Artificial Intelligence-Driven Diagnostic Processes and Comprehensive Multimodal Models in Pain Medicine
Abstract
1. Introduction
2. Computer-Aided Diagnosis
2.1. Automatic Pain Assessment
2.2. AI Strategies Implemented
2.3. Diagnosis of Low Back Pain
2.4. Large Language Models for Sentiment Analysis
2.5. Neurofunctional Investigations
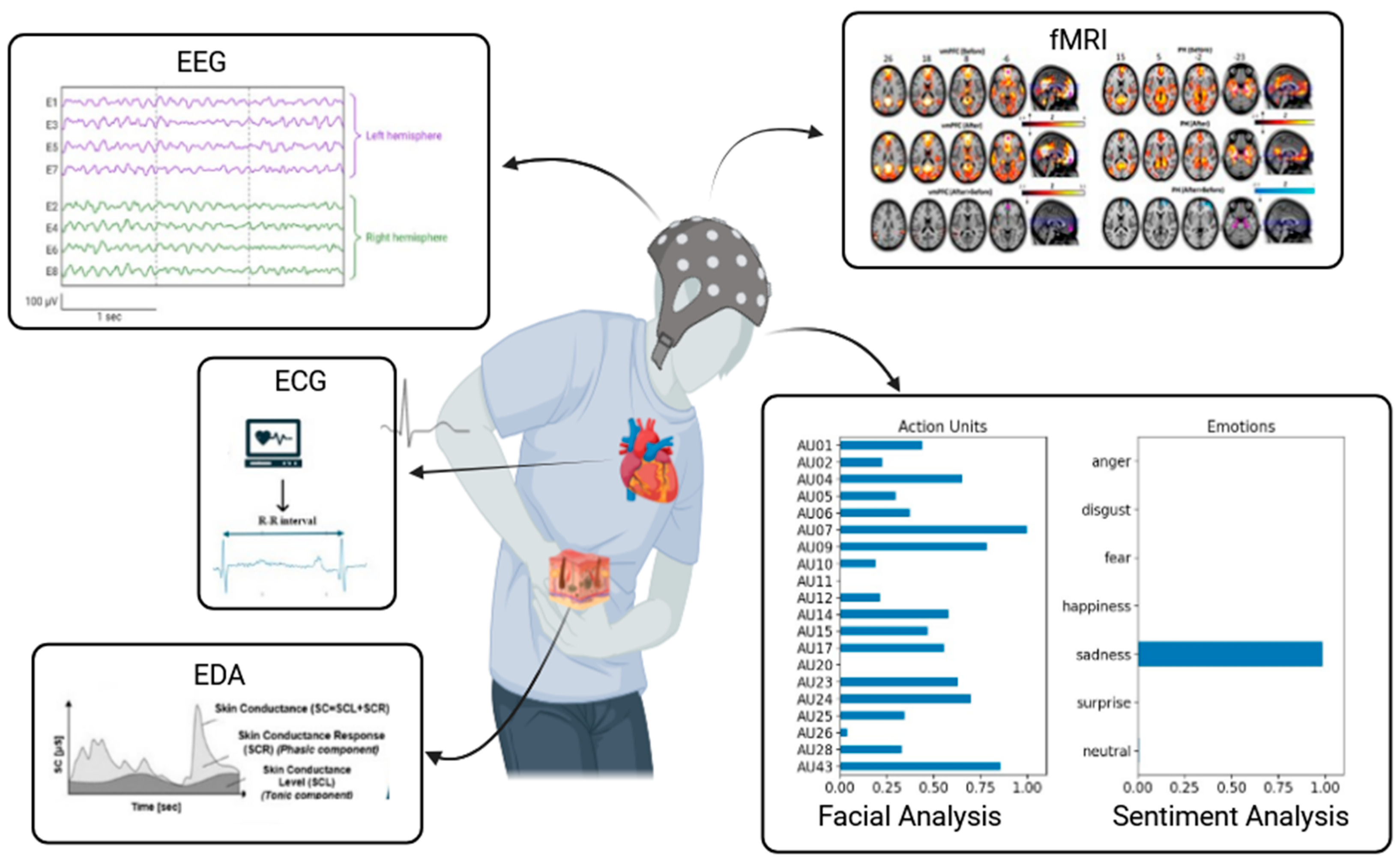
3. Integration of AI within a Comprehensive Multimodal Model
4. AI-Powered Assessment in Infants and Cognitively Impaired Populations
4.1. Pain Diagnosis in Newborn and Infant
4.2. Cognitively Impaired Elderly Individuals
5. Limitations, AI Ethics, and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, S.; Kidwai, A.; Rakhamimova, E.; Elias, M.; Caldwell, W.; Bergese, S.D. Clinical Diagnosis and Treatment of Chronic Pain. Diagnostics 2023, 13, 3689. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.-W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef]
- El-Tallawy, S.N.; Pergolizzi, J.V.; Vasiliu-Feltes, I.; Ahmed, R.S.; LeQuang, J.K.; El-Tallawy, H.N.; Varrassi, G.; Nagiub, M.S. Incorporation of “Artificial Intelligence” for Objective Pain Assessment: A Comprehensive Review. Pain Ther. 2024, 13, 293–317. [Google Scholar] [CrossRef]
- Mathew, B.; Morris, D.; Hendry, D.; Waddell, G. Artificial intelligence in the diagnosis of low-back pain and sciatica. Spine 1988, 13, 168–172. [Google Scholar] [CrossRef]
- Sturman, M.F.; Perez, M. Computer-assisted diagnosis of acute abdominal pain. Compr. Ther. 1989, 15, 26–35. [Google Scholar] [PubMed]
- Abd-Elsayed, A.; Robinson, C.L.; Marshall, Z.; Diwan, S.; Peters, T. Applications of Artificial Intelligence in Pain Medicine. Curr. Pain Headache Rep. 2024, 28, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Vitale, V.N.; Mariani, F.; Iuorio, M.; Cutugno, F. Development of a binary classifier model from extended facial codes toward video-based pain recognition in cancer patients. Scand. J. Pain 2023, 23, 638–645. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, M.; Yang, T.; Li, J.; Qin, C.; Wang, B.; Wang, L.; Li, B.; Liu, J. Identifying significant structural factors associated with knee pain severity in patients with osteoarthritis using machine learning. Sci. Rep. 2024, 14, 14705. [Google Scholar] [CrossRef]
- Cascella, M.; Scarpati, G.; Bignami, E.G.; Cuomo, A.; Vittori, A.; Di Gennaro, P.; Crispo, A.; Coluccia, S. Utilizing an artificial intelligence framework (conditional generative adversarial network) to enhance telemedicine strategies for cancer pain management. J. Anesth. Analg. Crit. Care 2023, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Semeraro, F.; Montomoli, J.; Bellini, V.; Piazza, O.; Bignami, E. The Breakthrough of Large Language Models Release for Medical Applications: 1-Year Timeline and Perspectives. J. Med. Syst. 2024, 48, 22. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Schiavo, D.; Cuomo, A.; Ottaiano, A.; Perri, F.; Patrone, R.; Migliarelli, S.; Bignami, E.G.; Vittori, A.; Cutugno, F. Artificial Intelligence for Automatic Pain Assessment: Research Methods and Perspectives. Pain Res. Manag. 2023, 2023, 6018736. [Google Scholar] [CrossRef]
- de Aberasturi, Y.L.d.A.-J.; la Cueva, A.V.-D.; Aretxabala-Cortajarena, N.; Quintano-Rodero, A.; Rodriguez-Nuñez, C.; Pelegrin-Gaspar, P.M.; Gil-Garcia, Z.I.; Margüello-Fernandez, A.A.; Aparicio-Cilla, L.; Parraza-Diez, N. Pupillary dilation reflex and behavioural pain scale: Study of diagnostic test. Intensiv. Crit. Care Nurs. 2023, 74, 103332. [Google Scholar] [CrossRef] [PubMed]
- Guitart-Vela, J.; Magrone, Á.; González, G.; Folch, J. Effectiveness and Safety of Sublingual Fentanyl in the Treatment of Breakthrough Cancer Pain in Older Patients with Cancer: Results from a Retrospective Observational Study. J. Pain Palliat. Care Pharmacother. 2024, 1–12. [Google Scholar] [CrossRef]
- Puntmann, V.O. How-to guide on biomarkers: Biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad. Med. J. 2009, 85, 538–545. [Google Scholar] [CrossRef]
- Fontaine, D.; Vielzeuf, V.; Genestier, P.; Limeux, P.; Santucci-Sivilotto, S.; Mory, E.; Darmon, N.; Lanteri-Minet, M.; Mokhtar, M.; Laine, M.; et al. Artificial intelligence to evaluate postoperative pain based on facial expression recognition. Eur. J. Pain 2022, 26, 1282–1291. [Google Scholar] [CrossRef]
- Bargshady, G.; Zhou, X.; Deo, R.C.; Soar, J.; Whittaker, F.; Wang, H. Ensemble neural network approach detecting pain intensity from facial expressions. Artif. Intell. Med. 2020, 109, 101954. [Google Scholar] [CrossRef]
- Hosseini, E.; Fang, R.; Zhang, R.; Chuah, C.-N.; Orooji, M.; Rafatirad, S.; Rafatirad, S.; Homayoun, H. Convolution Neural Network for Pain Intensity Assessment from Facial Expression. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, Scotland, UK, 11–15 July 2022; pp. 2697–2702. [Google Scholar] [CrossRef]
- Barua, P.D.; Baygin, N.; Dogan, S.; Baygin, M.; Arunkumar, N.; Fujita, H.; Tuncer, T.; Tan, R.-S.; Palmer, E.; Bin Azizan, M.M.; et al. Automated detection of pain levels using deep feature extraction from shutter blinds-based dynamic-sized horizontal patches with facial images. Sci. Rep. 2022, 12, 17297. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ashouri, S.; Abedi, M.; Azadeh-Fard, N.; Parnianpour, M.; Khalaf, K.; Rashedi, E. Using a motion sensor to categorize non-specific low back pain patients: A machine learning approach. Sensors 2020, 20, 3600. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Quddusi, A.; Klukowska, A.M.; Schröder, M.L. Initial classification of low back and leg pain based on objective functional testing: A pilot study of machine learning applied to diagnostics. Eur. Spine J. 2020, 29, 1702–1708. [Google Scholar] [CrossRef]
- Liew, B.X.W.; Rugamer, D.; De Nunzio, A.M.; Falla, D. Interpretable machine learning models for classifying low back pain status using functional physiological variables. Eur. Spine J. 2020, 29, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Liawrungrueang, W.; Park, J.-B.; Cholamjiak, W.; Sarasombath, P.; Riew, K.D. Artificial Intelligence-Assisted MRI Diagnosis in Lumbar Degenerative Disc Disease: A Systematic Review. Glob. Spine J. 2024, 21925682241274372. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Yu, K.; Xie, Z.; Liu, L.; Wang, P.; Zhang, W.; Wang, Y.; Wu, X. Differentiation of lumbar disc herniation and lumbar spinal stenosis using natural language processing–based machine learning based on positive symptoms. Neurosurg. Focus 2022, 52, E7. [Google Scholar] [CrossRef] [PubMed]
- Soin, A.; Hirschbeck, M.; Verdon, M.; Manchikanti, L. A Pilot Study Implementing a Machine Learning Algorithm to Use Artificial Intelligence to Diagnose Spinal Conditions. Pain Physician. 2022, 25, 171–178. [Google Scholar] [PubMed]
- Venerito, V.; Iannone, F. Large language model-driven sentiment analysis for facilitating fibromyalgia diagnosis. RMD Open 2024, 10, e004367. [Google Scholar] [CrossRef]
- Hughes, J.A.; Wu, Y.; Jones, L.; Douglas, C.; Brown, N.; Hazelwood, S.; Lyrstedt, A.-L.; Jarugula, R.; Chu, K.; Nguyen, A. Analyzing pain patterns in the emergency department: Leveraging clinical text deep learning models for real-world insights. Int. J. Med. Inform. 2024, 190, 105544. [Google Scholar] [CrossRef]
- Latypov, T.H.; So, M.C.; Hung, P.S.-P.; Tsai, P.; Walker, M.R.; Tohyama, S.; Tawfik, M.; Rudzicz, F.; Hodaie, M. Brain imaging signatures of neuropathic facial pain derived by artificial intelligence. Sci. Rep. 2023, 13, 10699. [Google Scholar] [CrossRef]
- Peng, K.; Karunakaran, K.D.; Green, S.; Borsook, D. Machines, mathematics, and modules: The potential to provide real-time metrics for pain under anesthesia. Neurophotonics 2024, 11, 010701. [Google Scholar] [CrossRef]
- Racek, A.; Hu, X.; Nascimento, T.; Bender, M.; Khatib, L.; Chiego, D.; Holland, G.; Bauer, P.; McDonald, N.; Ellwood, R.; et al. Different Brain Responses to Pain and Its Expectation in the Dental Chair. J. Dent. Res. 2015, 94, 998–1003. [Google Scholar] [CrossRef]
- Hu, X.; Racek, A.J.; Bellile, E.; Nascimento, T.D.; Bender, M.C.; Toback, R.L.; Burnett, D.; Khatib, L.; McMahan, R.; Kovelman, I.; et al. Brain Functional Changes before, during, and after Clinical Pain. J. Dent. Res. 2018, 97, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.T.; De Pretto, L.R.; Simis, M.; Fregni, F.; Battistella, L.R. Primary Motor Area Activity in Phantom Limb Imagery of Traumatic Unilateral Lower Limb Amputees with Phantom Limb Pain. Adv. Rehabil. Sci. Pract. 2024, 13, 27536351241261023. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Liang, M.; Peng, J.; Yu, Q.; Li, Y.; Yang, J.; Zhang, S.; Wang, C. Cortical Mechanisms Underlying Effects of Repetitive Peripheral Magnetic Stimulation on Dynamic and Static Postural Control in Patients with Chronic Non-Specific Low Back Pain: A Dou-ble-Blind Randomized Clinical Trial. Pain Ther. 2024, 13, 953–970. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, Y.; Fan, B.; Li, S.; Zhang, Z.; Fang, J. Global trends and performances of infrared imaging technology studies on acupuncture: A bibliometric analysis. Front. Neurosci 2024, 18, 1387752. [Google Scholar] [CrossRef]
- Lee, J.; Mawla, I.; Kim, J.; Loggia, M.L.; Ortiz, A.; Jung, C.; Chan, S.-T.; Gerber, J.; Schmithorst, V.J.; Edwards, R.R.; et al. Machine learning–based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. Pain 2019, 160, 550–560. [Google Scholar] [CrossRef]
- Gruss, S.; Treister, R.; Werner, P.; Traue, H.C.; Crawcour, S.; Andrade, A.; Walter, S. Pain Intensity Recognition Rates via Biopotential Feature Patterns with Support Vector Machines. PLoS ONE 2015, 10, e0140330. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, A.; Cascella, M.; Forte, C.A.; Bimonte, S.; Esposito, G.; De Santis, S.; Cavanna, L.; Fusco, F.; Dauri, M.; Natoli, S.; et al. Careful Breakthrough Cancer Pain Treatment through Rapid-Onset Transmucosal Fentanyl Improves the Quality of Life in Cancer Patients: Results from the BEST Multicenter Study. J. Clin. Med. 2020, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Di Gennaro, P.; Crispo, A.; Vittori, A.; Petrucci, E.; Sciorio, F.; Marinangeli, F.; Ponsiglione, A.M.; Romano, M.; Ovetta, C.; et al. Advancing the integration of biosignal-based automated pain assessment methods into a comprehensive model for addressing cancer pain. BMC Palliat. Care 2024, 23, 198. [Google Scholar] [CrossRef] [PubMed]
- Page, G.G. Are there long-term consequences of pain in newborn or very young infants? J. Perinat. Edu. 2004, 13, 10–17. [Google Scholar] [CrossRef]
- Brahnam, S.; Chuang, C.-F.; Shih, F.Y.; Slack, M.R. Machine recognition and representation of neonatal facial displays of acute pain. Artif. Intell. Med. 2006, 36, 211–222. [Google Scholar] [CrossRef]
- Pal, P.; Iyer, A.N.; Yantorno, R.E. Emotion detection from infant facial expressions and cries. In Proceedings of the 2006 IEEE International Conference on Acoustics Speed and Signal Processing, Toulouse, France, 14–19 May 2006; Volume 2, p. II. [Google Scholar]
- Zamzmi, G.; Pai, C.-Y.; Goldgof, D.; Kasturi, R.; Sun, Y.; Ashmeade, T. Automated pain assessment in neonates. In Proceedings of the Image Analysis: 20th Scandinavian Conference, Tromsø, Norway, 12–14 June 2017; pp. 350–361. [Google Scholar]
- Partala, T.; Surakka, V. Pupil size variation as an indication of affective processing. Int. J. Hum.-Comput. Stud. 2003, 59, 185–198. [Google Scholar] [CrossRef]
- Walter, S.; Gruss, S.; Limbrecht-Ecklundt, K.; Traue, H.C.; Werner, P.; Al-Hamadi, A.; Diniz, N.; da Silva, G.M.; Andrade, A.O. Automatic pain quantification using autonomic parameters. Psychol. Neurosci. 2014, 7, 363–380. [Google Scholar] [CrossRef]
- Ranger, M.; Glinas, C. Innovating in pain assessment of the critically ill: Exploring cerebral near-infrared spectroscopy as a bedside approach. Pain Manag. Nursing. 2014, 15, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Zamzmi, G.; Kasturi, R.; Goldgof, D.; Zhi, R.; Ashmeade, T.; Sun, Y. A Review of Automated Pain Assessment in Infants: Features, Classification Tasks, and Databases. IEEE Rev. Biomed. Eng. 2017, 11, 77–96. [Google Scholar] [CrossRef]
- Hughes, J.D.; Chivers, P.; Hoti, K. The Clinical Suitability of an Artificial Intelligence-Enabled Pain Assessment Tool for Use in Infants: Feasibility and Usability Evaluation Study. J. Med. Internet Res. 2023, 25, e41992. [Google Scholar] [CrossRef] [PubMed]
- Carlini, L.P.; Ferreira, L.A.; Coutrin, G.A.S.; Varoto, V.V.; Heiderich, T.M.; Balda, R.C.X.; Barros, M.C.M.; Guinsburg, R.; Thomaz, C.E. A Convolutional Neural Network-Based Mobile Application to Bedside Neonatal Pain Assessment. In Proceedings of the 34th SIBGRAPI Conference on Graphics, Patterns and Images (SIBGRAPI), Gramado, Brazil, 18–22 October 2021; pp. 394–401. [Google Scholar]
- Heiderich, T.M.; Leslie, A.T.F.S.; Guinsburg, R. Neonatal procedural pain can be assessed by computer software that has good sensitivity and specificity to detect facial movements. Acta Paediatr. 2014, 104, e63–e69. [Google Scholar] [CrossRef]
- Brahnam, S.; Chuang, C.-F.; Sexton, R.S.; Shih, F.Y. Machine assessment of neonatal facial expressions of acute pain. Decis. Support Syst. 2007, 43, 1242–1254. [Google Scholar] [CrossRef]
- Li, J.; Chen, K.; Yang, L.; Mutsaers, M.; Barakova, E. EGG: AI-Based Interactive Design Object for Managing Post-operative Pain in Children. In Artificial Intelligence for Neuroscience and Emotional Systems. IWINAC 2024; Lecture Notes in Computer Science; Ferrández Vicente, J.M., Val Calvo, M., Adeli, H., Eds.; Springer: Cham, Switzerland, 2024; Volume 14674. [Google Scholar] [CrossRef]
- Gholami, B.; Haddad, W.M.; Tannenbaum, A.R. Relevance vector machine learning for neonate pain intensity assessment using digital imaging. IEEE Trans. Biomed. Eng. 2010, 57, 1457–1466. [Google Scholar] [CrossRef]
- Smith, E.; Storch, E.A.; Vahia, I.; Wong, S.T.C.; Lavretsky, H.; Cummings, J.L.; Eyre, H.A. Affective computing for late-life mood and cognitive disorders. Front. Psychiatry 2021, 12, 782183. [Google Scholar] [CrossRef]
- Atee, M.; Hoti, K.; Hughes, J.D. Psychometric Evaluation of the Electronic Pain Assessment Tool: An Innovative Instrument for Individuals with Moderate-to-Severe Dementia. Dement. Geriatr. Cogn. Disord. 2018, 44, 256–267. [Google Scholar] [CrossRef]
- Babicova, I.; Cross, A.; Forman, D.; Hughes, J.; Hoti, K. Evaluation of the Psychometric Properties of PainChek R in UK Aged Care Residents with advanced dementia. BMC Geriatr. 2021, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Warden, V.; Hurley, A.C.; Volicer, L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) Scale. J. Am. Med. Dir. Assoc. 2003, 4, 9–15. [Google Scholar] [CrossRef]
- Gomutbutra, P.; Kittisares, A.; Sanguansri, A.; Choosri, N.; Sawaddiruk, P.; Fakfum, P.; Lerttrakarnnon, P.; Saralamba, S. Classification of elderly pain severity from automated video clip facial action unit analysis: A study from a Thai data repository. Front. Artif. Intell. 2022, 5, 942248. [Google Scholar] [CrossRef] [PubMed]
- Ak, R.; Vitelli, V.; Zio, E. An interval-valued neural network approach for uncertainty quantification in short-term wind speed prediction. IEEE Trans. Neural Netw. Learn. Syst. 2015, 26, 2787–2800. [Google Scholar] [CrossRef] [PubMed]
- Ozek, B.; Lu, Z.; Radhakrishnan, S.; Kamarthi, S. Uncertainty quantification in neural-network based pain intensity estimation. PLoS ONE 2024, 19, e0307970. [Google Scholar] [CrossRef]
- Benanti, P. The urgency of an algorethics. Discov. Artif. Intell. 2023, 3, 11. [Google Scholar] [CrossRef]
- Montomoli, J.; Bitondo, M.M.; Cascella, M.; Rezoagli, E.; Romeo, L.; Bellini, V.; Semeraro, F.; Gamberini, E.; Frontoni, E.; Agnoletti, V.; et al. Algor-ethics: Charting the ethical path for AI in critical care. J. Clin. Monit. Comput. 2024, 38, 931–939. [Google Scholar] [CrossRef]
- The White House. Executive Order on the Safe Secure and Trustworthy Development and Use of Artificial Intelligence. Available online: https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/ (accessed on 29 July 2024).
- European Parliament. Artificial Intelligence Act. Available online: https://ai-act-law.eu/ (accessed on 20 July 2024).
- Zednik, C. Solving the black box problem: A normative framework for explainable artificial intelligence. Philos. Technol. 2021, 34, 265–288. [Google Scholar] [CrossRef]
- Marcus, E.; Teuwen, J. Artificial intelligence and explanation: How, why, and when to explain black boxes. Eur. J. Radiol. 2024, 173, 111393. [Google Scholar] [CrossRef]
- Collins, G.S.; Dhiman, P.; Navarro, C.L.A.; Ma, J.; Hooft, L.; Reitsma, J.B.; Logullo, P.; Beam, A.L.; Peng, L.; Van Calster, B.; et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 2021, 11, e048008. [Google Scholar] [CrossRef]
- Kiran, N.; Sapna, F.; Kiran, F.; Kumar, D.; Raja, F.; Shiwlani, S.; Paladini, A.; Sonam, F.; Bendari, A.; Perkash, R.S.; et al. Digital Pathology: Transforming Diagnosis in the Digital Age. Cureus 2023, 15, e44620. [Google Scholar] [CrossRef] [PubMed]
- Banks, T.J.; Nguyen, T.D.; Uhlmann, J.K.; Nair, S.S.; Scherrer, J.F. Predicting opioid use disorder before and after the opioid prescribing peak in the United States: A machine learning tool using electronic healthcare records. Health Inform. J. 2023, 29, 14604582231168826. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, A.; Ciurleo, R.; Vecchione, C.; Turolla, A.; Piramide, N.; Ciccarelli, M.; Piramide, E.; Garofano, M. Telerehabilitation: A Solution for Patients after Hip Fracture? Transl. Med. UniSa 2024, 26, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Capuozzo, M.; Ferrara, F.; Ottaiano, A.; Perri, F.; Sabbatino, F.; Conti, V.; Santoriello, V.; Ponsiglione, A.M.; Romano, M.; et al. Two-year Opioid Prescription Trends in Local Sanitary Agency Naples 3 South, Campania Region, Italy. Descriptive Analyses and AI-based Translational Perspectives. Transl. Med. UniSa 2024, 26, 1. [Google Scholar] [CrossRef]
- Segal, Z.; Radinsky, K.; Elad, G.; Marom, G.; Beladev, M.; Lewis, M.; Ehrenberg, B.; Gillis, P.; Korn, L.; Koren, G. Development of a machine learning algorithm for early detection of opioid use disorder. Pharmacol. Res. Perspect. 2020, 8, e00669. [Google Scholar] [CrossRef]
| Application | Method, Setting/Aim | Key Findings | Ref. |
|---|---|---|---|
| AI for Diagnosing Pain Conditions | ResNet-18 CNN for postoperative pain. | Sensitivity: 89.7%, severe pain detection: 77.5%, pain intensity estimation: 53% | [17] |
| Ensemble deep learning model. | Accuracy°: 89%, AUC ROC^: 94% for shoulder pain | [18] | |
| Transfer learning with pre-trained CNN. | Identified seven-level pain thresholds from facial expressions | [19] | |
| DarkNet19 pre-trained on ImageNet1K. | Accuracy°: 95% for shoulder pain | [20] | |
| SVM and ANN. Chronic low back pain. | Classification accuracy°: SVM (75%), ANN (60%) for NSLBP | [21] | |
| Fuzzy-rule-based system. Low and leg pain. | Accuracy°: 96% using 5R-STS for lumbar conditions | [22] | |
| Functional data boosting. Low back pain. | AUC^: 90.4% (control vs. current pain), 91.2% (control vs. pain in remission) | [23] | |
| Different ML and DL architectures (systematic review). | Accuracy°, recall*, and specificity§ from 71.5% to 99% in DDD diagnosis | [24] | |
| LLM (Mistral-7B-Instruct-v0.2) for sentiment analysis in fibromyalgia. | Accuracy°: 87%, precision‡: 92%, recall*: 84%, specificity§: 82%, | [27] | |
| LSTM and XGBoost for distinguishing between lumbar spine stenosis and lumbar disc herniation. | LSTM: AUC ROC 0.84, accuracy 0.78, recall 0.90, F1 score of 0.81, and precision of 0.73. XGBoost: AUC ROC 0.75, accuracy 0.69, recall 0.73, F1 score 0.71, and precision 0.69 | [25] | |
| Comprehensive Pain Assessment | SVM and SVR. | Classification accuracy: 92.45%, AUC^: 0.97 | [36] |
| Biosignal-based pain recognition. SVM. | Accuracy°: 91% (baseline vs. pain tolerance threshold), 79% (baseline vs. pain threshold) | [37] | |
| Decision tree ML. Spinal conditions. | Accuracy°: 72% | [26] | |
| Random forest, logistic regression. Neuropathic facial pain. | Accuracy°: 95% | [29] | |
| Newborn/Infant | Facial expression: PCA, LDA, and SVM. | Pain versus non-pain (88.00%), pain versus rest (94.62%), pain versus cry (80.00%), pain versus air puff (83.33%), and pain versus friction (93.00%) | [41] |
| Facial expressions and crying | Emotion recognition | [42] | |
| Elderly/Non-verbal Patients | Various models and medical device applications (e.g., PainChek) | Enhanced pain assessment | [54,55,56,57,58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascella, M.; Leoni, M.L.G.; Shariff, M.N.; Varrassi, G. Artificial Intelligence-Driven Diagnostic Processes and Comprehensive Multimodal Models in Pain Medicine. J. Pers. Med. 2024, 14, 983. https://doi.org/10.3390/jpm14090983
Cascella M, Leoni MLG, Shariff MN, Varrassi G. Artificial Intelligence-Driven Diagnostic Processes and Comprehensive Multimodal Models in Pain Medicine. Journal of Personalized Medicine. 2024; 14(9):983. https://doi.org/10.3390/jpm14090983
Chicago/Turabian StyleCascella, Marco, Matteo L. G. Leoni, Mohammed Naveed Shariff, and Giustino Varrassi. 2024. "Artificial Intelligence-Driven Diagnostic Processes and Comprehensive Multimodal Models in Pain Medicine" Journal of Personalized Medicine 14, no. 9: 983. https://doi.org/10.3390/jpm14090983
APA StyleCascella, M., Leoni, M. L. G., Shariff, M. N., & Varrassi, G. (2024). Artificial Intelligence-Driven Diagnostic Processes and Comprehensive Multimodal Models in Pain Medicine. Journal of Personalized Medicine, 14(9), 983. https://doi.org/10.3390/jpm14090983







