Minimally Invasive Therapies for Knee Osteoarthritis
Abstract
1. Introduction
2. Osteoarthritis Background
2.1. Epidemiology, Etiology, and Risk Factors
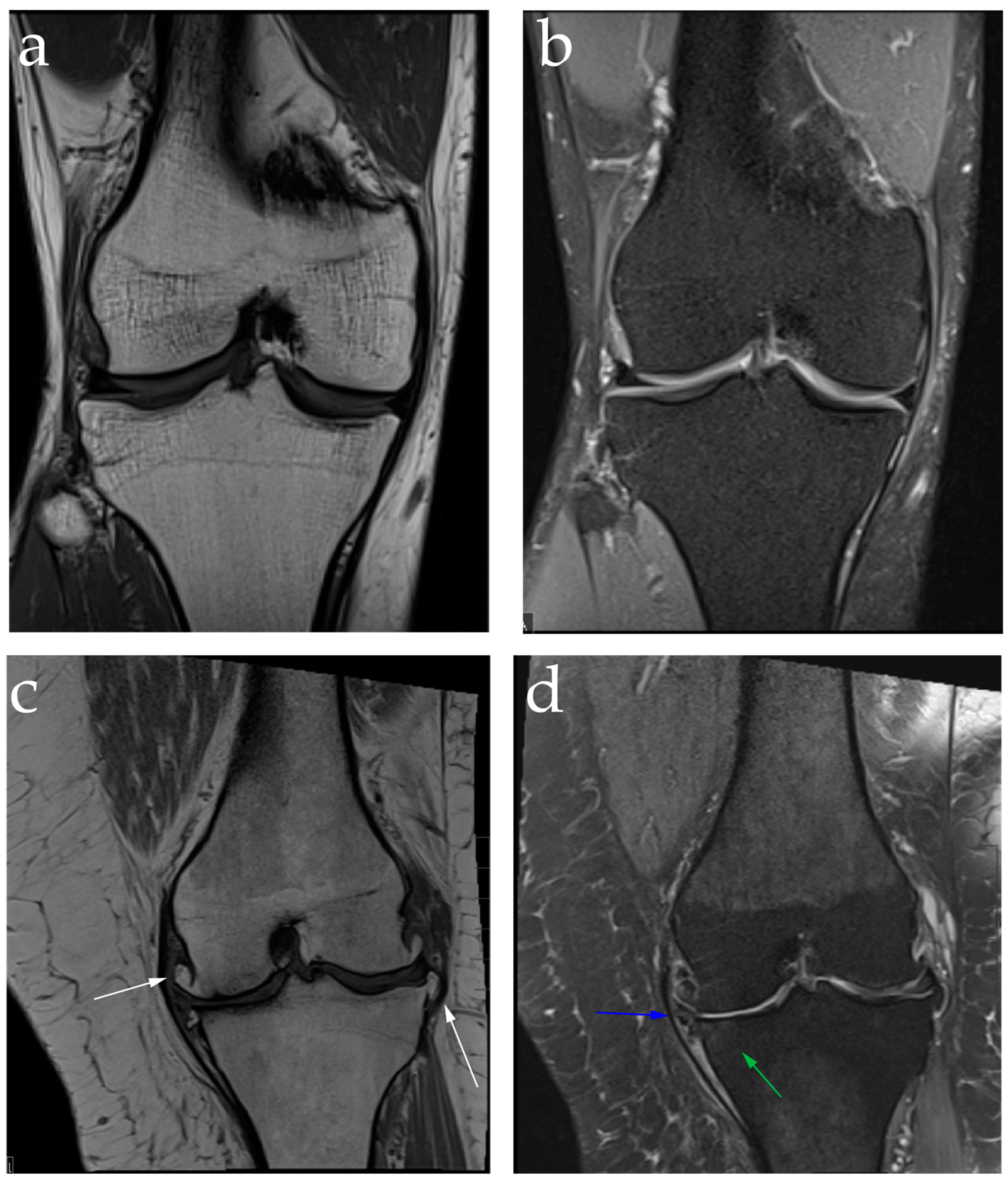
2.2. Diagnosis: Clinical Features and Image Findings
2.3. Current Management and Challenges in Treatments
3. Intra-Articular Steroid Injections
3.1. Background and Mechanism of Action of Intra-Articular Steroid Injections
3.2. Efficacy of Intra-Articular Steroid Injections
3.3. Complications of Intra-Articular Steroid Injections
3.4. Current Recommendations and Conclusion on Intra-Articular Steroid Injections
4. Intra-Articular Hyaluronic Acid Injection
4.1. Background and Mechanism of Action of Intra-Articular Hyaluronic Acid Injection
4.2. Efficacy of Intra-Articular Hyaluronic Acid Injection
4.3. Complications of Intra-Articular Hyaluronic Acid Injection
4.4. Recommendations and Conclusions on Intra-Articular Hyaluronic Acid Injection
5. Intra-Articular Platelet-Rich Plasma Injection
5.1. Background and Mechanism of Action of Intra-Articular Platelet-Rich Plasma Injection
5.2. Efficacy of Intra-Articular Platelet-Rich Plasma Injection
5.3. Preparation of Intra-Articular Platelet-Rich Plasma Injection
5.4. Complications of Intra-Articular Platelet-Rich Plasma Injection
5.5. Recommendations and Conclusions on Intra-Articular Platelet-Rich Plasma Injection
6. Technique and Approach to Intra-Articular Injections
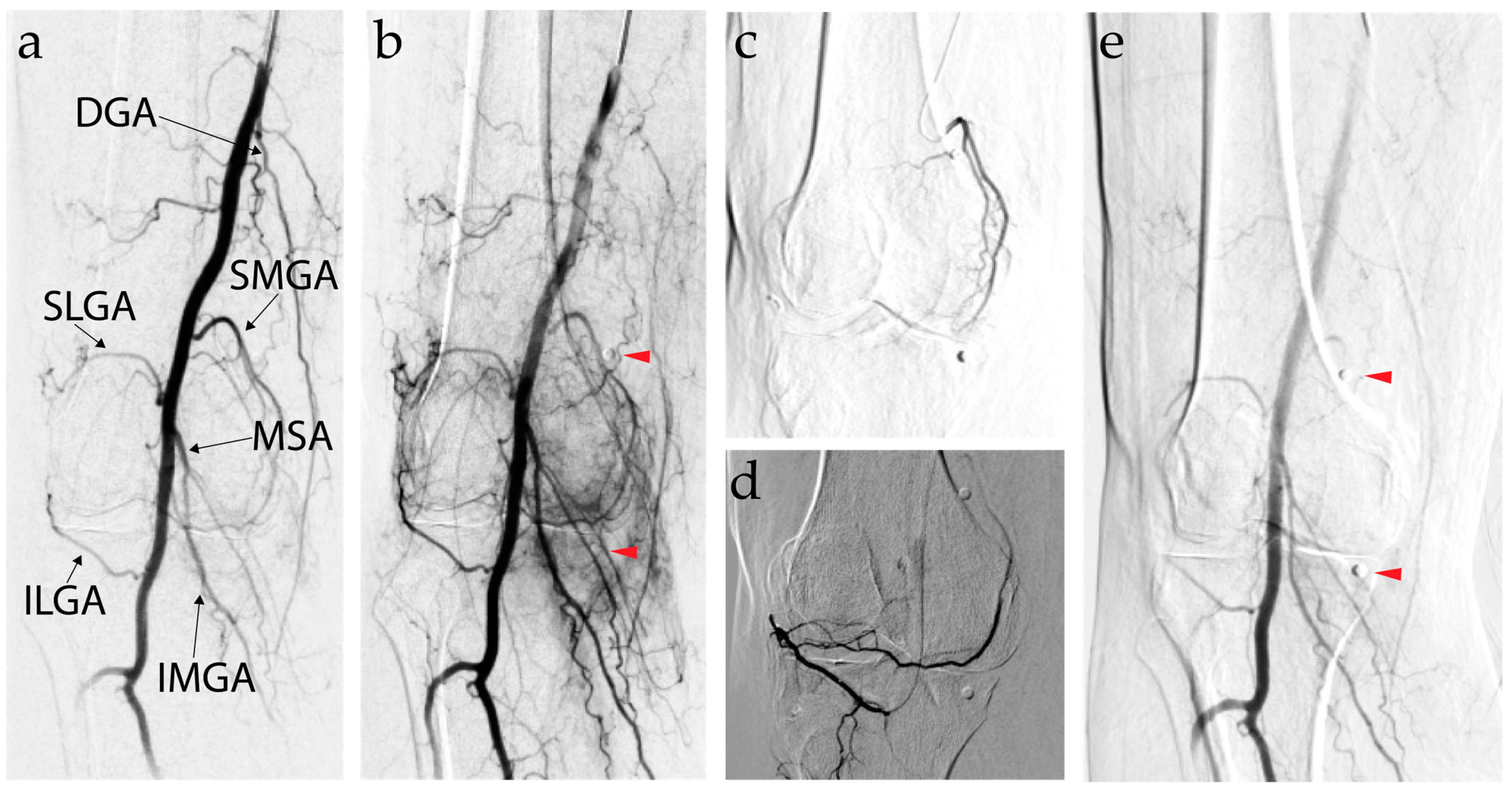
7. Genicular Artery Embolization
7.1. Background and Mechanism of Action of Genicular Artery Embolization
7.2. Efficacy of Genicular Artery Embolization
7.3. Genicular Artery Embolization Technique
7.4. Complications of Genicular Artery Embolization
7.5. Recommendations and Conclusions on Genicular Artery Embolization
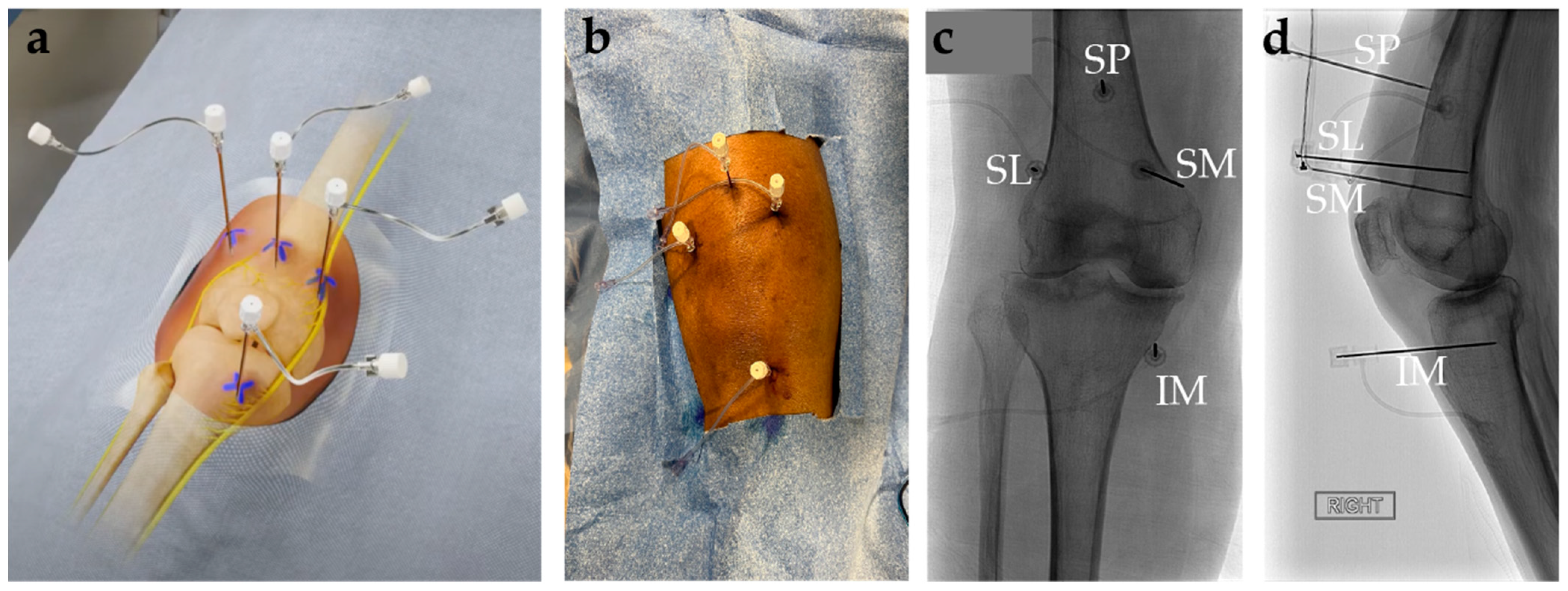
8. Genicular Nerve Ablation
8.1. Background and Mechanism of Action of Genicular Nerve Ablation
8.2. Efficacy of Genicular Nerve Ablation
8.3. Genicular Nerve Ablation Technique
8.4. Complications of Genicular Nerve Ablation
8.5. Recommendations and Conclusions on Genicular Nerve Ablation
9. Genicular Nerve Cryoneurolysis
9.1. Background and Mechanism of Action of Genicular Nerve Cryoneurolysis
9.2. Efficacy of Genicular Nerve Cryoneurolysis
9.3. Genicular Nerve Cryoneurolysis Technique
9.4. Complications of Genicular Nerve Cryoneurolysis
9.5. Recommendations and Conclusions on Genicular Nerve Cryoneurolysis
10. Regenerative and Experimental Therapies
10.1. Intra-Articular Collagen Injections
10.2. Mesenchymal Stem Cells
10.3. Hydrogels
11. Role of Artificial Intelligence in Decision-Making and Patient Selection
AI Recommendations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidari, B. Knee Osteoarthritis Prevalence, Risk Factors, Pathogenesis and Features: Part I. Casp. J. Intern. Med. 2011, 2, 205. [Google Scholar]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- KELLGREN, J.H.; LAWRENCE, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Bedson, J.; Croft, P.R. The Discordance between Clinical and Radiographic Knee Osteoarthritis: A Systematic Search and Summary of the Literature. BMC Musculoskelet. Disord. 2008, 9, 116. [Google Scholar] [CrossRef]
- Yusuf, E.; Kortekaas, M.C.; Watt, I.; Huizinga, T.W.J.; Kloppenburg, M. Do Knee Abnormalities Visualised on MRI Explain Knee Pain in Knee Osteoarthritis? A Systematic Review. Ann. Rheum. Dis. 2011, 70, 60–67. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The Long-Term Consequence of Anterior Cruciate Ligament and Meniscus Injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef]
- Faber, S.; Brown, A.; Cottreau, J. Safety of Oral and Topical Nonsteroidal Anti-Inflammatory Drugs. Orthop. Nurs. 2024, 43, 234–237. [Google Scholar] [CrossRef]
- Lim, W.B.; Al-Dadah, O. Conservative Treatment of Knee Osteoarthritis: A Review of the Literature. World J. Orthop. 2022, 13, 212. [Google Scholar] [CrossRef]
- DeRogatis, M.; Anis, H.K.; Sodhi, N.; Ehiorobo, J.O.; Chughtai, M.; Bhave, A.; Mont, M.A. Non-Operative Treatment Options for Knee Osteoarthritis. Ann. Transl. Med. 2019, 7, S245. [Google Scholar] [CrossRef]
- The American Academy of Orthopaedic Surgeons Board of Directors Management of Osteoarthritis of the Knee (Non-Arthroplasty) Evidence-Based Clinical Practice Guideline. Available online: https://www.aaos.org/oak3cpg (accessed on 16 March 2024).
- Rönn, K.; Reischl, N.; Gautier, E.; Jacobi, M. Current Surgical Treatment of Knee Osteoarthritis. Arthritis 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Imani, F.; Patel, V.B. Therapeutic Challenges for Knee Osteoarthritis. Anesthesiol. Pain Med. 2019, 9, 95377. [Google Scholar] [CrossRef] [PubMed]
- Jessar, R.A.; Ganzell, M.A.; Ragan, C. The Action of Hydrocortisone in Synovial Inflammation. J. Clin. Investig. 1953, 32, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular Injections (Corticosteroid, Hyaluronic Acid, Platelet Rich Plasma) for the Knee Osteoarthritis. World J. Orthop. 2014, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Arroll, B.; Goodyear-Smith, F. Corticosteroid Injections for Osteoarthritis of the Knee: Meta-Analysis. BMJ Br. Med. J. 2004, 328, 869. [Google Scholar] [CrossRef]
- Hepper, C.T.; Halvorson, J.J.; Duncan, S.T.; Gregory, A.J.M.; Dunn, W.R.; Spindler, K.P. The Efficacy and Duration of Intra-Articular Corticosteroid Injection for Knee Osteoarthritis: A Systematic Review of Level I Studies. J. Am. Acad. Orthop. Surg. 2009, 17, 638–646. [Google Scholar] [CrossRef]
- Godwin, M.; Dawes, M. Intra-Articular Steroid Injections for Painful Knees. Systematic Review with Meta-Analysis. Can. Fam. Physician 2004, 50, 241. [Google Scholar]
- Najm, A.; Alunno, A.; Gwinnutt, J.M.; Weill, C.; Berenbaum, F. Efficacy of Intra-Articular Corticosteroid Injections in Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Jt. Bone Spine 2021, 88, 105198. [Google Scholar] [CrossRef]
- Pyne, D.; Ioannou, Y.; Mootoo, R.; Bhanji, A. Intra-Articular Steroids in Knee Osteoarthritis: A Comparative Study of Triamcinolone Hexacetonide and Methylprednisolone Acetate. Clin. Rheumatol. 2004, 23, 116–120. [Google Scholar] [CrossRef]
- Buyuk, A.F.; Kilinc, E.; Camurcu, I.Y.; Camur, S.; Ucpunar, H.; Kara, A. Compared Efficacy of Intra-Articular Injection of Methylprednisolone and Triamcinolone. Acta Ortop. Bras. 2017, 25, 206. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Hunter, D.J.; Cohen, S.B.; Kraus, V.B.; Berenbaum, F.; Lieberman, J.R.; Jones, D.G.; Spitzer, A.I.; Jevsevar, D.S.; Katz, N.P.; et al. Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain: A Double-Blinded, Randomized, Placebo-Controlled, Multinational Study. J. Bone Jt. Surg. Am. Vol. 2018, 100, 666–677. [Google Scholar] [CrossRef]
- Langworthy, M.J.; Conaghan, P.G.; Ruane, J.J.; Kivitz, A.J.; Lufkin, J.; Cinar, A.; Kelley, S.D. Efficacy of Triamcinolone Acetonide Extended-Release in Participants with Unilateral Knee Osteoarthritis: A Post Hoc Analysis. Adv. Ther. 2019, 36, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Abdi, S. Complications of Joint, Tendon, and Muscle Injections. Tech. Reg. Anesth. Pain Manag. 2007, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Ibad, H.A.; Kasaeian, A.; Ghotbi, E.; Roemer, F.; Jarraya, M.; Ghazi-Sherbaf, F.; Dolatshahi, M.; Demehri, S.; Guermazi, A. Longitudinal MRI-Defined Cartilage Loss and Radiographic Joint Space Narrowing Following Intra-Articular Corticosteroid Injection for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Osteoarthr. Imaging 2023, 3, 100157. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Raynauld, J.P.; Abram, F.; Dorais, M.; Paiement, P.; Martel-Pelletier, J. Intra-Articular Corticosteroid Knee Injection Induces a Reduction in Meniscal Thickness with No Treatment Effect on Cartilage Volume: A Case–Control Study. Sci. Rep. 2020, 10, 13789. [Google Scholar] [CrossRef]
- Buelt, A.; Narducci, D.M. Osteoarthritis Management: Updated Guidelines from the American College of Rheumatology and Arthritis Foundation. Am. Fam. Physician 2021, 103, 120–121. [Google Scholar]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Stephens, M.B.; Beutler, A.I.; O’connor, F.G. Musculoskeletal Injections: A Review of the Evidence. Am. Fam. Physician 2008, 78, 971–976. [Google Scholar]
- Kim, Y.M.; Joo, Y.B.; Song, J.-H. Preoperative Intra-Articular Steroid Injections within 3 Months Increase the Risk of Periprosthetic Joint Infection in Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2023, 18, 148. [Google Scholar] [CrossRef]
- Migliore, A.; Procopio, S. Effectiveness and Utility of Hyaluronic Acid in Osteoarthritis. Clin. Cases Miner. Bone Metab. 2015, 12, 31–33. [Google Scholar] [CrossRef]
- Webner, D.; Huang, Y.; Hummer, C.D. Intraarticular Hyaluronic Acid Preparations for Knee Osteoarthritis: Are Some Better Than Others? Cartilage 2021, 13, 1619S–1636S. [Google Scholar] [CrossRef]
- Grishko, V.; Xu, M.; Ho, R.; Mates, A.; Watson, S.; Kim, J.T.; Wilson, G.L.; Pearsall, A.W. Effects of Hyaluronic Acid on Mitochondrial Function and Mitochondria-Driven Apoptosis Following Oxidative Stress in Human Chondrocytes. J. Biol. Chem. 2009, 284, 9132–9139. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Rabbani, S.A.; Wadhwa, T. Role and Effectiveness of Intra-Articular Injection of Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Systematic Review. Cureus 2022, 14, e24503. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The Mechanism of Action for Hyaluronic Acid Treatment in the Osteoarthritic Knee: A Systematic Review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Natov, N.S.; Dasi, U.R.; Schmid, C.H.; McAlindon, T.E. Therapeutic Trajectory Following Intra-Articular Hyaluronic Acid Injection in Knee Osteoarthritis–Meta-Analysis. Osteoarthr. Cartil. 2011, 19, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.V.; Jüni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapié, C.A.; da Costa, B.R. Viscosupplementation for Knee Osteoarthritis: Systematic Review and Meta-Analysis. BMJ 2022, 378, e069722. [Google Scholar] [CrossRef]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W.; Schuler, T.C. Understanding the Minimum Clinically Important Difference: A Review of Concepts and Methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef]
- Miller, L.E.; Bhattacharyya, S.; Parrish, W.R.; Fredericson, M.; Bisson, B.; Altman, R.D. Safety of Intra-Articular Hyaluronic Acid for Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized Trials Involving More than 8000 Patients. Cartilage 2021, 13, 351S–363S. [Google Scholar] [CrossRef]
- Kaniecki, T. Hyaluronic Acid. Available online: https://rheumatology.org/patients/hyaluronic-acid (accessed on 28 April 2024).
- Ong, K.L.; Farr, J.; Gudeman, A.S.; Murray, I.R.; McIntyre, L.F.; Hummer, C.D.; Ngai, W.; Lau, E.; Altman, R.D.; Sherman, S.L. Risk of Severe Acute Localized Reactions for Different Intraarticular Hyaluronic Acid Knee Injections in a Real-World Setting. Cartilage 2021, 13, 376S–386S. [Google Scholar] [CrossRef]
- Jain, N.K.; Gulati, M. Platelet-Rich Plasma: A Healing Virtuoso. Blood Res. 2016, 51, 3–5. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; Dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The Regenerative Mechanisms of Platelet-Rich Plasma: A Review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, P.; Liao, B.; You, H.; Cai, Y. Effects and Action Mechanisms of Individual Cytokines Contained in PRP on Osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 713. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Yudoh, K.; Masuko, K. The Potential Role of Vascular Endothelial Growth Factor (VEGF) in Cartilage: How the Angiogenic Factor Could Be Involved in the Pathogenesis of Osteoarthritis? Osteoarthr. Cartil. 2008, 16, 279–286. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M. Differential Role of Transforming Growth Factor-Beta in an Osteoarthritic or a Healthy Joint. J. Bone Metab. 2018, 25, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Luo, L.; Gui, T.; Yu, F.; Yan, L.; Yao, L.; Zhong, L.; Yu, W.; Han, B.; Patel, J.M.; et al. Targeting Cartilage EGFR Pathway for Osteoarthritis Treatment. Sci. Transl. Med. 2021, 13, eabb3946. [Google Scholar] [CrossRef]
- Degen, R.M.; Bernard, J.A.; Oliver, K.S.; Dines, J.S. Commercial Separation Systems Designed for Preparation of Platelet-Rich Plasma Yield Differences in Cellular Composition. HSS J. 2017, 13, 75–80. [Google Scholar] [CrossRef]
- Magalon, J.; Bausset, O.; Serratrice, N.; Giraudo, L.; Aboudou, H.; Veran, J.; Magalon, G.; Dignat-Georges, F.; Sabatier, F. Characterization and Comparison of 5 Platelet-Rich Plasma Preparations in a Single-Donor Model. Arthroscopy 2014, 30, 629–638. [Google Scholar] [CrossRef]
- Castillo, T.N.; Pouliot, M.A.; Kim, H.J.; Dragoo, J.L. Comparison of Growth Factor and Platelet Concentration from Commercial Platelet-Rich Plasma Separation Systems. Am. J. Sports Med. 2011, 39, 266–271. [Google Scholar] [CrossRef]
- Chiu, Y.M.; Wang, D.; McCormick, Z.; Diwan, S.; Candido, K.D.; Chang Chien, G.C. Safety of Intra-Articular Platelet Rich Plasma Injections for Large Joint Osteoarthritis: A Review Article. Curr. Orthop. Pract. 2022, 33, 480–486. [Google Scholar] [CrossRef]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr. Rev. Musculoskelet. Med. 2018, 11, 624–634. [Google Scholar] [CrossRef]
- Di Martino, A.; Boffa, A.; Andriolo, L.; Romandini, I.; Altamura, S.A.; Cenacchi, A.; Roverini, V.; Zaffagnini, S.; Filardo, G. Leukocyte-Rich versus Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Trial. Am. J. Sports Med. 2022, 50, 609–617. [Google Scholar] [CrossRef]
- Irrgang, J.J.; Anderson, A.F.; Boland, A.L.; Harner, C.D.; Kurosaka, M.; Neyret, P.; Richmond, J.C.; Shelborne, K.D. Development and Validation of the International Knee Documentation Committee Subjective Knee Form. Am. J. Sports Med. 2001, 29, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dhillon, M.S.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment with Platelet-Rich Plasma Is More Effective than Placebo for Knee Osteoarthritis: A Prospective, Double-Blind, Randomized Trial. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-Articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 2021, 326, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 2023, 10, 1204144. [Google Scholar] [CrossRef]
- Saita, Y.; Kobayashi, Y.; Nishio, H.; Wakayama, T.; Fukusato, S.; Uchino, S.; Momoi, Y.; Ikeda, H.; Kaneko, K. Predictors of Effectiveness of Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 4514. [Google Scholar] [CrossRef]
- Maricar, N.; Parkes, M.J.; Callaghan, M.J.; Felson, D.T.; O’Neill, T.W. Where and How to Inject the Knee—A Systematic Review. Semin. Arthritis Rheum. 2013, 43, 195–203. [Google Scholar] [CrossRef]
- Lockman, L.E.; Chb, M. Knee Joint Injections and Aspirations: The Triangle Technique. Can. Fam. Physician 2006, 52, 1403. [Google Scholar]
- Epelboym, Y.; Mandell, J.C.; Collins, J.E.; Burch, E.; Shiang, T.; Killoran, T.; Macfarlane, L.; Guermazi, A. Genicular Artery Embolization as a Treatment for Osteoarthritis Related Knee Pain: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2023, 46, 760–769. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial Inflammation in Osteoarthritis Progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Ro, D.H.; Jang, M.; Koh, J.; Choi, W.S.; Kim, H.-C.; Han, H.-S.; Choi, J.W. Mechanism of Action of Genicular Artery Embolization in a Rabbit Model of Knee Osteoarthritis. Eur. Radiol. 2022, 33, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Landers, S.; Hely, R.; Hely, A.; Harrison, B.; Page, R.S.; Maister, N.; Gwini, S.M.; Gill, S.D. Genicular Artery Embolization for Early-Stage Knee Osteoarthritis: Results from a Triple-Blind Single-Centre Randomized Controlled Trial. Bone Jt. Open 2023, 4, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Korchi, A.M.; Shinjo, T.; Kato, S. Transcatheter Arterial Embolization as a Treatment for Medial Knee Pain in Patients with Mild to Moderate Osteoarthritis. Cardiovasc. Interv. Radiol. 2015, 38, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Taslakian, B.; Miller, L.E.; Mabud, T.S.; Macaulay, W.; Samuels, J.; Attur, M.; Alaia, E.F.; Kijowski, R.; Hickey, R.; Sista, A.K. Genicular Artery Embolization for Treatment of Knee Osteoarthritis Pain: Systematic Review and Meta-Analysis. Osteoarthr. Cartil. Open 2023, 5, 100342. [Google Scholar] [CrossRef]
- Sterbis, E.; Casadaban, L. Genicular Artery Embolization Technique. Tech. Vasc. Interv. Radiol. 2023, 26, 100878. [Google Scholar] [CrossRef]
- Sapoval, M.; Querub, C.; Pereira, H.; Pellerin, O.; Boeken, T.; Di Gaeta, A.; Ahmar, M.A.; Lefevre-Colau, M.-M.; Nguyen, C.; Daste, C.; et al. Genicular Artery Embolization for Knee Osteoarthritis: Results of the LipioJoint-1 Trial. Diagn. Interv. Imaging 2024, 105, 144–150. [Google Scholar] [CrossRef]
- Little, M.W.; Gibson, M.; Briggs, J.; Speirs, A.; Yoong, P.; Ariyanayagam, T.; Davies, N.; Tayton, E.; Tavares, S.; MacGill, S.; et al. Genicular artEry embolizatioN in patiEnts with oSteoarthrItiS of the Knee (GENESIS) Using Permanent Microspheres: Interim Analysis. Cardiovasc. Interv. Radiol. 2021, 44, 931–940. [Google Scholar] [CrossRef]
- Padia, S.A.; Genshaft, S.; Blumstein, G.; Plotnik, A.; Kim, G.H.J.; Gilbert, S.J.; Lauko, K.; Stavrakis, A.I. Genicular Artery Embolization for the Treatment of Symptomatic Knee Osteoarthritis. JBJS Open Access 2021, 6, e21.00085. [Google Scholar] [CrossRef]
- Wang, B.; Tai, T.-W.; Liang, K.-W.; Wang, C.-K.; Liu, Y.-S.; Huang, M.-T.; Chang, C.-W. Short-Term Effects of Genicular Artery Embolization on Symptoms and Bone Marrow Abnormalities in Patients with Refractory Knee Osteoarthritis. J. Vasc. Interv. Radiol. 2023, 34, 1126–1134.e2. [Google Scholar] [CrossRef]
- Liu, S.; Swilling, D.; Morris, E.M.; Macaulay, W.; Golzarian, J.; Hickey, R.; Taslakian, B. Genicular Artery Embolization: A Review of Essential Anatomic Considerations. J. Vasc. Interv. Radiol. 2024, 35, 487–496.e6. [Google Scholar] [CrossRef]
- Tran, J.; Peng, P.W.H.; Chan, V.W.S.; Agur, A.M.R. Overview of Innervation of Knee Joint. Phys. Med. Rehabil. Clin. N. Am. 2021, 32, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-J.; Hwang, S.-J.; Song, J.-G.; Leem, J.-G.; Kang, Y.-U.; Park, P.-H.; Shin, J.-W. Radiofrequency Treatment Relieves Chronic Knee Osteoarthritis Pain: A Double-Blind Randomized Controlled Trial. Pain 2011, 152, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Pritzlaff, S.; Jung, M.J.; Ghosh, P.; Hagedorn, J.M.; Tate, J.; Scarfo, K.; Strand, N.; Chakravarthy, K.; Sayed, D.; et al. Latest Evidence-Based Application for Radiofrequency Neurotomy (LEARN): Best Practice Guidelines from the American Society of Pain and Neuroscience (ASPN). J. Pain Res. 2021, 14, 2807–2831. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Singam, A.; Chakole, V.; Das, S.; Sharma, V. Efficacy and Safety of Cryoablation Compared with Cooled Radiofrequency Ablation of Genicular Nerves in Advanced Osteoarthritis of the Knee: A Study Protocol of Single-Centric, Assessor-Blinded, Randomized, Parallel-Group, Non-Inferiority Study. Cardiovasc. Interv. Radiol. 2024, 47, 508–514. [Google Scholar] [CrossRef]
- Risso, R.C.; Ferraro, L.H.C.; Nouer Frederico, T.; Peng, P.W.H.; Luzo, M.V.; Debieux, P.; Sakata, R.K. Chemical Ablation of Genicular Nerve with Phenol for Pain Relief in Patients with Knee Osteoarthritis: A Prospective Study. Pain Pract. 2021, 21, 438–444. [Google Scholar] [CrossRef]
- Kidd, V.D.; Strum, S.R.; Strum, D.S.; Shah, J. Genicular Nerve Radiofrequency Ablation for Painful Knee Arthritis: The Why and the How. JBJS Essent. Surg. Tech. 2019, 9, e10. [Google Scholar] [CrossRef]
- Conger, A.; Gililland, J.; Anderson, L.; Pelt, C.E.; Peters, C.; McCormick, Z.L. Genicular Nerve Radiofrequency Ablation for the Treatment of Painful Knee Osteoarthritis: Current Evidence and Future Directions. Pain Med. 2021, 22, S20–S23. [Google Scholar] [CrossRef]
- Wong, P.K.-W.; Kokabi, N.; Guo, Y.; Reiter, D.; Reimer, N.B.; Oskouei, S.; Gonzalez, F.M. Safety and Efficacy Comparison of Three- vs Four-Needle Technique in the Management of Moderate to Severe Osteoarthritis of the Knee Using Cooled Radiofrequency Ablation. Skelet. Radiol. 2021, 50, 739–750. [Google Scholar] [CrossRef]
- Kim, S.Y.; Le, P.U.; Kosharskyy, B.; Kaye, A.D.; Shaparin, N.; Downie, S.A. Is Genicular Nerve Radiofrequency Ablation Safe? A Literature Review and Anatomical Study. Pain Physician 2016, 19, E697–E705. [Google Scholar]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-Articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef]
- Sag, A.A.; Bittman, R.; Prologo, F.; Friedberg, E.B.; Nezami, N.; Ansari, S.; Prologo, J.D. Percutaneous Image-Guided Cryoneurolysis: Applications and Techniques. Radiographics 2022, 42, 1776–1794. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, S. A Classification of Peripheral Nerve Injuries Producing Loss of Function. Brain 1951, 74, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Finneran Iv, J.J.; Ilfeld, B.M. Percutaneous Cryoneurolysis for Acute Pain Management: Current Status and Future Prospects. Expert Rev. Med. Devices 2021, 18, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kambin, P.; Casey, K.F.; Bonner, F.J.; O’Brien, E.; Shao, Z.; Ou, S. Mechanism Research of Cryoanalgesia. Neurol. Res. 1995, 17, 307–311. [Google Scholar] [CrossRef]
- Trescot, A.M. Cryoanalgesia in Interventional Pain Management. Pain Physician 2003, 6, 345–360. [Google Scholar] [CrossRef]
- Wallace, A.N.; Hillen, T.J.; Friedman, M.V.; Zohny, Z.S.; Stephens, B.H.; Greco, S.C.; Talcott, M.R.; Jennings, J.W. Percutaneous Spinal Ablation in a Sheep Model: Protective Capacity of an Intact Cortex, Correlation of Ablation Parameters with Ablation Zone Size, and Correlation of Postablation MRI and Pathologic Findings. AJNR Am. J. Neuroradiol. 2017, 38, 1653–1659. [Google Scholar] [CrossRef]
- Mihalko, W.M.; Kerkhof, A.L.; Ford, M.C.; Crockarell, J.R.; Harkess, J.W.; Guyton, J.L. Cryoneurolysis before Total Knee Arthroplasty in Patients With Severe Osteoarthritis for Reduction of Postoperative Pain and Opioid Use in a Single-Center Randomized Controlled Trial. J. Arthroplast. 2021, 36, 1590–1598. [Google Scholar] [CrossRef]
- Nygaard, N.-P.B.; Koch-Jensen, C.; Vægter, H.B.; Wedderkopp, N.; Blichfeldt-Eckhardt, M.; Gram, B. Cryoneurolysis for the Management of Chronic Pain in Patients with Knee Osteoarthritis; a Double-Blinded Randomized Controlled Sham Trial. BMC Musculoskelet. Disord. 2021, 22, 228. [Google Scholar] [CrossRef]
- Eyre, D.R.; Weis, M.A.; Wu, J.J. Articular Cartilage Collagen: An Irreplaceable Framework? Eur. Cells Mater. 2006, 12, 57–63. [Google Scholar] [CrossRef]
- Ouyang, Z.; Dong, L.; Yao, F.; Wang, K.; Chen, Y.; Li, S.; Zhou, R.; Zhao, Y.; Hu, W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. Int. J. Mol. Sci. 2023, 24, 9841. [Google Scholar] [CrossRef]
- Tarantino, D.; Mottola, R.; Palermi, S.; Sirico, F.; Corrado, B.; Gnasso, R. Intra-Articular Collagen Injections for Osteoarthritis: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 4390. [Google Scholar] [CrossRef] [PubMed]
- Martin Martin, L.S.; Massafra, U.; Bizzi, E.; Migliore, A. A Double Blind Randomized Active-Controlled Clinical Trial on the Intra-Articular Use of Md-Knee versus Sodium Hyaluronate in Patients with Knee Osteoarthritis (“Joint”). BMC Musculoskelet. Disord. 2016, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.; Murphy, M. Mesenchymal Stem Cells in Joint Disease and Repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Thoene, M.; Bejer-Olenska, E.; Wojtkiewicz, J. The Current State of Osteoarthritis Treatment Options Using Stem Cells for Regenerative Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 8925. [Google Scholar] [CrossRef]
- Iijima, H.; Isho, T.; Kuroki, H.; Takahashi, M.; Aoyama, T. Effectiveness of Mesenchymal Stem Cells for Treating Patients with Knee Osteoarthritis: A Meta-Analysis toward the Establishment of Effective Regenerative Rehabilitation. npj Regen. Med. 2018, 3, 15. [Google Scholar] [CrossRef]
- Migliorini, F.; Rath, B.; Colarossi, G.; Driessen, A.; Tingart, M.; Niewiera, M.; Eschweiler, J. Improved Outcomes after Mesenchymal Stem Cells Injections for Knee Osteoarthritis: Results at 12-Months Follow-up: A Systematic Review of the Literature. Arch. Orthop. Trauma Surg. 2020, 140, 853–868. [Google Scholar] [CrossRef]
- Agarwal, N.; Mak, C.; Bojanic, C.; To, K.; Khan, W. Meta-Analysis of Adipose Tissue Derived Cell-Based Therapy for the Treatment of Knee Osteoarthritis. Cells 2021, 10, 1365. [Google Scholar] [CrossRef]
- Muthu, S.; Patil, S.C.; Jeyaraman, N.; Jeyaraman, M.; Gangadaran, P.; Rajendran, R.L.; Oh, E.J.; Khanna, M.; Chung, H.Y.; Ahn, B.C. Comparative Effectiveness of Adipose-Derived Mesenchymal Stromal Cells in the Management of Knee Osteoarthritis: A Meta-Analysis. World J. Orthop. 2023, 14, 23. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Z.; Zhu, W.; Weng, X. Recent Developments and Current Applications of Hydrogels in Osteoarthritis. Bioengineering 2022, 9, 132. [Google Scholar] [CrossRef]
- Duan, W.L.; Zhang, L.N.; Bohara, R.; Martin-Saldaña, S.; Yang, F.; Zhao, Y.Y.; Xie, Y.; Bu, Y.Z.; Pandit, A. Adhesive Hydrogels in Osteoarthritis: From Design to Application. Mil. Med. Res. 2023, 10, 4. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.Y.; Meng, T.; Ma, X.W.; Li, H.; Li, K. Role of Hydrogels in Osteoarthritis: A Comprehensive Review. Int. J. Rheum. Dis. 2023, 26, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced Hydrogels for the Repair of Cartilage Defects and Regeneration. Bioact. Mater. 2021, 6, 998. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Liu, W.; Wang, B.; Li, J.J.; Zhao, Y.; Li, H.; Liu, A.; Du, Y.; Lin, J. Intra-Articular Injection of Cell-Laden 3D Microcryogels Empower Low-Dose Cell Therapy for Osteoarthritis in a Rat Model. Cell Transplant. 2020, 29, 0963689720932142. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Anggelia, M.R.; Liu, S.C.; Lin, C.F.; Lin, C.H. Enhancing Immunomodulatory Function of Mesenchymal Stromal Cells by Hydrogel Encapsulation. Cells 2024, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ha, C.; Lee, C.; Yoon, Y.C.; Park, Y. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613. [Google Scholar] [CrossRef]
- Song, J.S.; Hong, K.T.; Kim, N.M.; Hwangbo, B.H.; Yang, B.S.; Victoroff, B.N.; Choi, N.H. Clinical and Magnetic Resonance Imaging Outcomes After Human Cord Blood-Derived Mesenchymal Stem Cell Implantation for Chondral Defects of the Knee. Orthop. J. Sports Med. 2023, 11, 23259671231158391. [Google Scholar] [CrossRef]
- Jung, S.H.; Nam, B.J.; Choi, C.H.; Kim, S.; Jung, M.; Chung, K.; Park, J.; Jung, Y.; Kim, S.H. Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cell Implantation versus Microdrilling Combined with High Tibial Osteotomy for Cartilage Regeneration. Sci. Rep. 2024, 14, 3333. [Google Scholar] [CrossRef]
- Matas, J.; García, C.; Poblete, D.; Vernal, R.; Ortloff, A.; Luque-Campos, N.; Hidalgo, Y.; Cuenca, J.; Infante, C.; Cadiz, M.I.; et al. A Phase I Dose-Escalation Clinical Trial to Assess the Safety and Efficacy of Umbilical Cord-Derived Mesenchymal Stromal Cells in Knee Osteoarthritis. Stem Cells Transl. Med. 2024, 13, 193. [Google Scholar] [CrossRef]
- Vinikoor, T.; Dzidotor, G.K.; Le, T.T.; Liu, Y.; Kan, H.M.; Barui, S.; Chorsi, M.T.; Curry, E.J.; Reinhardt, E.; Wang, H.; et al. Injectable and Biodegradable Piezoelectric Hydrogel for Osteoarthritis Treatment. Nat. Commun. 2023, 14, 6257. [Google Scholar] [CrossRef]
- Mohammadi, S.; Salehi, M.A.; Jahanshahi, A.; Shahrabi Farahani, M.; Zakavi, S.S.; Behrouzieh, S.; Gouravani, M.; Guermazi, A. Artificial Intelligence in Osteoarthritis Detection: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2024, 32, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Chan, P.K.; Wen, C.; Fung, W.C.; Cheung, A.; Chan, V.W.K.; Cheung, M.H.; Fu, H.; Yan, C.H.; Chiu, K.Y. Artificial Intelligence in Diagnosis of Knee Osteoarthritis and Prediction of Arthroplasty Outcomes: A Review. Arthroplasty 2022, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Bastick, A.N.; Belo, J.N.; Runhaar, J.; Bierma-Zeinstra, S.M.A. What Are the Prognostic Factors for Radiographic Progression of Knee Osteoarthritis? A Meta-Analysis. Clin. Orthop. Relat. Res. 2015, 473, 2969. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.; Yu, C.; Zhang, W.; Morelli, J.N.; Kacher, D.; Li, X. MR Imaging of Knee Osteoarthritis and Correlation of Findings with Reported Patient Pain. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2010, 30, 248–254. [Google Scholar] [CrossRef]
- Bianchi, J.; Ruellas, A.; Prieto, J.C.; Li, T.; Soroushmehr, R.; Najarian, K.; Gryak, J.; Deleat-Besson, R.; Le, C.; Yatabe, M.; et al. Decision Support Systems in Temporomandibular Joint Osteoarthritis: A Review of Data Science and Artificial Intelligence Applications. Semin. Orthod. 2021, 27, 78–86. [Google Scholar] [CrossRef]
- Pongsakonpruttikul, N.; Angthong, C.; Kittichai, V.; Chuwongin, S.; Puengpipattrakul, P.; Thongpat, P.; Boonsang, S.; Tongloy, T. Artificial Intelligence Assistance in Radiographic Detection and Classification of Knee Osteoarthritis and Its Severity: A Cross-Sectional Diagnostic Study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1549–1558. [Google Scholar] [CrossRef]
- Abdullah, S.S.; Rajasekaran, M.P. Automatic Detection and Classification of Knee Osteoarthritis Using Deep Learning Approach. Radiol. Med. 2022, 127, 398–406. [Google Scholar] [CrossRef]
- Smolle, M.A.; Goetz, C.; Maurer, D.; Vielgut, I.; Novak, M.; Zier, G.; Leithner, A.; Nehrer, S.; Paixao, T.; Ljuhar, R.; et al. Artificial Intelligence-Based Computer-Aided System for Knee Osteoarthritis Assessment Increases Experienced Orthopaedic Surgeons’ Agreement Rate and Accuracy. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1053–1062. [Google Scholar] [CrossRef]
- Lenskjold, A.; Willadsen, M.; Nybing, J.; Engell, L.; Gudbergsen, H.; Troelsen, A.; Moller, A.; Boesen, M. Mass-Diagnosing Knee Osteoarthritis in a Consecutive Clinical Cohort with Artificial Intelligence. Osteoarthr. Cartil. 2022, 30, S247–S248. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Qiu, K.; Yang, F.; Wu, W. Artificial Intelligence for Detecting Temporomandibular Joint Osteoarthritis Using Radiographic Image Data: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy. PLoS ONE 2023, 18, e0288631. [Google Scholar] [CrossRef]
- Neubauer, M.; Moser, L.; Neugebauer, J.; Raudner, M.; Wondrasch, B.; Führer, M.; Emprechtinger, R.; Dammerer, D.; Ljuhar, R.; Salzlechner, C.; et al. Artificial-Intelligence-Aided Radiographic Diagnostic of Knee Osteoarthritis Leads to a Higher Association of Clinical Findings with Diagnostic Ratings. J. Clin. Med. 2023, 12, 744. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Poduval, M.; Bagaria, V. Evaluation of Artificial Intelligence Models for Osteoarthritis of the Knee Using Deep Learning Algorithms for Orthopedic Radiographs. World J. Orthop. 2022, 13, 603–614. [Google Scholar] [CrossRef]
- Hayashi, D.; Roemer, F.W.; Guermazi, A. Magnetic resonance imaging assessment of knee osteoarthritis: Current and developing new concepts and techniques. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S1), 88–95. [Google Scholar] [PubMed]
- Calivà, F.; Namiri, N.K.; Dubreuil, M.; Pedoia, V.; Ozhinsky, E.; Majumdar, S. Studying osteoarthritis with artificial intelligence applied to magnetic resonance imaging. Nature reviews. Rheumatology 2022, 18, 112–121. [Google Scholar] [CrossRef]
- Parisi, L.; RaviChandran, N.; Lanzillotta, M. Supervised Machine Learning for Aiding Diagnosis of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. TechRxiv 2020. [Google Scholar] [CrossRef]
- Sekiya, I.; Katano, H.; Guermazi, A.; Miura, Y.; Okanouchi, N.; Tomita, M.; Masumoto, J.; Kitazume, Y.; Koga, H.; Ozeki, N. Association of AI-Determined Kellgren–Lawrence Grade with Medial Meniscus Extrusion and Cartilage Thickness by AI-Based 3D MRI Analysis in Early Knee Osteoarthritis. Sci. Rep. 2023, 13, 20093. [Google Scholar] [CrossRef] [PubMed]
- Kiran, A.; Suhail, Z.; Amjad, A.; Arshed, M.A.; Zafar, Z. AI-Powered Radiology: Enhancing Efficiency and Accuracy in Knee Osteoarthritis Diagnosis through Automated Bone Segmentation. J. Comput. Biomed. Inform. 2024, 6, 89–98. [Google Scholar]
- Roemer, F.W.; Jarraya, M.; Hayashi, D.; Crema, M.D.; Haugen, I.K.; Hunter, D.J.; Guermazi, A. A Perspective on the Evolution of Semi-Quantitative MRI Assessment of Osteoarthritis: Past, Present and Future. Osteoarthr. Cartil. 2024, 32, 460–472. [Google Scholar] [CrossRef]
- Nelson, A.E. How Feasible Is the Stratification of Osteoarthritis Phenotypes by Means of Artificial Intelligence? Expert Rev. Precis. Med. Drug Dev. 2021, 6, 83–85. [Google Scholar] [CrossRef]
- Lee, K.S.; Kwak, H.J.; Oh, J.M.; Jha, N.; Kim, Y.J.; Kim, W.; Baik, U.B.; Ryu, J.J. Automated Detection of TMJ Osteoarthritis Based on Artificial Intelligence. J. Dent. Res. 2020, 99, 1363–1367. [Google Scholar] [CrossRef]
- Kokkotis, C.; Moustakidis, S.; Papageorgiou, E.; Giakas, G.; Tsaopoulos, D.E. Machine Learning in Knee Osteoarthritis: A Review. Osteoarthr. Cartil. Open 2020, 2, 100069. [Google Scholar] [CrossRef]
- Parisi, L.; RaviChandran, N.; Lanzillotta, M. Artificial Intelligence for Clinical Gait Diagnostics of Knee Osteoarthritis: An Evidence-based Review and Analysis. TechRxiv. 2020. [Google Scholar] [CrossRef]
- Phan Trung, H.; Nguyen Thiet, S.; Nguyen Trung, T.; Le Tan, L.; Tran Minh, T.; Quan Thanh, T. OsteoGA: An Explainable AI Framework for Knee Osteoarthritis Severity Assessment. In Proceedings of the 12th International Symposium on Information and Communication Technology, Ho Chi Minh, Vietnam, 7–8 December 2023; Association for Computing Machinery: New York, NY, USA, 2023; pp. 639–646. [Google Scholar]
- Yeoh, P.S.Q.; Lai, K.W.; Goh, S.L.; Hasikin, K.; Hum, Y.C.; Tee, Y.K.; Dhanalakshmi, S. Emergence of Deep Learning in Knee Osteoarthritis Diagnosis. Comput. Intell. Neurosci. 2021, 2021, 4931437. [Google Scholar] [CrossRef] [PubMed]
- Binvignat, M.; Pedoia, V.; Butte, A.J.; Louati, K.; Klatzmann, D.; Berenbaum, F.; Mariotti-Ferrandiz, E.; Sellam, J. Use of Machine Learning in Osteoarthritis Research: A Systematic Literature Review. RMD Open 2022, 8, e001998. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.; Hudson, C.; Small, J.; Mak, T.; Richardson, P.; Taddei, A. Targeting Senescent Chondrocytes in Osteoarthritis: Artificial Intelligence Powered Target Identification and Hypothesis Validation Strategy. Osteoarthr. Cartil. 2021, 29, S419–S420. [Google Scholar] [CrossRef]
- Olsson, S.; Akbarian, E.; Lind, A.; Razavian, A.S.; Gordon, M. Automating Classification of Osteoarthritis According to Kellgren-Lawrence in the Knee Using Deep Learning in an Unfiltered Adult Population. BMC Musculoskelet. Disord. 2021, 22, 844. [Google Scholar] [CrossRef]
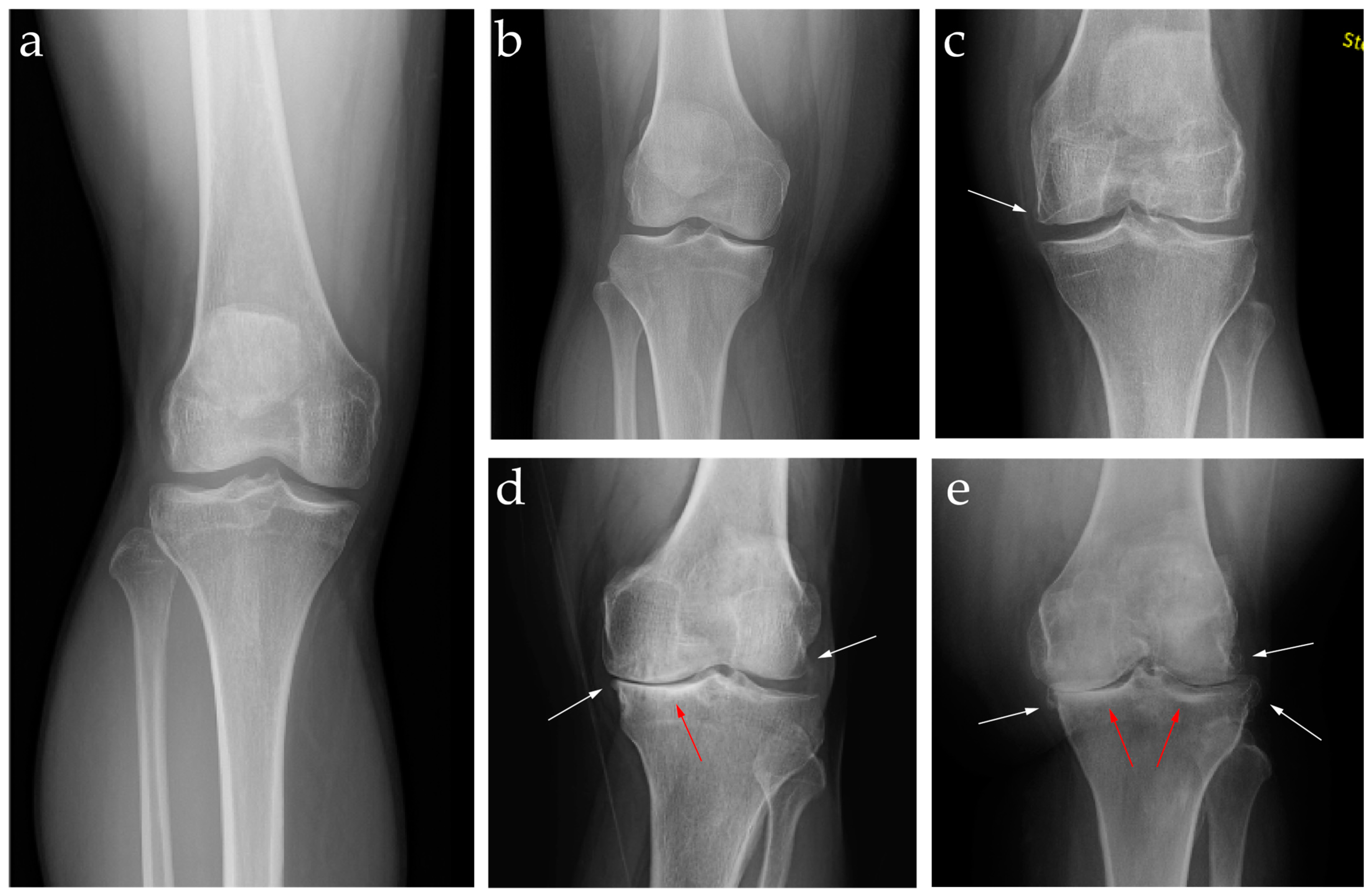

| KL Grade | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Classification | Normal | Doubtful | Mild | Moderate | Severe |
| Description | No radiographic features of OA | Minimal joint space narrowing with possible osteophytes | Evidence of at least one osteophyte with definite joint space narrowing | Multiple osteophytes, definite joint space narrowing and some evidence of sclerosis with or without deformity of the bone contour | Large osteophytes, complete obliteration of the joint space, severe sclerosis, and definite deformity of the bone contour |
| Invasiveness | Type | Method |
|---|---|---|
| Non-invasive | Nonpharmacologic | Weight loss if overweight or obese (5–10% of body weight) |
| Physical therapy | ||
| Pharmacologic | oral analgesics (e.g., oral NSAIDs, duloxetine) | |
| topical analgesics (e.g., NSAIDs, capsaicin) | ||
| Invasive | Operative | arthroscopic procedures |
| total knee arthroplasty | ||
| Minimally invasive | Corticosteroid injections | |
| Hyaluronic acid injections | ||
| Platelet-rich plasma injections | ||
| Genicular nerve ablation | ||
| Genicular artery embolization |
| References | HD Data Analyzed | Methods | Multivariate Data |
|---|---|---|---|
| AI et al., 2010 [116] | MRI | Kellgren–Lawrence scores, Fisher exact test, correlation analysis | - |
| Hayashi et al., 2019 [125] | MRI | CNN | - |
| Mohammadi et al., 2023 [113] | X-ray | Meta-regression | - |
| Bianchi et al., 2021 [117] | CBCT | Random forest, support vector machines, light GBM, XGboost, UNet, ResNet, | - |
| Parisi et al., 2023 [127] | - | Multi-layer perceptron (MLP) | The UARTA star-rating quality assessment scale |
| Pongsakonpruttikul et al., 2022 [118] | X-ray | Modified-YOLO | - |
| Sekiya et al., 2023 [128] | MRI | Statistical analysis | - |
| Abdullah and Rajasekaran 2022 [119] | X-ray | Faster RCNN, ResNet-50 with transfer learning and AlexNet | - |
| Kiran et al., 2023 [129] | Radiography | UNet | - |
| Lee et al., 2022 [114] | PubMed and EMBASE databases | Autoencoder | - |
| Calivà et al., 2022 [126] | MRI | Deep neural networks | - |
| Smolle et al., 2023 [120] | X-ray | KL grade, joint space narrowing (JSN), sclerosis and osteophyte OARSI grade by computerized methods | - |
| Lenskjold et al., 2022 [121] | X-ray | KL and OARSI grades | - |
| Xu et al., 2023 [122] | CBCT | KNN and convolutional neural networks (CNN) | - |
| Roemer et al., 2024 [130] | MRI | Whole-Organ Magnetic Resonance Imaging Score (WORMS) | - |
| Neubauer et al., 2023 [123] | X-ray | Correlation analysis | - |
| Tiwari et al., 2022 [124] | X-ray | ResNet50, VGG-16, InceptionV3, MobilnetV2, EfficientnetB7, DenseNet201, Xception and NasNetMobile | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osuala, U.; Goh, M.H.; Mansur, A.; Smirniotopoulos, J.B.; Scott, A.; Vassell, C.; Yousefi, B.; Jain, N.K.; Sag, A.A.; Lax, A.; et al. Minimally Invasive Therapies for Knee Osteoarthritis. J. Pers. Med. 2024, 14, 970. https://doi.org/10.3390/jpm14090970
Osuala U, Goh MH, Mansur A, Smirniotopoulos JB, Scott A, Vassell C, Yousefi B, Jain NK, Sag AA, Lax A, et al. Minimally Invasive Therapies for Knee Osteoarthritis. Journal of Personalized Medicine. 2024; 14(9):970. https://doi.org/10.3390/jpm14090970
Chicago/Turabian StyleOsuala, Uchenna, Megan H. Goh, Arian Mansur, John B. Smirniotopoulos, Arielle Scott, Christine Vassell, Bardia Yousefi, Neil K. Jain, Alan A. Sag, Allison Lax, and et al. 2024. "Minimally Invasive Therapies for Knee Osteoarthritis" Journal of Personalized Medicine 14, no. 9: 970. https://doi.org/10.3390/jpm14090970
APA StyleOsuala, U., Goh, M. H., Mansur, A., Smirniotopoulos, J. B., Scott, A., Vassell, C., Yousefi, B., Jain, N. K., Sag, A. A., Lax, A., Park, K. W., Kheradi, A., Sapoval, M., Golzarian, J., Habibollahi, P., Ahmed, O., Young, S., & Nezami, N. (2024). Minimally Invasive Therapies for Knee Osteoarthritis. Journal of Personalized Medicine, 14(9), 970. https://doi.org/10.3390/jpm14090970









