Ocular Ultrasound in the Diagnosis of Optic Neuropathies: A Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
3. Ultrasound Technique
4. Ocular Ultrasound in Glaucoma
5. Ocular Ultrasound in Acute Optic Neuritis
6. Ocular Ultrasound in Optic Neuritis Related to Demyelinating Disorders
7. Ocular Ultrasound in Other Optic Neuropathies
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biousse, V.; Newman, N.J. Diagnosis and clinical features of common optic neuropathies. Lancet Neurol. 2016, 15, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Gupta, V.K.; Li, J.C.; Klistorner, A.; Graham, S.L. Optic neuropathies: Characteristic features and mechanisms of retinal ganglion cell loss. Rev. Neurosci. 2013, 24, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Sandbach, J.M.; Newman, N.J. Retinal masqueraders of optic nerve disease. Ophthalmol. Clin. N. Am. 2001, 14, 41–59. [Google Scholar]
- Weerasinghe, D.; Lueck, C. Mimics and chameleons of optic neuritis. Pract. Neurol. 2016, 16, 96–110. [Google Scholar] [CrossRef]
- Shapey, J.; Sabin, H.I.; Danesh-Meyer, H.V.; Kaye, A.H. Diagnosis and management of optic nerve sheath meningiomas. J. Clin. Neurosci. 2013, 20, 1045–1056. [Google Scholar] [CrossRef]
- Gaier, E.D.; Boudreault, K.; Rizzo, J.F., 3rd; Falardeau, J.; Cestari, D.M. Atypical Optic Neuritis. Curr. Neurol. Neurosci. Rep. 2015, 15, 76. [Google Scholar] [CrossRef]
- Rizzo, J.F., 3rd; Lessell, S. Optic neuritis and ischemic optic neuropathy. Overlapping clinical profiles. Arch. Ophthalmol. 1991, 109, 1668–1672. [Google Scholar] [CrossRef]
- Lochner, P.; Leone, M.A.; Coppo, L.; Nardone, R.; Zedde, M.L.; Cantello, R.; Brigo, F. B-mode transorbital ultrasononography for the diagnosis of acute optic neuritis. A systematic review. Clin. Neurophysiol. 2016, 127, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Rosa, N.; De Bernardo, M.; Di Stasi, M.; Cione, F.; Capaldo, I. A-Scan Ultrasonographic Evaluation of Patients with Idiopathic Intracranial Hypertension: Comparison of Optic Nerves. J. Clin. Med. 2022, 11, 6153. [Google Scholar] [CrossRef]
- Rosa, N.; De Bernardo, M.; Abbinante, G.; Vecchio, G.; Cione, F.; Capasso, L. Optic Nerve Drusen Evaluation: A Comparison between Ultrasound and OCT. J. Clin. Med. 2022, 11, 3715. [Google Scholar] [CrossRef]
- De Bernardo, M.; Vitiello, L.; Rosa, N. A-scan ultrasonography and optic nerve sheath diameter assessment during acute elevations in intra-abdominal pressure. Surgery 2020, 167, 1023–1024. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Vitiello, L.; Rosa, N. Optic nerve sheath diameter ultrasonography in differentiation of ischemic and hemorrhagic strokes. Am. J. Emerg. Med. 2019, 37, 1384–1385. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Vitiello, L.; Capone, M.; Rosa, N. A-scan ultrasonography and optic nerve sheath diameter evaluation in children with acute liver failure. Liver Int. 2020, 40, 1504. [Google Scholar] [CrossRef]
- De Bernardo, M.; Vitiello, L.; Rosa, N. Ocular ultrasonography to detect intracranial pressure in aneurysmal subarachnoid hemorrhage. Ann. Clin. Transl. Neurol. 2020, 7, 1459–1460. [Google Scholar] [CrossRef]
- Ossoinig, K.C. Standardized echography of the optic nerve. In Documenta Ophthalmologica Proceedings Series; Ophthalmic Echography 13; Till, P., Ed.; Springer: Dordrecht, The Netherlands, 1990; Volume 55, pp. 3–99. [Google Scholar]
- De Bernardo, M.; Vitiello, L.; De Pascale, I.; Capasso, L.; Cornetta, P.; Rosa, N. Optic Nerve Ultrasound Evaluation in Idiopathic Intracranial Hypertension. Front. Med. 2022, 9, 845554. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Vitiello, L.; Rosa, N. Ultrasound optic nerve sheath diameter measurement in optic neuritis. Am. J. Emerg. Med. 2019, 37, 1595–1596. [Google Scholar] [CrossRef]
- De Bernardo, M.; Vitiello, L.; Rosa, N. Ultrasound optic nerve sheath diameter evaluation in patients undergoing robot-assisted laparoscopic pelvic surgery. J. Robot. Surg. 2019, 13, 709–710. [Google Scholar] [CrossRef]
- Abbinante, G.; Vitiello, L.; Coppola, A.; Salerno, G.; Gagliardi, V.; Pellegrino, A. Optic Nerve Ultrasound Evaluation in Children: A Review. Diagnostics 2023, 13, 535. [Google Scholar] [CrossRef]
- Vitiello, L.; De Bernardo, M.; Capasso, L.; Cornetta, P.; Rosa, N. Optic Nerve Ultrasound Evaluation in Animals and Normal Subjects. Front. Med. 2022, 8, 797018. [Google Scholar] [CrossRef]
- De Bernardo, M.; Vitiello, L.; Rosa, N. Optic nerve ultrasound measurement in multiple sclerosis. Acta Neurol. Scand. 2019, 139, 399–400. [Google Scholar] [CrossRef]
- De Bernardo, M.; Vitiello, L.; De Luca, M.; La Marca, A.; Rosa, N. Optic Nerve Changes Detected with Ocular Ultrasonography during Different Surgical Procedures: A Narrative Review. J. Clin. Med. 2022, 11, 5467. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, L.; Salerno, G.; De Bernardo, M.; D’Aniello, O.; Capasso, L.; Marotta, G.; Rosa, N. Ultrasound Detection of Intracranial Hypertension in Brain Injuries. Front. Med. 2022, 9, 870808. [Google Scholar] [CrossRef]
- Kerlen, C.H. B-scan ultrasonography in optic nerve lesions. Doc. Ophthalmol. 1982, 52, 317–325. [Google Scholar] [CrossRef]
- Abegão Pinto, L.; Vandewalle, E.; Pronk, A.; Stalmans, I. Intraocular pressure correlates with optic nerve sheath diameter in patients with normal tension glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Willekens, K.; Abegão Pinto, L.; Vandewalle, E.; Marques-Neves, C.; Stalmans, I. Higher optic nerve sheath diameters are associated with lower ocular blood flow velocities in glaucoma patients. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 477–483. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Ma, T.; Shi, W.; Zhu, Q.; Kang, J.; Wang, N. Measurement and Associations of the Optic Nerve Subarachnoid Space in Normal Tension and Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 2018, 186, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Omatiga, A.G.; Onakpoya, O.H.; Idowu, B.M.; Asaleye, C.M.; Adegbehingbe, B.O.; Aderibigbe, A.S. B-mode sonographic evaluation of optic nerve sheath diameter and lens thickness in Nigerian adults with glaucoma. Afr. Health Sci. 2018, 18, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.J.; Carroll, F.D. Evaluation of optic neuropathy with B-scan ultrasonography. Am. J. Ophthalmol. 1972, 74, 915–920. [Google Scholar] [CrossRef]
- Dees, C.; Buimer, R.; Dick, A.D.; Atta, H.R. Ultrasonographic investigation of optic neuritis. Eye 1995, 9 Pt 4, 488–494. [Google Scholar] [CrossRef][Green Version]
- Elvin, A.; Andersson, T.; Söderström, M. Optic neuritis. Doppler ultrasonography compared with MR and correlated with visual evoked potential assessments. Acta Radiol. 1998, 39, 243–248. [Google Scholar]
- Karaali, K.; Senol, U.; Aydin, H.; Cevikol, C.; Apaydin, A.; Lüleci, E. Optic neuritis: Evaluation with orbital Doppler sonography. Radiology 2003, 226, 355–358. [Google Scholar] [CrossRef]
- Karami, M.; Janghorbani, M.; Dehghani, A. Orbital Doppler evaluation of blood flow velocities in optic neuritis. Korean J. Ophthalmol. 2012, 26, 116–122. [Google Scholar] [CrossRef]
- Lochner, P.; Cantello, R.; Fassbender, K.; Lesmeister, M.; Nardone, R.; Siniscalchi, A.; Clemente, N.; Naldi, A.; Coppo, L.; Brigo, F.; et al. Longitudinal Assessment of Transorbital Sonography, Visual Acuity, and Biomarkers for Inflammation and Axonal Injury in Optic Neuritis. Dis. Markers 2017, 2017, 5434310. [Google Scholar] [CrossRef]
- Lochner, P.; Cantello, R.; Brigo, F.; Coppo, L.; Nardone, R.; Tezzon, F.; Raymkulova, O.; Strigaro, G.; Comi, C.; Leone, M.A. Transorbital sonography in acute optic neuritis: A case-control study. AJNR Am. J. Neuroradiol. 2014, 35, 2371–2375. [Google Scholar] [CrossRef] [PubMed]
- Lochner, P.; Leone, M.A.; Fassbender, K.; Cantello, R.; Coppo, L.; Nardone, R.; Zorzi, G.; Lesmeister, M.; Comi, C.; Brigo, F. Transorbital Sonography and Visual Outcome for the Diagnosis and Monitoring of Optic Neuritis. J. Neuroimaging 2017, 27, 92–96. [Google Scholar] [CrossRef]
- Wayman, D.; Carmody, K.A. Optic neuritis diagnosed by bedside emergency physician-performed ultrasound: A case report. J. Emerg. Med. 2014, 47, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Badron, J.; Ong, G.Y. Bedside Transorbital Ultrasound in the Clinical Evaluation of Pediatric Optic Neuritis in the Emergency Department. J. Emerg. Med. 2019, 56, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.P.; Kashani, S.; Mailhot, T.; Omer, T. More than meets the eye: Point-of-care ultrasound diagnosis of acute optic neuritis in the emergency department. Am. J. Emerg. Med. 2019, 37, 177.e1–177.e4. [Google Scholar] [CrossRef]
- Rovira, À.; Vidal-Jordana, A.; Auger, C.; Sastre-Garriga, J. Optic Nerve Imaging in Multiple Sclerosis and Related Disorders. Neuroimaging Clin. N. Am. 2024, 34, 399–420. [Google Scholar] [CrossRef]
- Titlić, M.; Erceg, I.; Kovacević, T.; Gabrić, N.; Karaman, K.; Zuljan, I.; Orsolić, K.; Kalajzić, J. The correlation of changes of the optic nerve diameter in the acute retrobulbar neuritis with the brain changes in multiple sclerosis. Coll. Antropol. 2005, 29, 633–636. [Google Scholar]
- Stefanović, I.B.; Jovanović, M.; Krnjaja, B.D.; Veselinović, D.; Jovanović, P. Influence of retrobulbar neuritis and papillitis on echographically measured optic nerve diameter. Vojnosanit. Pregl. 2010, 67, 32–35. [Google Scholar] [CrossRef]
- Raeesmohammadi, L.; Esmaeili, S.; Abbasi, M.H.; Mehrpour, M.; Mirzaasgari, Z.; Baradaran, H.R.; Deilami, P.; Motamed, M.R. Transbulbar B-mode sonography in multiple sclerosis without optic neuritis; clinical relevance. Brain Res. 2020, 1734, 146723. [Google Scholar] [CrossRef] [PubMed]
- De Masi, R.; Orlando, S.; Conte, A.; Pasca, S.; Scarpello, R.; Spagnolo, P.; Muscella, A.; De Donno, A. Transbulbar B-Mode Sonography in Multiple Sclerosis: Clinical and Biological Relevance. Ultrasound Med. Biol. 2016, 42, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Katsanos, A.H.; Ayzenberg, I.; Schwake, C.; Gahlen, A.; Tsivgoulis, G.; Voumvourakis, K.; Gold, R.; Krogias, C. Atrophy of optic nerve detected by transorbital sonography in patients with demyelinating diseases of the central nervous system. Eur. J. Neurol. 2020, 27, 626–632. [Google Scholar] [CrossRef]
- Elkholy, S.H.; El-Jaafary, S.I.; Kotb, M.S.; El Gohary, A.M.; Elbhy, B.A. Trans-orbital sonography versus visual evoked potentials in acute demyelinating optic neuritis. Mult. Scler. Relat. Disord. 2020, 40, 101934. [Google Scholar] [CrossRef]
- Saigh, M.P.; Plauché, H.M.; Butts, C.; Karam, A.K.; Suau, S.J.; Moreno-Walton, L. Acute Optic Neuritis Diagnosed by Bedside Ultrasound in an Emergency Department. J. Emerg. Med. 2019, 57, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Kim, Y.H.; Baek, S.H.; Son, M.H.; Lee, J.H.; Kim, B.J. Transorbital ultrasonography in acute optic neuritis: Can it be a supportive diagnostic tool? Mult. Scler. Relat. Disord. 2019, 31, 54–58. [Google Scholar] [CrossRef]
- Koraysha, N.A.; Kishk, N.; Hassan, A.; Samy El Gendy, N.M.; Shehata, H.S.; Al-Azayem, S.A.; Kamal, Y.S. Evaluating optic nerve diameter as a possible biomarker for disability in patients with multiple sclerosis. Neuropsychiatr. Dis. Treat. 2019, 15, 2571–2578. [Google Scholar] [CrossRef]
- Krogias, C.; Ayzenberg, I.; Schroeder, C.; Grüter, T.; Gold, R.; Yoon, M.S. Transorbital sonography in CIDP patients: No evidence for optic nerve hypertrophy. J. Neurol. Sci. 2016, 362, 206–208. [Google Scholar] [CrossRef]
- Candeliere Merlicco, A.; Gabaldón Torres, L.; Villaverde González, R.; Fernández Romero, I.; Aparicio Castro, E.; Lastres Arias, M.C. Transorbital ultrasonography for measuring optic nerve atrophy in multiple sclerosis. Acta Neurol. Scand. 2018, 138, 388–393. [Google Scholar] [CrossRef]
- Gerling, J.; Janknecht, P.; Hansen, L.L.; Kommerell, G. Diameter of the optic nerve in idiopathic optic neuritis and in anterior ischemic optic neuropathy. Int. Ophthalmol. 1997, 21, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, A.; Giti, M.; Akhlaghi, M.R.; Karami, M.; Salehi, F. Ultrasonography in distinguishing optic neuritis from nonarteritic anterior ischemic optic neuropathy. Adv. Biomed. Res. 2012, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xiao, W.; Ye, H.; Chen, R.; Wu, J.; Mao, Y.; Yang, H. Ultrasonographic measurement of the optic nerve sheath diameter in dysthyroid optic neuropathy. Eye 2021, 35, 568–574. [Google Scholar] [CrossRef] [PubMed]
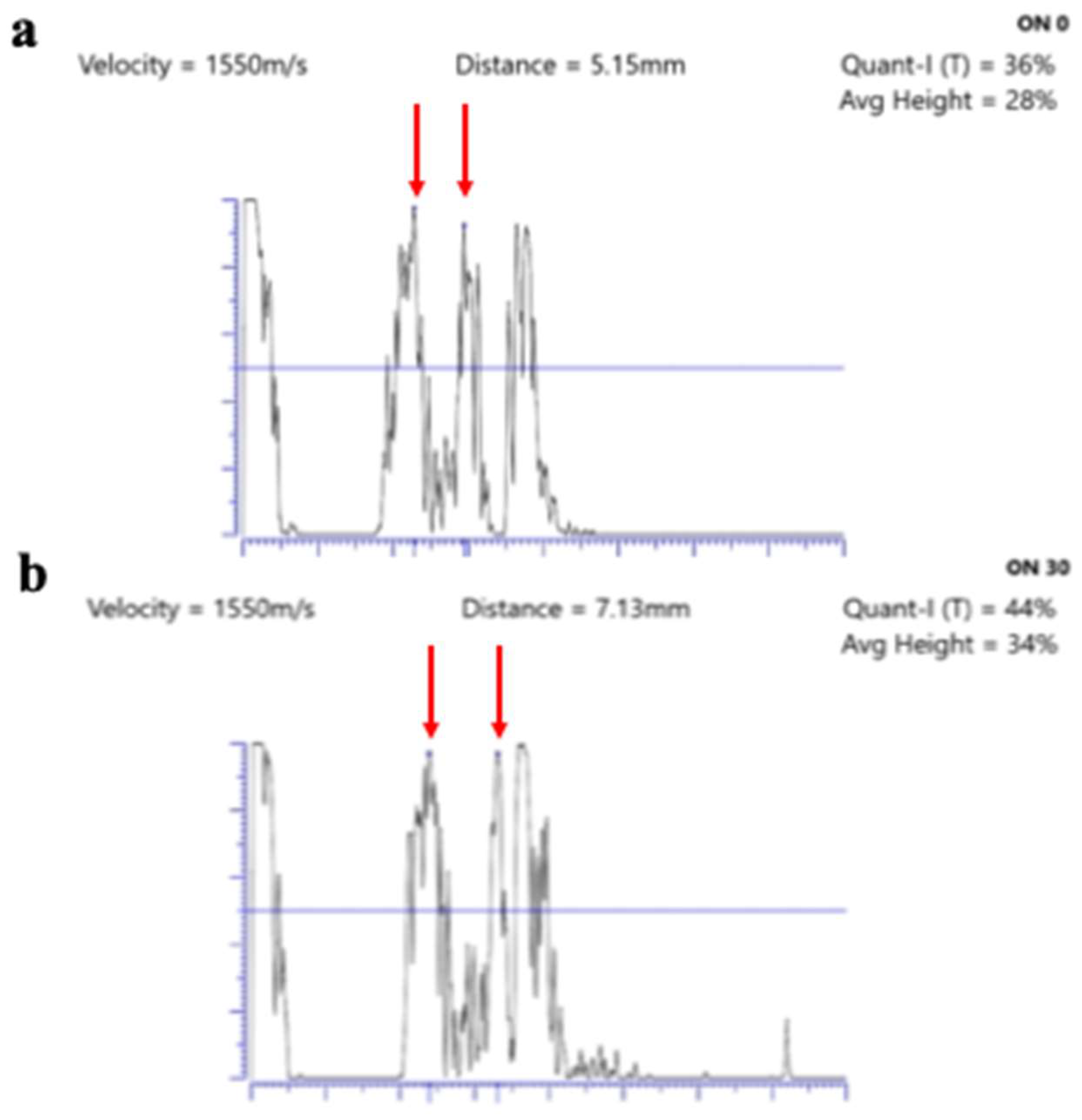
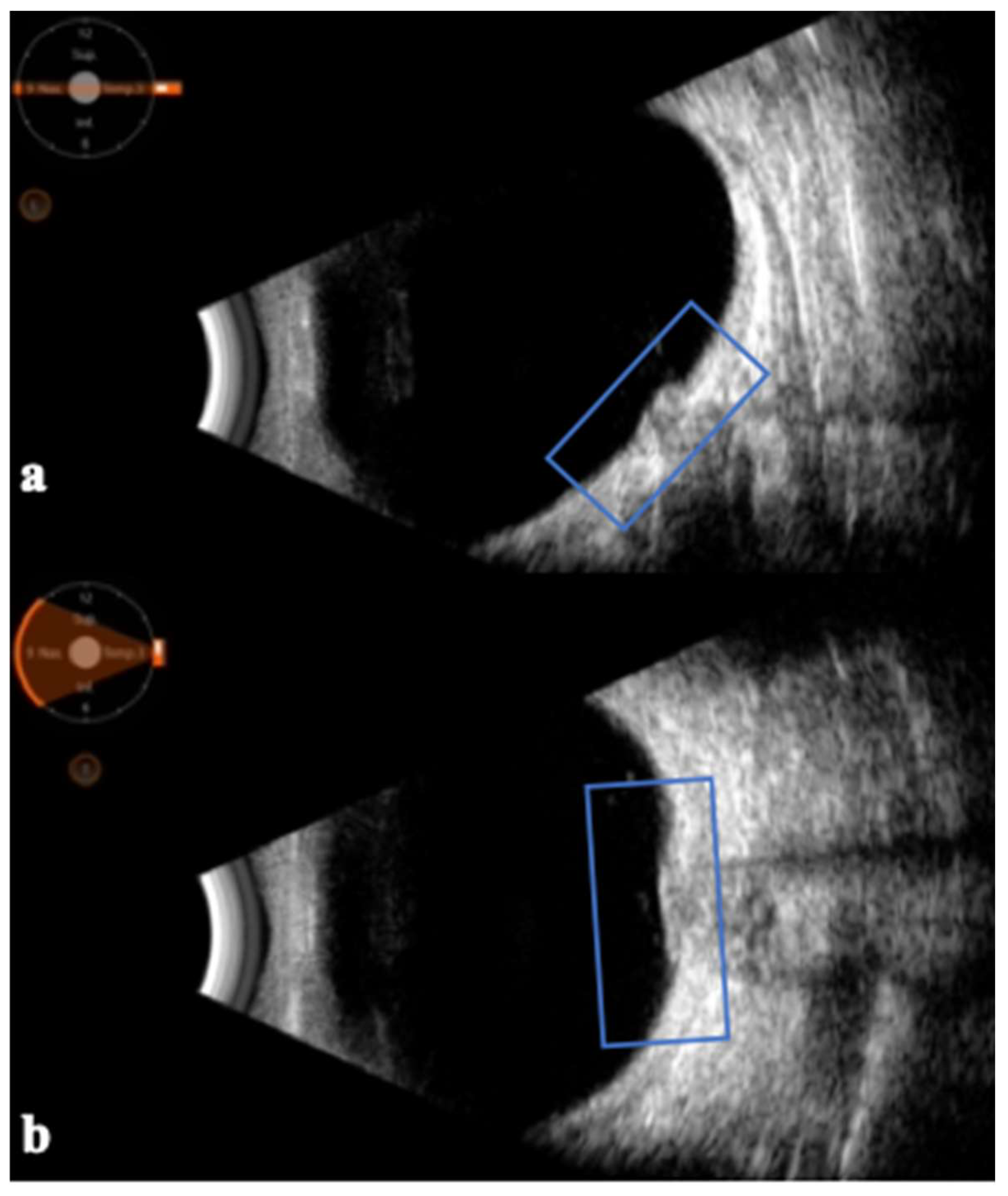
| Glaucoma |
|
Inflammatory - Idiopathic inflammatory optic neuritis (often associated with multiple sclerosis) - Neuromyelitis optica - Systemic inflammatory and autoimmune diseases - Infectious diseases |
|
Vascular - Anterior or posterior - Arteritic or non-arteritic - Post-radiation therapy |
|
Compressive or infiltrative
- Neoplastic - Non-neoplastic |
| Paraneoplastic |
| Toxic |
| Nutritional |
| Hereditary |
| Traumatic |
| Authors (Year) | Ref. | N. Patients | Ultrasound Technique | Outcomes |
|---|---|---|---|---|
| Dees et al. (1995) | [30] | 27 patients with idiopathic optic neuritis | B-scan and standardized A-scan techniques | Substantial increase in optic nerve diameter in 74% of cases and a positive correlation between nerve swelling and the severity of initial visual loss. |
| Elvin et al. (1998) | [31] | 18 patients with a clinical diagnosis of optic neuritis | Doppler ultrasonography | A statistically significant difference was found in the optic nerve diameter and in the resistance to flow in the central retinal artery between the affected and unaffected eyes, confirming the possibility of using Doppler as an indicator of optic nerve inflammation activity. |
| Karaali et al. (2003) | [32] | 20 patients with acute unilateral optic neuritis | Doppler ultrasonography | Peak systolic and end diastolic velocities in the ophthalmic artery were significantly increased in the affected eyes. |
| Karami et al. (2012) | [33] | 23 patients with acute unilateral optic neuritis | Doppler ultrasonography | No significant differences were found in orbital blood flow parameters between the eye with optic neuritis and the healthy contralateral eye. |
| Lochner et al. (2017) | [34] | 23 patients with unilateral optic neuritis and 19 sex- and age-matched healthy controls | B-scan ultrasonography | Optic nerve sheath diameter and optic nerve diameter are increased in the affected eye. |
| Lochner et al. (2014) | [35] | 21 patients with unilateral optic neuritis and 21 sex- and age-matched healthy controls | B-scan ultrasonography | The median optic nerve sheath diameter and optic nerve diameter were thicker on the affected side compared with the nonaffected side and controls. |
| Lochner et al. (2017) | [36] | 45 patients with newly diagnosed optic neuritis | B-scan ultrasonography | The median optic nerve sheath diameter was thicker on the affected side compared with the nonaffected side. |
| Wayman and Carmody (2014) | [37] | 37-year-old diabetic patient complaining of visual loss in the left eye | B-scan ultrasonography | Optic nerve sheath diameter was found to be 6.21 mm with an elevation of the optic disc. The diagnosis of optic neuritis was then confirmed following an ophthalmological and neurological consultation, also performing a brain magnetic resonance imaging. |
| Badron and Ong (2019) | [38] | 15-year-old girl with optic neuritis | B-scan ultrasonography | Transorbital ultrasound revealed an irregularly enlarged optic nerve sheath with increased optic nerve sheath diameter (5.1 mm) and an elevated optic disc height (0.5 mm). The ultrasound findings correlated well with her magnetic resonance imaging of her orbit. |
| Yee et al. (2019) | [39] | A 35-year-old man, a 48-year-old man, a 34-year-old female, and a 28-year-old female with optic neuritis | B-scan ultrasonography | Optic nerve sheath diameters of the affected eyes were all found to be more than 5.5 mm. |
| Titlić et al. (2005) | [41] | 20 patients with multiple sclerosis and retrobulbar neuritis | B-scan ultrasonography | The diameter of the optic nerve with retrobulbar neuritis showed statistically significant differences from the healthy one and correlated significantly with the number of multiple sclerosis brain lesions. |
| Stefanović et al. (2010) | [42] | 23 patients presenting with retrobulbar optic neuritis and papillitis | B-scan and standardized A-scan techniques | The retrobulbar region of the optic nerve was found to be thicker in 94% of the patients with retrobulbar neuritis and in all the patients with papillitis, also finding a correlation between the reduction in visual acuity and the thickening of the retrobulbar region of the optic nerve. |
| Raeesmohammadi et al. (2020) | [43] | 60 patients affected by multiple sclerosis who had not recently received an optic neuritis diagnosis, and 60 healthy sex- and age-matched volunteers. | B-scan ultrasonography | Patients affected by multiple sclerosis who were not currently experiencing an optic neuritis had considerably lower levels of optic nerve sheath diameter and optic nerve diameter than their healthy controls, indicating a gradual subclinical axonal loss. |
| De Masi et al. (2016) | [44] | 60 unselected relapse-free multiple sclerosis patients and 35 matched healthy controls | B-scan ultrasonography | Optic nerve diameter and optic nerve sheath diameter evaluated with ocular ultrasound were found to be smaller in patients affected by multiple sclerosis compared to healthy controls, suggesting a chronic depletion of axons. |
| Schroeder et al. (2020) | [45] | 78 patients with a history of demyelinating disorders | B-scan ultrasonography | Optic nerve diameter and optic nerve sheath diameter evaluated with ocular ultrasound were found to be smaller in patients affected by multiple sclerosis compared to healthy controls, suggesting a chronic depletion of axons. |
| Elkholy et al. (2020) | [46] | 25 patients with a first attack of an acute demyelinating optic neuritis compared to 25 healthy subjects | B-scan ultrasonography | A significant thickening of optic nerve sheath diameter was found compared to controls. Furthermore, optic nerve sheath diameter thickness and visual acuity were found to be significantly inversely correlated. |
| Saigh et al. (2019) | [47] | 59-year-old female patient with progressive unilateral visual loss while she was receiving outpatient treatment for relapsing–remitting multiple sclerosis | B-scan ultrasonography | Transorbital ultrasound revealed a disparity between the optic nerve sheath diameters of the affected and nonaffected eyes and striking optic nerve edema in the affected eye, thus diagnosing an acute optic neuritis. |
| Kwon et al. (2019) | [48] | 17 patients with first-attack unilateral acute optic neuritis | B-scan ultrasonography | Ocular ultrasonography revealed thickening of the optic nerve diameter and optic nerve sheath diameter on the affected side compared with the unaffected side. |
| Koraysha et al. (2019) | [49] | 23 patients affected by relapsing–remitting multiple sclerosis with a history of optic neuritis, 26 patients affected by relapsing–remitting multiple sclerosis without a history of optic neuritis, and 50 age- and sex-matched healthy controls | B-scan ultrasonography | No significant differences of optic nerve diameter and optic nerve sheath diameter were found between the patients’ group and control group. |
| Krogias et al. (2016) | [50] | 17 patients affected by chronic inflammatory demyelinating polyradiculoneuropathy compared to 15 healthy subjects | B-scan ultrasonography | Optic nerve diameter and optic nerve sheath diameter showed no significant differences between the two study groups. |
| Candeliere Merlicco et al. (2018) | [51] | 59 patients diagnosed with relapsing–remitting multiple sclerosis and 36 healthy controls | B-scan ultrasonography | The ultrasound optic nerve sheath diameter was found to be smaller in patients with multiple sclerosis and to be correlated with the Expanded Disability Status Scale and with the duration of the disease without being interfered by the previous history of optic neuritis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, A.; Abbinante, G.; De Pascale, I.; Gagliardi, V.; Salerno, G.; Pellegrino, A.; Vitiello, L. Ocular Ultrasound in the Diagnosis of Optic Neuropathies: A Review of the Literature. J. Pers. Med. 2024, 14, 949. https://doi.org/10.3390/jpm14090949
Coppola A, Abbinante G, De Pascale I, Gagliardi V, Salerno G, Pellegrino A, Vitiello L. Ocular Ultrasound in the Diagnosis of Optic Neuropathies: A Review of the Literature. Journal of Personalized Medicine. 2024; 14(9):949. https://doi.org/10.3390/jpm14090949
Chicago/Turabian StyleCoppola, Alessia, Giulia Abbinante, Ilaria De Pascale, Vincenzo Gagliardi, Giulio Salerno, Alfonso Pellegrino, and Livio Vitiello. 2024. "Ocular Ultrasound in the Diagnosis of Optic Neuropathies: A Review of the Literature" Journal of Personalized Medicine 14, no. 9: 949. https://doi.org/10.3390/jpm14090949
APA StyleCoppola, A., Abbinante, G., De Pascale, I., Gagliardi, V., Salerno, G., Pellegrino, A., & Vitiello, L. (2024). Ocular Ultrasound in the Diagnosis of Optic Neuropathies: A Review of the Literature. Journal of Personalized Medicine, 14(9), 949. https://doi.org/10.3390/jpm14090949







