Evaluating the Survival Benefits of Perioperative Chemotherapy in Frail and Morbid Muscle-Invasive Bladder Cancer Patients †
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Frailty and Morbidity Assessment
2.3. Perioperative Chemotherapy
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Perioperative Chemotherapy
3.3. Survival Analyses
3.4. Postoperative Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Ginkel, N.; Hermans, T.J.N.; Meijer, D.; Boormans, J.L.; Voortman, J.; Mertens, L.; van Beek, S.C.; Vis, A.N.; the Dutch Cystectomy Snapshot Research Group. Survival outcomes of patients with muscle-invasive bladder cancer according to pathological response at radical cystectomy with or without neo-adjuvant chemotherapy: A case-control matching study. Int. Urol. Nephrol. 2022, 54, 3145–3152. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; de Vere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- Hamid, A.R.A.H.; Ridwan, F.R.; Parikesit, D.; Widia, F.; Mochtar, C.A.; Umbas, R. Meta-analysis of neoadjuvant chemotherapy compared to radical cystectomy alone in improving overall survival of muscle-invasive bladder cancer patients. BMC Urol. 2020, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Shariat, S.F.; Lasky, R.E.; Skinner, E.C.; Novara, G.; Lerner, S.P.; Fradet, Y.; Bastian, P.J.; Kassouf, W.; Karakiewicz, P.I.; et al. The effectiveness of off-protocol adjuvant chemotherapy for patients with urothelial carcinoma of the urinary bladder. Clin. Cancer Res. 2010, 16, 4461–4467. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, M.; Kaul, S.; Fleishman, A.; Korets, R.; Chang, P.; Wagner, A.; Kim, S.; Bellmunt, J.; Kaplan, I.; Olumi, A.F.; et al. Adjuvant chemotherapy versus observation following radical cystectomy for locally advanced urothelial carcinoma of the bladder. Urol. Oncol. 2022, 40, 274.e15–274.e23. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Skoneczna, I.; Kerst, J.M.; Albersd, P.; Fossa, S.D.; Agerbaek, M.; Dumez, H.; de Santis, M.; Théodore, C.; Leahy, M.G.; et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): An intergroup, open-label, randomised phase 3 trial. Lancet. Oncol. 2015, 16, 76–86. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Yagoda, A.; Scher, H.I.; Watson, R.C.; Ahmed, T.; Weiselberg, L.R.; Geller, N.; Hollander, P.S.; Herr, H.W.; Sogani, P.C.; et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J. Urol. 1985, 133, 403–407. [Google Scholar] [CrossRef]
- Grimberg, D.C.; Shah, A.; Molinger, J.; Whittle, J.; Gupta, R.T.; Wischmeyer, P.E.; McDonald, S.R.; Inman, B.A. Assessments of frailty in bladder cancer. Urol. Oncol. 2020, 38, 698–705. [Google Scholar] [CrossRef]
- Mottet, N.; Ribal, M.J.; Boyle, H.; De Santis, M.; Caillet, P.; Choudhury, A.; Garg, T.; Nielsen, M.; Wüthrich, P.; Gust, K.M.; et al. Management of bladder cancer in older patients: Position paper of a SIOG Task Force. J. Geriatr. Oncol. 2020, 11, 1043–1053. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA. Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef]
- Baltussen, J.C.; de Glas, N.A.; van Holstein, Y.; van der Elst, M.; Trompet, S.; Boogaard, A.U.D.; van der Plas-Krijgsman, W.; Labots, G.; Holterhues, C.; van der Bol, J.M.; et al. Chemotherapy-Related Toxic Effects and Quality of Life and Physical Functioning in Older Patients. JAMA Netw. open 2023, 6, E2339116. [Google Scholar] [CrossRef]
- Ben-David, R.; Pellegrino, F.; Alerasool, P.; Tillu, N.; Lavallee, E.; Attalla, K.; Waingankar, N.; John, S.P.; Mehrazin, R.; Moschini, M.; et al. Robotic-assisted radical cystectomy with cutaneous ureterostomies: A contemporary multicenter analysis. World J. Urol. 2024, 42, 251. [Google Scholar] [CrossRef]
- Savin, Z.; Hertzberg, H.; Schreter, E.; Ben David, R.; Bar-Yosef, Y.; Sofer, M.; Beri, A.; Yossepowitch, O.; Mano, R. Radical cystectomy and perioperative chemotherapy in octogenarians with bladder cancer. Can. Urol. Assoc. J. 2021, 15, E465–E470. [Google Scholar] [CrossRef]
- Shariat, S.F.; Milowsky, M.; Droller, M.J. Bladder cancer in the elderly. Urol. Oncol. 2009, 27, 653–667. [Google Scholar] [CrossRef]
- Vikram, R.; Sandler, C.M.; Ng, C.S. Imaging and staging of transitional cell carcinoma: Part 1, lower urinary tract. AJR. Am. J. Roentgenol. 2009, 192, 1481–1487. [Google Scholar] [CrossRef]
- Ahmadi, H.; Duddalwar, V.; Daneshmand, S. Diagnosis and Staging of Bladder Cancer. Hematol. Oncol. Clin. North Am. 2021, 35, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Psutka, S.P.; Carrasco, A.; Schmit, G.D.; Moynagh, M.R.; Boorjian, S.A.; Frank, I.; Stewart, S.B.; Thapa, P.; Tarrell, R.F.; Cheville, J.C.; et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer 2014, 120, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- McFerrin, C.; Raza, S.J.; May, A.; Davaro, F.; Siddiqui, S.; Hamilton, Z. Charlson comorbidity score is associated with readmission to the index operative hospital after radical cystectomy and correlates with 90-day mortality risk. Int. Urol. Nephrol. 2019, 51, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Chappidi, M.R.; Kates, M.; Patel, H.D.; Tosoian, J.J.; Kaye, D.R.; Sopko, N.A.; Lascano, D.; Liu, J.-J.; McKiernan, J.; Bivalacqua, T.J. Frailty as a marker of adverse outcomes in patients with bladder cancer undergoing radical cystectomy. Urol. Oncol. 2016, 34, 256.e1–256.e6. [Google Scholar] [CrossRef] [PubMed]
- Koppie, T.M.; Serio, A.M.; Vickers, A.J.; Vora, K.; Dalbagni, G.; Donat, S.M.; Herr, H.W.; Bochner, B.H. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 2008, 112, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Gupta, S.; Kitchlu, A.; Meraz-Munoz, A.; North, S.A.; Alimohamed, N.S.; Blais, N.; Sridhar, S.S. Defining cisplatin eligibility in patients with muscle-invasive bladder cancer. Nat. Rev. Urol. 2021, 18, 104–114. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Ornaghi, P.I.; Afferi, L.; Antonelli, A.; Cerruto, M.A.; Mordasini, L.; Mattei, A.; Baumeister, P.; Marra, G.; Krajewski, W.; Mari, A.; et al. Frailty impact on postoperative complications and early mortality rates in patients undergoing radical cystectomy for bladder cancer: A systematic review. Arab J. Urol. 2020, 19, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Savin, Z.; Yossepowitch, O.; Lazarovich, A.; Rosenzwieg, B.; Shashar, R.; Hoffman, A.; Gal, J.; Haifler, M.; Pilosov, I.; Frifeld, Y.; et al. 11-item modified frailty index and outcomes after radical cystectomy. J. Geriatr. Oncol. 2023, 14, 101627. [Google Scholar] [CrossRef] [PubMed]
- Woldu, S.L.; Sanli, O.; Clinton, T.N.; Lotan, Y. Validating the predictors of outcomes after radical cystectomy for bladder cancer. Cancer 2019, 125, 223–231. [Google Scholar] [CrossRef]

| Variables | F-M Cohort (n = 102) | Non-Frail, Healthier Patients (n = 189) | p-Value |
|---|---|---|---|
| Age at surgery [years], median (IQR) | 72 (IQR 68–79) | 67 (61–73) | <0.001 |
| eGFR at presentation [mL/min/1.73 m2], median (IQR) | 59 (IQR 44–87) | 78 (59–96) | <0.001 |
| Albumin [g/dL], median (IQR) | 39 (36–43) | 40 (36–43) | 0.58 |
| CCI score, median (IQR) | 6 (5–8) | 3 (2–5) | <0.001 |
| mFI score, median (IQR) | 2 (2–3) | 1 (0–1) | <0.001 |
| Orthotopic diversion, n (%) | 4 (4%) | 39 (21%) | <0.001 |
| Perioperative chemotherapy, n (%) | 45 (44%) | 97 (51%) | 0.39 |
| NAC, n (%) | 32 (31%) | 76 (40%) | 0.20 |
| AC, n (%) Both, n (%) | 19 (19%) 6 (6%) | 33 (17%) 12 (6%) | 0.75 1.00 |
| Variables | Non-POC (n = 57) | POC (n = 45) | p-Value |
|---|---|---|---|
| Clinical variables | |||
| Age at surgery [years], median (IQR) | 74 (69–81) | 72 (66–76) | 0.004 |
| eGFR at presentation [mL/min/1.73 m2], median (IQR) | 58 (36–86) | 66 (48–99) | 0.04 |
| Male-to-female ratio | 10:1 | 2.5:1 | 0.01 |
| CCI score, median (IQR) | 7 (5–8) | 5 (4–6) | <0.001 |
| mFI score, median (IQR) | 2 (2–3) | 2 (2–3) | 0.27 |
| Albumin [g/dL], median (IQR) Orthotopic diversion, n (%) | 39 (36–43) 2 (4%) | 39 (36–43) 2 (7%) | 0.54 1.00 |
| Preoperative oncological variables | |||
| Clinical T stage | 0.08 | ||
| cT1-2, n (%) | 49 (86%) | 32 (71%) | |
| cT3-4, n (%) | 8 (14%) | 13 (29%) | |
| Clinical N stage | 0.2 | ||
| cN0, n (%) | 53 (93%) | 38 (84%) | |
| cN+, n (%) | 4 (7%) | 7 (16%) | |
| Pathological variables | |||
| Pathological T stage | 0.01 | ||
| pT0, n (%) | 7 (12%) | 11 (24%) | |
| pT1-2, n (%) | 25 (44%) | 8 (18%) | |
| pT3-4, n (%) | 25 (44%) | 26 (58%) | |
| Pathological N stage | 0.02 | ||
| pN0, n (%) | 46 (80%) | 26 (58%) | |
| pN+, n (%) | 11 (20%) | 19 (42%) | |
| Prostate cancer, n (%) Grade 1 Grade 2+ | 6 (11%) 5 (9%) 1 (2%) | 8 (18%) 5 (11%) 3 (7%) | 0.39 |
| Variables | HR | 95% CI | p-Value |
|---|---|---|---|
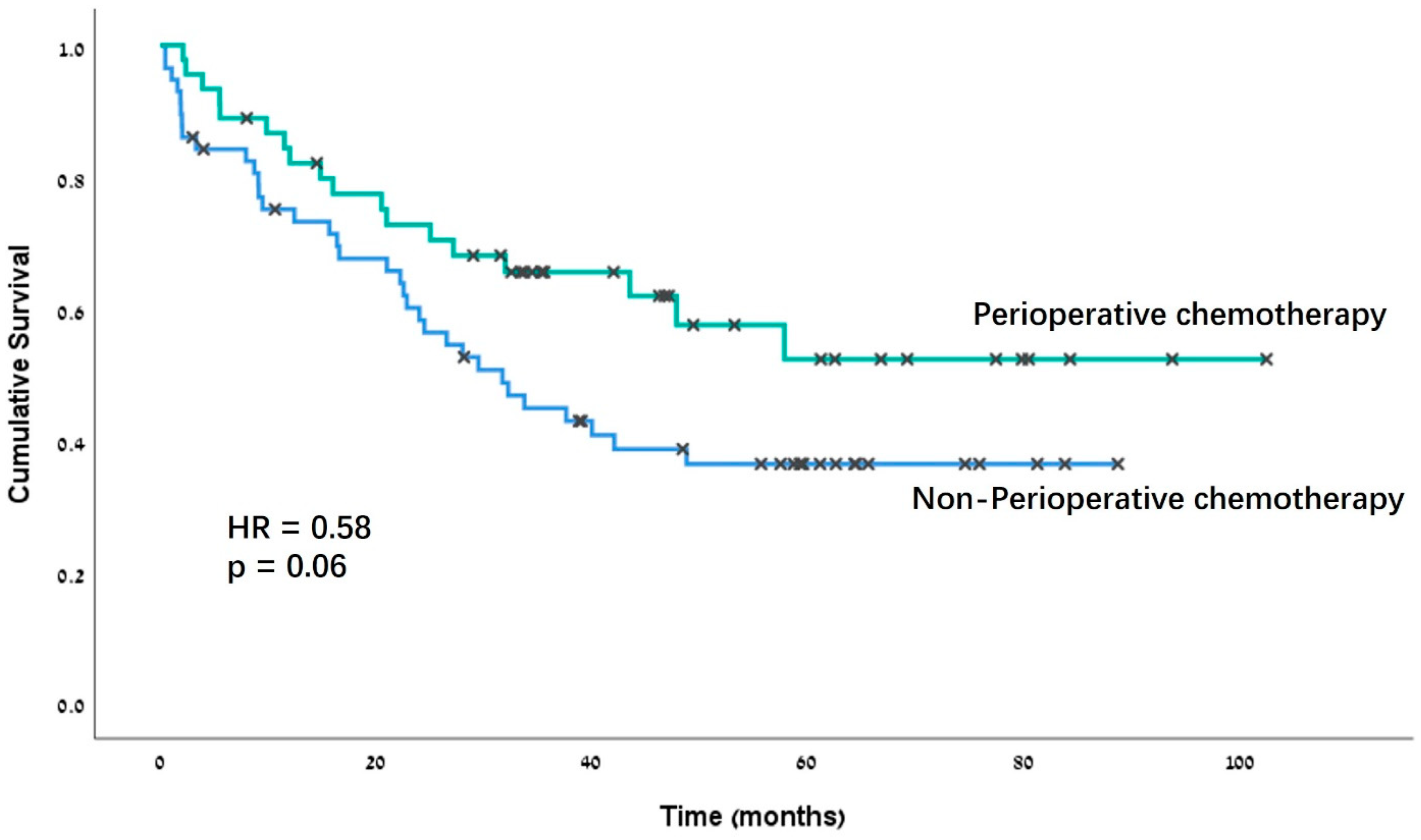
| Perioperative chemotherapy | 0.79 | 0.4–1.56 | 0.50 |
| Age | 1.08 | 1.03–1.14 | 0.002 |
| eGFR | 0.99 | 0.98–1.01 | 0.59 |
| Gender | 2.99 | 1.06–8.42 | 0.03 |
| CCI | 1.06 | 0.92–1.21 | 0.40 |
| Clinical T staging | 1.57 | 1.08–2.27 | 0.01 |
| Clinical N staging | 1.72 | 0.89–3.3 | 0.10 |
| Variables | NAC (n = 32) | Non-NAC (n = 70) | p-Value |
|---|---|---|---|
| Overall complications, n (%) | 15 (47%) | 47 (67%) | 0.20 |
| Major complications, n (%) | 2 (6%) | 13 (19%) | 0.14 |
| Minor complications, n (%) | 13 (41%) | 34 (48%) | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savin, Z.; Levin, L.; Lazarovich, A.; Rosenzweig, B.; Shashar, R.; Hoffman, A.; Gal, J.; Haifler, M.; Pilosov, I.; Freifeld, Y.; et al. Evaluating the Survival Benefits of Perioperative Chemotherapy in Frail and Morbid Muscle-Invasive Bladder Cancer Patients. J. Pers. Med. 2024, 14, 954. https://doi.org/10.3390/jpm14090954
Savin Z, Levin L, Lazarovich A, Rosenzweig B, Shashar R, Hoffman A, Gal J, Haifler M, Pilosov I, Freifeld Y, et al. Evaluating the Survival Benefits of Perioperative Chemotherapy in Frail and Morbid Muscle-Invasive Bladder Cancer Patients. Journal of Personalized Medicine. 2024; 14(9):954. https://doi.org/10.3390/jpm14090954
Chicago/Turabian StyleSavin, Ziv, Lin Levin, Alon Lazarovich, Barak Rosenzweig, Reut Shashar, Azik Hoffman, Jonathan Gal, Miki Haifler, Ilona Pilosov, Yuval Freifeld, and et al. 2024. "Evaluating the Survival Benefits of Perioperative Chemotherapy in Frail and Morbid Muscle-Invasive Bladder Cancer Patients" Journal of Personalized Medicine 14, no. 9: 954. https://doi.org/10.3390/jpm14090954





