Tooth Loss in Periodontitis Patients—A Risk Factor for Mild Cognitive Impairment: A Systematic Review and Meta—Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Search Design
2.3. Inclusion Criteria
- Longitudinal prospective cohort and cross-sectional human studies published up to and including April 2023, with limitations to the English language.
- The exposure of interest were chronic periodontal disease and tooth loss, ensuring that at least one of these or both had been assessed and recorded by means of a clinical examination by a qualified dental professional.
- The outcome of interest was individuals diagnosed with mild cognitive deterioration through verified tests such as the mini-mental state examination (MMSE), delayed word recall (DWR) and the digit symbol substitution test (DSST). No restrictions were imposed on the age, gender, socioeconomic status or ethnic status of the subjects.
2.4. Exclusion Criteria
- Studies which assessed the effect of dementia or Alzheimer’s disease on overall dental health were excluded from this review.
- Those studies where the outcome differed from mild cognitive impairment or where the exposure factors were other than periodontitis and tooth loss, were not included in this review (such as smoking, diabetes and other risk factors common to both diseases). In this case, we could consider these as hidden confounding factors and hence a limitation for this review. Additionally, studies where information was gathered by means other than clinical examinations, such as surveys or interviews, were also excluded.
- Case–control studies, case reports, reviews and animal studies were excluded.
2.5. Selection Process
2.6. Data Extraction
- General information: The study’s title, authors, year of publication, publisher/source of study and study design;
- Population and setting: Description of the population, location of the study and the method of recruiting subjects;
- Methods: Aim of the study, date of the start of data collection and the end date, total duration of observations, ethical approval obtained, and written consent obtained from participants;
- Participants: Total number of participants at baseline, withdrawals and dropouts by the time of follow up, age at baseline recruitment and gender;
- Exposure: Methods to evaluate periodontal health such as measurements of probing depth and alveolar bone height in radiographs, and the measures used to assess the extent of severity of disease; the number of remaining teeth at baseline were counted and then again at follow up; and the number of missing teeth were measured as the difference between the two readings;
- Outcome: Tests to assess cognitive function;
- Conclusion: A summary of conclusions as derived by the authors from individual studies;
- Others: Funding sources and conflicts of interest.
2.7. Assessment of Quality
- Good/high quality: 3 or 4 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 2 or 3 stars in the outcome/exposure domain;
- Fair/moderate quality: 2 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 2 or 3 stars in the outcome/exposure domain.
- Low/poor quality: 0 or 1 star in the selection domain OR 0 stars in the comparability domain OR 0 or 1 stars in the outcome/exposure domain [41].
2.8. Quantitative Analysis
3. Results
3.1. Data Synthesis
3.2. Meta-Analysis
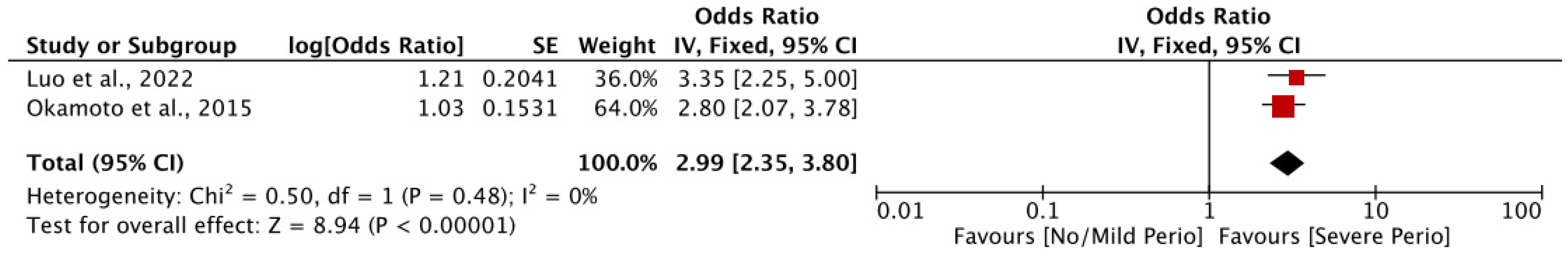
3.2.1. Association of Periodontal Disease and Cognitive Decline
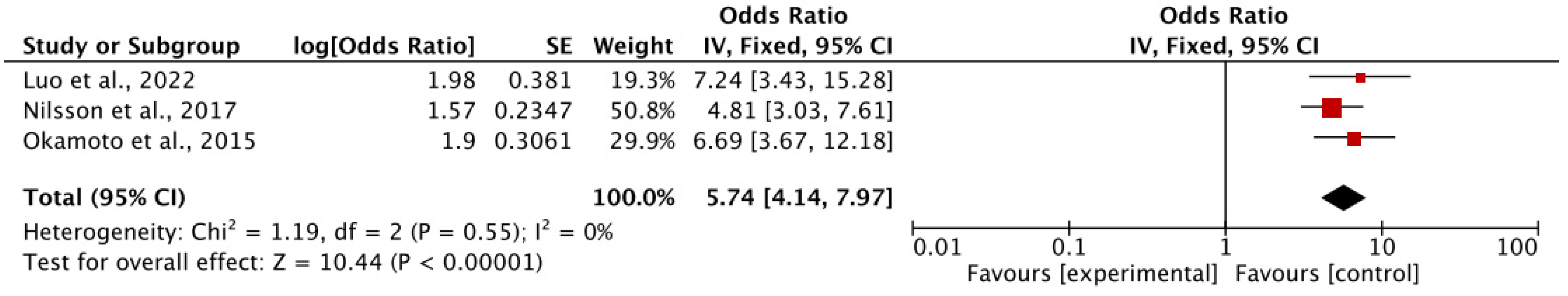
3.2.2. Association of Tooth Loss and Cognitive Decline
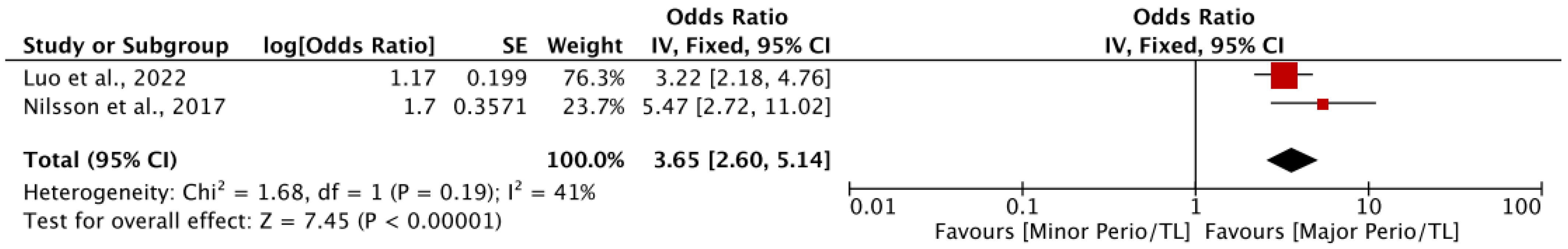
3.2.3. Association of Periodontal Disease and Tooth Loss Together with Cognitive Decline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, W.-L.; Jiang, M.-J.; Gu, B.-B.; Wei, Y.-M.; Fan, S.-N.; Liao, W.; Zheng, Y.-Q.; Liao, S.-W.; Xiong, Y.; Li, Y.; et al. Tooth loss as a risk factor for dementia: Systematic review and meta-analysis of 21 observational studies. BMC Psychiatry 2018, 18, 345. [Google Scholar] [CrossRef]
- Pais, R.; Ruano, L.; Carvalho, O.P.; Barros, H. Global cognitive impairment prevalence and incidence in community dwelling older adults—A systematic review. Geriatrics 2020, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Abd-Allah, F.; Abdoli, A.; Abu-Gharbieh, E.; Alipour, V.; Almustanyir, S.; Amu, H.; Arabloo, J.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ikeda, M. Mild cognitive impairment in a population-based epidemiological study. Psychogeriatrics 2007, 7, 104–108. [Google Scholar] [CrossRef]
- Bradfield, N.I. Mild Cognitive Impairment: Diagnosis and Subtypes. Clin. EEG Neurosci. 2023, 54, 4–11. [Google Scholar] [CrossRef]
- Heinik, J. Cognitive domains in the dsm-5 era and their assessment by physicians in cognitively impaired elderlies. Harefuah 2022, 161, 506–514. [Google Scholar] [PubMed]
- Wang, Y.; Li, M.; Haughton, D.; Kazis, L.E. Transition of mild cognitive impairment to Alzheimer’s disease: Medications as modifiable risk factors. PLoS ONE 2024, 19, e0306270. [Google Scholar] [CrossRef]
- Falk, N.; Cole, A.; Meredith, T.J. Evaluation of Suspected Dementia. Am. Fam. Physician 2018, 97, 398–405. [Google Scholar]
- Hu, C.; Wang, L.; Guo, Y.; Cao, Z.; Lu, Y.; Qin, H. Study of the Risk and Preventive Factors for Progress of Mild Cognitive Impairment to Dementia. Am. J. Alzheimer’s Dis. Other Dementiasr 2020, 35, 1533317520925324. [Google Scholar] [CrossRef]
- Rasmussen, I.J.; Frikke-Schmidt, R. Modifiable cardiovascular risk factors and genetics for targeted prevention of dementia. Eur. Heart J. 2023, 44, 2526–2543. [Google Scholar] [CrossRef]
- Hu, C.; Wang, L.; Zhao, X.; Zhu, B.; Tian, M.; Qin, H. Investigation of risk factors for the conversion of mild cognitive impairment to dementia. Int. J. Neurosci. 2021, 131, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Gatz, M.; Mortimer, J.A.; Fratiglioni, L.; Johansson, B.; Berg, S.; Reynolds, C.A.; Pedersen, N.L. Potentially modifiable risk factors for dementia in identical twins. Alzheimer’s Dement. 2006, 2, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Lee, H.-J.; Suh, S.W.; Lee, J.R.; Byun, S.; Kim, K.S.; Kim, S.Y.; Lee, J.-T.; Yoo, E.; Chang, N.-H.; et al. Loss of Functional Dentition is Associated with Cognitive Impairment. J. Alzheimer’s Dis. 2020, 73, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Alawaji, Y.N.; Alshammari, A.; Mostafa, N.; Carvalho, R.M.; Aleksejuniene, J. Periodontal disease prevalence, extent, and risk associations in untreated individuals. Clin. Exp. Dent. Res. 2022, 8, 380–394. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Jiang, Z.; Marka, N.; Wolff, L.F. Periodontal Disease, Tooth Loss, and Systemic Conditions: An Exploratory Study. Int. Dent. J. 2024, 74, 207–215. [Google Scholar] [CrossRef]
- Desta, N.T. Pathophysiological association between periodontal disease and Alzheimer’s disease: Importance of periodontal health in the elderly. J. Oral Biosci. 2021, 63, 351–359. [Google Scholar] [CrossRef]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Lo Russo, L.; Lo Muzio, L. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020, 9, 495. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Caloro, G.A.; Capocasale, G.; Zhurakivska, K.; Troiano, G.; Lo Russo, L.; Lo Muzio, L. The Association between Tooth Loss and Alzheimer’s Disease: A Systematic Review with Meta-Analysis of Case Control Studies. Dent. J. 2019, 7, 49. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Periodontol. 2018, 89, S140–S158. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.L.S.; Mountjoy-Venning, W.; Anjomshoa, M.; Banoub, J.A.M.; Yasin, Y.J.; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study (vol 392, pg 1789, 2018). Lancet (Br. Ed.) 2019, 393, E44. [Google Scholar]
- Cichońska, D.; Mazuś, M.; Kusiak, A. Recent Aspects of Periodontitis and Alzheimer’s Disease—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 2612. [Google Scholar] [CrossRef]
- Trumble, B.C.; Schwartz, M.; Ozga, A.T.; Schwartz, G.T.; Stojanowski, C.M.; Jenkins, C.L.; Kraft, T.S.; Garcia, A.R.; Cummings, D.K.; Hooper, P.L.; et al. Poor Oral Health Is Associated With Inflammation, Aortic Valve Calcification, and Brain Volume Among Forager-Farmers. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2024, 79, glae013. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Singhrao, S.K.; Olsen, I.; Singhrao, S.K.; Olsen, I.; Singhrao, S.K. Importance of heterogeneity in Porhyromonas gingivalis lipopolysaccharide lipid A in tissue specific inflammatory signalling. J. Oral Microbiol. 2018, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Ganbaatar, U.; Erdeneochir, U.; Byambajav, P.; Jadamba, T.; Byambasukh, O.; Dagvajantsan, B. Relationship of tooth loss to mild cognitive impairment among middle-aged Mongolians: Mon-timeline study. J. Neurol. Sci. Conf. World Congr. Neurol. (WCN 2021) 2021, 4, 119731. [Google Scholar] [CrossRef]
- Oh, B.; Han, D.-H.; Han, K.-T.; Liu, X.; Ukken, J.; Chang, C.; Dounis, K.; Yoo, J.W. Association between residual teeth number in later life and incidence of dementia: A systematic review and meta-analysis. BMC Geriatr. 2018, 18, 48. [Google Scholar] [CrossRef]
- Nakamura, T.; Zou, K.; Shibuya, Y.; Michikawa, M. Oral dysfunctions and cognitive impairment/dementia. J. Neurosci. Res. 2021, 99, 518–528. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Yang, D.; Yang, S.; Zhao, Y.; Jiang, M.; Wang, X.; Zhao, L.; Liu, Q.; Lu, Z.; et al. Tooth loss and the risk of cognitive decline and dementia: A meta-analysis of cohort studies. Front. Neurol. 2023, 14, 1103052. [Google Scholar] [CrossRef]
- Xu, S.; Huang, X.; Gong, Y.; Sun, J. Association between tooth loss rate and risk of mild cognitive impairment in older adults: A population-based longitudinal study. Aging 2021, 13, 21599–21609. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Li, F.-R.; Chen, P.-L.; Cheng, X.; Mao, C.; Wu, X.-B. Tooth Loss, Denture Use, and Cognitive Impairment in Chinese Older Adults: A Community Cohort Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 77, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Tonsekar, P.P.; Jiang, S.S.; Yue, G. Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology 2017, 34, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.; Stephen, R.; Mäntylä, P.; Suominen, A.L.; Solomon, A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J. Am. Geriatr. Soc. 2022, 70, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, P.C.; Castro, M.M.L.; Magno, M.B.; Almeida, A.P.C.P.S.C.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. Association Between Periodontitis and Cognitive Impairment in Adults: A Systematic Review. Front. Neurol. 2019, 10, 323. [Google Scholar] [CrossRef]
- Guo, H.; Chang, S.; Pi, X.; Hua, F.; Jiang, H.; Liu, C.; Du, M. The effect of periodontitis on dementia and cognitive impairment: A meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 6823. [Google Scholar] [CrossRef]
- Al-Nasser, L.; Lamster, I.B. Prevention and management of periodontal diseases and dental caries in the older adults. Periodontology 2000 2020, 84, 69–83. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, S.-N.; Lee, J.-H. Severe periodontitis with tooth loss as a modifiable risk factor for the development of Alzheimer, vascular, and mixed dementia: National Health Insurance Service-National Health Screening Retrospective Cohort 2002–2015. J. Periodontal Implant. Sci. 2020, 50, 303–312. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- ElKholy, N.; Tawfik, H.M.; Ebeid, S.; Madkor, O.R.E.; Hamza, S.A. A model of cognitive evaluation battery for diagnosis of mild cognitive impairment and dementia in educated and illiterate Egyptian elderly people. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 95. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro, A., III; Dietrich, T.; Garcia, R. Tooth Loss and Periodontal Disease Predict Poor Cognitive Function in Older Men. J. Am. Geriatr. Soc. 2010, 58, 713–718. [Google Scholar] [CrossRef]
- Okamoto, N.; Morikawa, M.; Tomioka, K.; Yanagi, M.; Amano, N.; Kurumatani, N. Association between tooth loss and the development of mild memory impairment in the elderly: The Fujiwara-kyo Study. J. Alzheimer’s Dis. 2015, 44, 777–786. [Google Scholar] [CrossRef]
- Luo, H.; Wu, B.; González, H.M.; Stickel, A.; Kaste, L.M.; Tarraf, W.; Daviglus, M.L.; Sanders, A.E.; Cai, J. Tooth loss, periodontal disease, and mild cognitive impairment among hispanic/latino immigrants: The moderating effects of age at immigration. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 78, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.; Berglund, J.S.; Renvert, S. Periodontitis, tooth loss and cognitive functions among older adults. Clin. Oral Investig. 2018, 22, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, Q.; Yu, T. Kappa statistic considerations in evaluating inter-rater reliability between two raters: Which, when and context matters. BMC Cancer 2023, 23, 799. [Google Scholar] [CrossRef]
- Egashira, R.; Umezaki, Y.; Mizutani, S.; Obata, T.; Yamaguchi, M.; Tamai, K.; Yoshida, M.; Makino, M.; Naito, T. Relationship between cerebral atrophy and number of present teeth in elderly individuals with cognitive decline. Exp. Gerontol. 2021, 144, 111189. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, B.; Zhao, Q.; Guo, Q.; Meng, H.; Yu, L.; Zheng, L.; Hong, Z.; Ding, D. Association between tooth loss and cognitive function among 3063 Chinese older adults: A community-based study. PLoS ONE 2015, 10, e0120986. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Fujiwara, T.; Murayama, H.; Machida, M.; Inoue, S.; Shobugawa, Y. Differences in brain volume by tooth loss and cognitive function in older Japanese adults. Am. J. Geriatr. Psychiatry 2022, 30, 1271–1279. [Google Scholar] [CrossRef]
- Panzarella, V.; Mauceri, R.; Baschi, R.; Maniscalco, L.; Campisi, G.; Monastero, R. Oral health status in subjects with amnestic mild cognitive impairment and Alzheimer’s disease: Data from the Zabut Aging Project. J. Alzheimer’s Dis. 2022, 87, 173–183. [Google Scholar] [CrossRef]
- Avlund, K.; HolmPedersen, P.; Morse, D.E.; Viitanen, M.; Winblad, B. Tooth loss and caries prevalence in very old Swedish people: The relationship to cognitive function and functional ability. Gerodontology 2004, 21, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Naorungroj, S.; Slade, G.D.; Beck, J.D.; Mosley, T.H.; Gottesman, R.F.; Alonso, A.; Heiss, G. Cognitive decline and oral health in middle-aged adults in the ARIC study. J. Dent. Res. 2013, 92, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Germen, M.; Baser, U.; Lacin, C.C.; Fıratlı, E.; İşsever, H.; Yalcin, F. Periodontitis Prevalence, Severity, and Risk Factors: A Comparison of the AAP/CDC Case Definition and the EFP/AAP Classification. Int. J. Environ. Res. Public Health 2021, 18, 3459. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué-Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Cosp, X.B.; Cullum, S. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2021, 7, CD010783. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kimura, Y.; Ogawa, H.; Yamaga, T.; Ansai, T.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Fujisawa, M.; Okumiya, K.; et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: A 5-year cohort study. J. Periodontal Res. 2019, 54, 233–240. [Google Scholar] [CrossRef]
- Blazer, D.G. From Mouth to Brain: Tooth Loss and Cognitive Impairment. Am. J. Geriatr. Psychiatry 2022, 30, 1280–1282. [Google Scholar] [CrossRef]
- Rivier, C.A.; Renedo, D.B.; de Havenon, A.; Sunmonu, N.A.; Gill, T.M.; Payabvash, S.; Sheth, K.N.; Falcone, G.J. Association of Poor Oral Health with Neuroimaging Markers of White Matter Injury in Middle-Aged Participants in the UK Biobank. Neurology 2024, 102, e208010. [Google Scholar] [CrossRef]
- Kim, T.-H. Effects of masticatory exercise on cognitive function in community-dwelling older adults. Technol. Health Care 2021, 29, 125–131. [Google Scholar] [CrossRef]
- Gracht, I.; Derks, A.; Haselhuhn, K.; Wolfart, S. EMG correlations of edentulous patients with implant overdentures and fixed dental prostheses compared to conventional complete dentures and dentates: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2017, 28, 765–773. [Google Scholar] [CrossRef]
- Terasawa, H.; Hirai, T.; Ninomiya, T.; Ikeda, Y.; Ishijima, T.; Yajima, T.; Hamaue, N.; Nagase, Y.; Kang, Y.; Minami, M. Influence of tooth-loss and concomitant masticatory alterations on cholinergic neurons in rats: Immunohistochemical and biochemical studies. Neurosci. Res. 2002, 43, 373–379. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Jiang, Q. Tooth Loss-Associated Mechanisms That Negatively Affect Cognitive Function: A Systematic Review of Animal Experiments Based on Occlusal Support Loss and Cognitive Impairment. Front. Neurosci. 2022, 16, 811335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Wang, D.; Zhang, J.; Zhang, F. NSAID Exposure and Risk of Alzheimer’s Disease: An Updated Meta-Analysis from Cohort Studies. Front. Aging Neurosci. 2018, 10, 83. [Google Scholar] [CrossRef]
- Nemergut, M.; Batkova, T.; Vigasova, D.; Bartos, M.; Hlozankova, M.; Schenkmayerova, A.; Liskova, B.; Sheardova, K.; Vyhnalek, M.; Hort, J.; et al. Increased occurrence of Treponema spp. and double-species infections in patients with Alzheimer’s disease. Sci. Total Environ. 2022, 844, 157114. [Google Scholar] [CrossRef]
- Fang, Y.-C.; Hsieh, Y.-C.; Hu, C.-J.; Tu, Y.-K. Endothelial Dysfunction in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 2909. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Q.; Wang, L.; Ding, H.; Wang, Y.; Liu, Y.; Gong, T. The interaction between oral microbiota and gut microbiota in atherosclerosis. Front. Cardiovasc. Med. 2024, 11, 1406220. [Google Scholar] [CrossRef]
- Bailey, M.; Ilchovska, Z.G.; Hosseini, A.A.; Jung, J. The impact of APOE ε4 in Alzheimer’s disease: A meta-analysis of voxel-based morphometry studies. medRxiv Prepr. Serv. Health Sci. 2024; preprint. [Google Scholar] [CrossRef]
- Reissmann, D.R.; Wolfart, S.; John, M.T.; Marré, B.; Walter, M.; Kern, M.; Kohal, R.; Nothdurft, F.; Stark, H.; Schierz, O.; et al. Impact of shortened dental arch on oral health-related quality of life over a period of 10 years—A randomized controlled trial. J. Dent. 2019, 80, 55–62. [Google Scholar] [CrossRef]
- Ryder, M.I.; Xenoudi, P. Alzheimer disease and the periodontal patient: New insights, connections, and therapies. Periodontology 2000 2021, 87, 32–42. [Google Scholar] [CrossRef]
- Laugisch, O.; Johnen, A.; Buergin, W.; Eick, S.; Ehmke, B.; Duning, T.; Sculean, A. Oral and Periodontal Health in Patients with Alzheimer’s Disease and Other Forms of Dementia—A Cross-sectional Pilot Study. Oral Health Prev. Dent. 2021, 19, 255–261. [Google Scholar] [CrossRef]
- Dibello, V.; Custodero, C.; Cavalcanti, R.; Lafornara, D.; Dibello, A.; Lozupone, M.; Daniele, A.; Pilotto, A.; Panza, F.; Solfrizzi, V. Impact of periodontal disease on cognitive disorders, dementia, and depression: A systematic review and meta-analysis. GeroScience 2024, 46, 5133–5169. [Google Scholar] [CrossRef]
- McLister, C.; Donnelly, M.; Cardwell, C.R.; Moore, C.; O’neill, C.; Brocklehurst, P.; McKenna, G. Effectiveness of prosthodontic interventions and survival of remaining teeth in adult patients with shortened dental arches—A systematic review. J. Dent. 2018, 78, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Dreyhaupt, J.; Mundt, T.; Kohal, R.; Kern, M.; Rauch, A.; Nothdurft, F.; Hartmann, S.; Böning, K.; Boldt, J.; et al. Periodontal health in shortened dental arches: A 10-year RCT. J. Prosthodont. Res. 2020, 64, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Möllers, T.; Stocker, H.; Perna, L.; Rujescu, D.; Holleczek, B.; Schöttker, B.; Brenner, H. Subjective short-term memory difficulties at ages 50–75 predict dementia risk in a community-based cohort followed over 17 years. Age Ageing 2022, 51, afac113. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Capodiferro, S.; Toia, M.; Cantore, S.; Favia, G.; De Frenza, G.; Grassi, F. Evidence-Based Dentistry: What’s New? Int. J. Med. Sci. 2007, 4, 174–178. [Google Scholar] [CrossRef][Green Version]

| Author and Publication Year | Kaye et al., [43] | Okamoto et al., [44] | Luo et al., [45] | Nilsson et al., [46] |
|---|---|---|---|---|
| Population Description | Male members of US Deparment of VA DLS receiving medical and dental care in private sector | Men and Women aged 65 and over who were residents of Nara prefecture | Immigrant Population from Hispanic/Latino population in 4 centres from US. | Older adults from Swedish National Study on Ageing and Care |
| Location of Study | Boston, Massachusetts | Japan | Newyork, Chicago, Miami and San Diego | Sweden –Blekinge |
| Participants | ||||
| Number | 1231 | 3696 | 6377 | 775 |
| Age at baseline | 28–70 years | Over 65 years | 50–74 years | Over 60 years |
| Sex | Male | Male & Female | Male & Female | Male & Female |
| Withdrawals & Dropouts | 634 (51.5 %) | 1361 (36.8%) | 668 (10.5%) | N/A |
| Study Design | Prospective Observational | Prospective Observational | Longitudinal prospective cohort | cross –sectional |
| Data collection in: | 8 Years | 5 Years | ongoing since 2006 | |
| Start Date | 1993 | 2007 | 2006 | |
| End Date | 2001 | 2012 | ongoing | 2007–2009 |
| Methods to assess Periodontal Health: | Number of teeth showing increase in PPD by ≥2 mm from baseline | CPI code (WHO probe) on 10 representative teeth in 6 segments of oral cavity (tooth: 11, 16, 17, 26, 27, 31, 36, 37, 46 & 47) codes 0–4. Highest code noted as maximum CPI code for individual | No/mild/moderate/severe disease based on interproximal attachment loss (PPD) and ABL according to CDC-AAP definition | Proportion of teeth with ≥5 mm PPD (from gingival margin to base of sulcus) on >30% of teeth & ABL (distance from CEJ to alveolar bone) > 4 mm on OPG at ≥30% of sites |
| Number of teeth showing decrease in bone height by ≥40% from baseline | ||||
| Methods to assess number of teeth: | Number of teeth lost/decade of follow up | Remaining teeth categorised into 5 groups at baseline and follow up: 0/1–8/9–16/17–24/25–32 | STL counted as 8 or more teeth lost at follow up compared to baseline | Number of teeth remaining categorised into 2 groups: 1–19/≥20 |
| Mild Cognitive Impairment accessed by: | MMSE (MCI < 25) | MMSE (MMI ≥ 24) | Various neuropsychiatric battery tests including six item screener, verbal learning tests, word fluency, Digit symbol tests, trail making tests | MMSE (MCI < 25) |
| Spacial copying task (MCI < 10) | Word recall test (MMI –0/1) | Clock test (MCI < 8) | ||
| GDS score (MMI ≤ 5) | ||||
| Conclusion | Risk of cognitive decline in older men increases as more teeth are lost. Periodontal disease and caries (major reasons for tooth loss) are also related to cognitive decline. | Tooth loss predicts the development of Mild memory impairment in the elderly. | Significant tooth loss is a significant risk factor for mild cognitive impairment. | A history of periodontitis and tooth loss may be of importance for cognitive function among older adults. |
| Criteria | Kaye et al. [43] | Okamoto et al. [44] | Nilsson et al. [46] | Luo et al. [45] |
|---|---|---|---|---|
| Representativeness of the exposed cohort | * | * | * | |
| Selection of the non-exposed cohort | * | * | * | |
| Ascertainment of exposure (periodontitis and/or tooth loss) | * | * | * | |
| Demonstration that the outcome (dementia/cognitive impairment) was not present at the start of the study | * | * | ||
| Comparability of cohorts according to the design and analysis | * | * | * | |
| The study controls for additional factors (age, diet, smoking, education, socioeconomic factors, etc.) | * | * | * | * |
| Assessment of mild-cognitive impairment using validated assessment tools such as MMSE or the six-item screener test | * | * | * | |
| Follow-up was long enough for the outcomes to occur (≥5 years) | * | * | * | * |
| Adequacy of follow-up of cohorts | * | |||
| Total (maximum possible score = 9) | 8 | 5 | 5 | 8 |
| High | Low | Moderate | High |
| Number | Study | Reason |
|---|---|---|
| 1. | Han, J.H., Lee, H.J., Han, J.W., Suh, S.W., Lee, J.R., Byun, S., Kim, K.S., Kim, S.Y., Lee, J.T., Yoo, E., Chang, N.H., Kim, T.H. and Kim, K.W. (2020) ‘Loss of functional dentition is associated with cognitive impairment.’, Journal of Alzheimer’s Disease, 73(4), pp. 1313–1320. [13] | There was no consideration of periodontal disease as the cause for tooth loss; it studied functional occlusal units or teeth, not just remaining natural teeth. The exposure was different. |
| 2. | Egashira, R., Umezaki, Y., Mizutani, S., Obata, T., Yamaguchi, M., Tamai, K., Yoshida, M., Makino, M. and Naito, T. (2021) ‘Relationship between cerebral atrophy and number of present teeth in elderly individuals with cognitive decline’, Experimental gerontology, 144, pp. 111189 Available at: 10.1016/j.exger.2020.111189. [48] | This study investigated the relationship of increased tooth loss with the severity of brain atrophy. No neuropshychological tests were employed. The exposure was different. |
| 3. | Luo, J., Wu, B., Zhao, Q., Guo, Q., Meng, H., Yu, L., Zheng, L., Hong, Z. and Ding, D. (2015) ‘Association between tooth loss and cognitive function among 3063 Chinese older adults: A community-based study.’, PLoS ONE, 10(3) (pagination), pp. Arte Number: e0120986. ate of Pubaton: 24 Mar 2015. [49] | This study collected self-reported data on periodontal disease as the reason for tooth loss but did not report it as variables/exposure, as the authors did not consider the data to be reliable. The exposure was not fully reported. |
| 4. | Matsuyama, Y., Fujiwara, T., Murayama, H., Machida, M., Inoue, S. and Shobugawa, Y. (2022) ‘Differences in brain volume by tooth loss and cognitive function in older Japanese adults.’, American Journal of Geriatric Psychiatry, 30(12), pp. 1271–1279. [50] | In this study, there was no consideration of periodontitis as a reason for tooth loss. The exposure was different. |
| 5. | Panzarella, V., Mauceri, R., Baschi, R., Maniscalco, L., Campisi, G. and Monastero, R. (2022) ‘Oral health status in subjects with amnestic mild cognitive impairment and Alzheimer’s disease: Data from the Zabut Aging Project.’, Journal of Alzheimer’s Disease, 87(1), pp. 173–183. [51] | The exposure was amnestic mild cognitive impairment + Alzheimer’s disease, and the outcomes measured were oral health parameters such as caries and periodontal health. The exposure and outcome were different. |
| 6. | Ganbaatar, U., Erdeneochir, U., Byambajav, P., Jadamba, T., Byambasukh, O. and Dagvajantsan, B. (2021) ‘Relationship of tooth loss to mild cognitive impairment among middle-aged Mongolians: Mon-timeline study.’, Journal of the Neurological Sciences, Conference: World Congress of Neurology (WCN 2021), pp. Arte Number: 119731. ate of Pubaton: Otober 2021. [27] | This study mainly concentrated on dental caries as the primary reason for increased tooth loss in the Mongolian middle-aged population. The exposure was different. |
| 7. | Xu, S., Huang, X., Gong, Y. and Sun, J. (2021) ‘Association between tooth loss rate and risk of mild cognitive impairment in older adults: a population-based longitudinal study.’, Aging, 13(17), pp. 21599–21609. [31] | This study reported that a limitation was that they did not know the periodontal health of subjects, as no dental exam was carried out. Only self-reported number of teeth and information on denture use were collected. The exposure and the method used to assess exposure were different. |
| 8. | Avlund, K., HolmPedersen, P., Morse, D.E., Viitanen, M. and Winblad, B. (2004) ‘Tooth loss and caries prevalence in very old Swedish people: The relationship to cognitive function and functional ability.’, Gerodontology, 21(1), pp. 17–26. [52] | In this study, the exposure was cognitive decline and outcome variable measured was teeth lost. The exposure and outcome were different. |
| 9. | Naorungroj, S., Slade, G.D., Beck, J.D., Mosley, T.H., Gottesman, R.F., Alonso, A. and Heiss, G. (2013) ‘Cognitive decline and oral health in middle-aged adults in the ARIC study’, Journal of Dental Research, 92(9), pp. 795–801 Available at: 10.1177/0022034513497960. [53] | In this study, the exposure was cognitive decline, and the outcome variable measured was teeth lost and periodontitis. The exposure and outcome were different. |
| Study | Sample Size | Age at Baseline | Main Exposure | Exposure Cut-Off Point | Pooled Ratio | 95% Lower Limit | 95% Upper Limit | p |
|---|---|---|---|---|---|---|---|---|
| Kaye et al. [43] | 1231 | 28–70 | Each additional tooth with ABL progression/decade | 40% increase from baseline or teeth lost | 1.03 (HR) | 1.00 | 1.07 | n/a |
| Each additional tooth with PPD progression/decade | ≥2 mm from baseline or teeth lost | 1.04 (HR) | 1.01 | 1.09 | ||||
| Okamoto et al. [44] | 3696 | >65 | CPI codes 0–4 | Code 4 | 1.03 (AOR) | 0.73 | 1.45 | 0.871 |
| Nilsson et al. [46] | 775 | >60 | Distance from CEJ to bone level | ≥4 mm on ≥30% sites | 2.7 (AOR) | 1.2 | 5.9 | 0.013 |
| Luo et al. [45] | 6377 | >50 | % of teeth with attachment loss, and % of teeth with increased PPD | ≥6 mm on ≥2 sites, and ≥5 mm on ≥2 interproximal sites | 1.02 (AOR) | 0.67 | 1.54 | 0.93 |
| Study | Sample Size | Age at Baseline | Exposure Cut-Off Point | Pooled Ratio | 95% Lower Limit | 95% Upper Limit | p |
|---|---|---|---|---|---|---|---|
| Kaye et al. [43] | 1231 | 28–70 | Each additional tooth lost/decade | 1.09 (HR) | 1.01 | 1.18 | n/a |
| Okamoto et al. [44] | 3696 | >65 | Each tooth lost at follow-up | 1.01 (AOR) | 1.00 | 1.03 | 0.051 |
| Edentulous | 2.32 (AOR) | 1.44 | 3.74 | 0.001 | |||
| Nilsson et al. [46] | 775 | >60 | 1–19 teeth | 2.0 (AOR) | 1.1 | 3.6 | 0.03 |
| Luo et al. [45] | 6377 | >50 | ≥8 teeth | 1.46 (AOR) | 1.09 | 1.95 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, B.; Bizzoca, M.E.; Musella, G.; De Vito, D.; Lo Muzio, L.; Ballini, A.; Cantore, S.; Pisani, F. Tooth Loss in Periodontitis Patients—A Risk Factor for Mild Cognitive Impairment: A Systematic Review and Meta—Analysis. J. Pers. Med. 2024, 14, 953. https://doi.org/10.3390/jpm14090953
Agarwal B, Bizzoca ME, Musella G, De Vito D, Lo Muzio L, Ballini A, Cantore S, Pisani F. Tooth Loss in Periodontitis Patients—A Risk Factor for Mild Cognitive Impairment: A Systematic Review and Meta—Analysis. Journal of Personalized Medicine. 2024; 14(9):953. https://doi.org/10.3390/jpm14090953
Chicago/Turabian StyleAgarwal, Bhawna, Maria Eleonora Bizzoca, Gennaro Musella, Danila De Vito, Lorenzo Lo Muzio, Andrea Ballini, Stefania Cantore, and Flavio Pisani. 2024. "Tooth Loss in Periodontitis Patients—A Risk Factor for Mild Cognitive Impairment: A Systematic Review and Meta—Analysis" Journal of Personalized Medicine 14, no. 9: 953. https://doi.org/10.3390/jpm14090953
APA StyleAgarwal, B., Bizzoca, M. E., Musella, G., De Vito, D., Lo Muzio, L., Ballini, A., Cantore, S., & Pisani, F. (2024). Tooth Loss in Periodontitis Patients—A Risk Factor for Mild Cognitive Impairment: A Systematic Review and Meta—Analysis. Journal of Personalized Medicine, 14(9), 953. https://doi.org/10.3390/jpm14090953











