; Teodoreanu, R.-N.; et al. Clinicopathological Findings and Comprehensive Review of Buschke–Lowenstein Tumors Based on a Case Study. J. Pers. Med. 2024, 14, 887. https://doi.org/10.3390/jpm14080887
Grosu-Bularda A, Hariga C-S, Dumitru C-S, Calcaianu N, Creanga C-A, Enache V, Tache S-E, Bordeanu-Diaconescu E-M, Ratoiu V-A, Teodoreanu R-N, et al. Clinicopathological Findings and Comprehensive Review of Buschke–Lowenstein Tumors Based on a Case Study. Journal of Personalized Medicine. 2024; 14(8):887. https://doi.org/10.3390/jpm14080887
Chicago/Turabian StyleGrosu-Bularda, Andreea, Cristian-Sorin Hariga, Catalina-Stefania Dumitru, Nicolae Calcaianu, Cosmin-Antoniu Creanga, Valentin Enache, Silvia-Elena Tache, Eliza-Maria Bordeanu-Diaconescu, Vladut-Alin Ratoiu, Razvan-Nicolae Teodoreanu, and et al. 2024. "Clinicopathological Findings and Comprehensive Review of Buschke–Lowenstein Tumors Based on a Case Study" Journal of Personalized Medicine 14, no. 8: 887. https://doi.org/10.3390/jpm14080887




