Concussion and the Autonomic, Immune, and Endocrine Systems: An Introduction to the Field and a Treatment Framework for Persisting Symptoms
Abstract
:1. Introduction
2. Concussion and Regulatory Systems in the Body
3. An Introduction to the Regulatory Systems of the Body
3.1. The Autonomic Nervous System
3.2. The Immune System
3.3. The Endocrine (Hormonal) System
3.3.1. The Hypothalamic–Pituitary–Adrenal Axis (HPA Axis)
3.3.2. Circadian Rhythm Hormones
3.3.3. Other Pituitary Hormones
4. Interaction Effects Between Regulatory Systems in the Body
- (a)
- There is evidence of dysregulation of the autonomic nervous system and other regulatory body systems following concussion.
- (b)
- There is a large overlap of symptoms of concussion with other medical conditions that also impact the regulatory systems of the body.
- (c)
- Therefore, efforts to stabilize the regulatory systems in the body have the potential to reduce symptom burden and improve patient functioning.
5. Other Neurophysiology with Potential Relevance in Post-Concussive Complaints
- Cerebral blood flow anomalies, which in part are regulated by autonomic networks [124,125,126,127,128,129,130,131,132,133,134]. See also reviews of cerebral perfusion anomalies associated with various scanning modalities, including single photon emission computed tomography (SPECT) scanning [135,136], transcranial doppler ultrasound [137], functional magnetic resonance imaging blood flow markers [138], and arterial spin labeling MRI [139].
- Concussion can involve multiple aspects and markers of neuronal functioning,
- Neuronal anomalies likely evolve and change over time as a patient moves from the acute to chronic stages of recovery (neurotrauma is a process and not a single event) [140],
- The neural physiology of concussion is very complex and likely varies from one individual to another.
6. The Status of Current Treatment Research in Concussion
6.1. In the Absence of Well-Developed Treatment Research, What Do Clinicians Draw on to Guide Treatment Decisions?
6.2. Comment Regarding the Use of Medications in Concussion Rehabilitation
- (a)
- Some medications commonly used in concussion management have the potential for the unintended side effect of hampering the functioning of the autonomic nervous system, potentially impeding overall recovery even though some symptom relief may occur. Classes of medication where caution is indicated include SNRI and (to a lesser extent) SSRI medications, tricyclic antidepressants, antihistamines, antipsychotics, and beta blockers [163,164,165,166].
- (b)
- Some classes of medication present risks in the context of mTBI recovery. For example, some sleep-inducing medications (such as benzodiazepines and atypical gamma-aminobutyric acid (GABA) agonists such as zolpidem) could potentially prolong recovery from TBI, as there is evidence that they may adversely affect cognition and neuroplasticity [167]; analgesic overuse can precipitate headache symptoms [168]; and use of opiates following TBI can increase neural inflammation, impair plasticity, decrease myelin repair, increase neurodegeneration, and present risks for dependency in a vulnerable population [169].
- (c)
- Some patients are involved in occupational or athletic settings where drug testing is employed, which precludes participation for people using certain classes of medication (prescribed or otherwise). It is important for prescribers to be aware of restrictions as part of a cautious approach. For example, in the USA, the National Collegiate Athletic Association, which regulates student sports in university-aged athletes, includes the following banned medication classes: stimulants, beta blockers, various hormones, and beta-2 agonists.
7. Implications for Clinical Assessment and Treatment Rationale for Patients
- Review of injury history details and associated acute features at a level that enables the clinician to diagnose concussion based on current diagnostic criteria. This can help avoid iatrogenic impacts associated with misdiagnosis of concussion when one has not occurred.
- Physical and psychosocial stressors.
- Psychiatric history, including screens for depression, posttraumatic stress symptoms, and other anxiety symptoms.
- Chronic pain status, including screening for cervical strain symptoms, and history of pain/fatigue conditions.
- Sleep quality, including sleep apnea screening.
- Orthostatic intolerance symptom history, including vulnerability to presyncope and syncope.
- Pre-injury and post-injury physical exercise involvement and tolerance.
7.1. Brief Comment on Benign Paroxysmal Positional Vertigo
7.2. Brief Comment on Comorbid Pain Conditions
7.3. Brief Comment on Comorbid Psychological Features
7.4. The Treatment Rationale
- A basic overview of the autonomic nervous system and its functions,
- A brief discussion regarding the potential impact of concussion on the autonomic nervous system,
- Information regarding the overlap of concussion symptoms with other conditions that impact autonomic functioning,
- An individualized formulation outlining the factors that are potentially contributing to their post-concussive-type complaints,
- A discussion of the treatment rationale and initial focus.
8. Sleep Module
8.1. Neuroanatomical Principles: Sleep and Concussion
8.2. Sleep and Regulatory Systems in the Body
8.3. Sleep and the Glymphatic System
8.4. Sleep and the Restorative Systems in the Brain
8.5. Evidence: Sleep Treatment in Concussion
8.6. Clinical Judgment: Sleep Treatment in Concussion
- Set up a regular sleep block of about 8 h in duration. Be consistent in your bed and wake times.
- Get at least 30 min of direct light (preferably outside) sometime in the morning. (As an aside, in addition to supporting sleep, sunlight exposure may also improve vitamin D levels, which can reduce chronic inflammation, upregulate neurotrophic factors, and regulate oxidative stress following brain injury [307].)
- Avoid bright lights and blue-green tinted light at night by doing the following:
- Closing curtains and dimming lights a few hours before your bedtime.
- Using red-colored bulbs in your lamps at night.
- Installing a warm-colored night light in places like bathrooms and hallways
- Avoiding screens for an hour or two before bed (alternatively, experiment with using blue-green light-blocking glasses—the most effective are the 99% blockers, which are deep orange or red in color).
- Avoid stressful activities and have a wind-down period for at least an hour before your bedtime.
- Use the following activities to support your mind and body to unwind (a list of activities is generated in discussion with the patient).
9. Fatigue Module
9.1. Neuroanatomical Principles: Fatigue and Concussion
9.1.1. The Risks of Underexertion and Overexertion
9.2. Evidence: Fatigue and Concussion
“In concussion of the brain, as soon as the blow which strikes the skull has caused the symptoms of concussion, the physical disturbance of the brain, whatever it may be, has been produced… Such a disturbed brain is defective—if not in structure, certainly in its vital endowments, and is therefore unequal to even its ordinary duties. It recovers itself slowly; it then soon becomes fatigued from use; and if claims are made upon it too soon after the injury—that is, before structural and physical integrity is reacquired—the patient is very likely to suffer from a serious disease of the brain. Cerebral exercise or mental occupation should always in such cases be short of fatigue. The brain requires absence from occupation, or rest, for its complete recovery, and this should be in proportion to the severity and duration of the symptoms of concussion…”
9.3. Clinical Judgment: Fatigue and Concussion
- Evaluation of activity tolerance.
- Development of a daily schedule with activity periods, rest periods, and a structure for specific tasks to work on and a breakdown of those tasks into manageable portions.
- Adjusting the timing of demanding activities to periods where patients are typically most energized.
- Gradual increases in activity and work hours as dictated by patient symptoms.
- Education of the patient so that they are aware that progress is rarely linear; episodic setbacks are expected, even in cases where an overall improving trajectory is apparent.
- Emotional support to manage the stresses associated with setbacks and limitations.
9.3.1. The Importance of Tracking
10. Exercise Module
10.1. Neuroanatomical Principles: Exercise and Concussion
- Upregulation of endocrine functioning, including regulation of cortisol levels,
- Improved balance between the sympathetic and parasympathetic branches of the autonomic nervous system,
- Reduced neuroinflammation and upregulation of neuroprotective mechanisms,
- Improved brain blood flow regulation (cerebrovascular autoregulation),
- Improved mood, emotional regulation, and reduced physical pain, possibly due to synergistic effects of exercise-induced increases in the concentrations of dopamine, serotonin, endogenous opioids, and endogenous endocannabinoids,
- Increased brain-derived neurotrophic factor, which promotes neurogenesis (neuron production) and synaptic plasticity, learning, and memory,
- Increased vascular endothelial growth factor, which promotes proliferation of blood vessels in the brain (angiogenesis) and protects against neuronal cell death (apoptosis),
- Increased insulin growth factor, which fosters increased vasculature and neuron production (neurogenesis) in locations of the brain such as the hippocampus,
- Upregulation of mitochondrial density and production,
- Reduced oxidative stress,
- Reduced cognitive decline.
10.2. Evidence: Exercise and Concussion
- Some include interval training components, based on research in general populations, which display some advantages of interval training over continuous training for aerobic capacity, cardiovascular health, mitochondrial biogenesis, and vascular function [425,426,427,428]. Wu et al. [429] found modest benefits for adding blood flow restriction and body-cooling apparatus to a moderate-intensity interval training program for persisting concussion symptoms in adults.
- Evaluation using a march-in-place protocol of increasing metronome speed rather than treadmill or bicycle assessment was used by Haider et al. [430] to evaluate exercise tolerance in a military concussion sample. Adding a graduated aerobic exercise program based on the marching test results to the recovery process reduced average recovery time from 24 days to 17 days.
- Use of lower body negative pressure during aerobic exercise and a supine tilt during cycling to prolong exercise tolerance post-concussion [431].
- Combined aerobic–resistance exercises (light weight circuit) have been proposed but not yet tested [432].
10.2.1. Comment Regarding Orthostatic Intolerance and Concussion
10.3. Clinical Judgment: Exercise and Concussion
11. Nutrition Module
11.1. Neuroanatomical Principles: Nutrition and Concussion
- The previous discussion on inflammation and the capacity for diet to be a powerful mediator of unhelpful inflammatory responses and other secondary injury cascades post-injury.
- Suboptimal nutrition’s capacity to dysregulate the homeostatic regulatory systems we have discussed in this article.
- The brain’s energy needs, which can be compromised by the injury-related impairment of energy generating systems in the brain.
11.1.1. A Brief Overview of the Gut Microbiome
11.1.2. Nutrition, Brain Injury, and Neuroinflammation
11.1.3. Nutrition, Brain Injury, and Regulatory Systems
11.1.4. Nutrition, Brain Injury, and Energy Systems
11.1.5. Dietary Patterns to Promote Health and Healing
11.2. Evidence: Nutrition and Concussion
11.2.1. Supplements in Concussion
11.2.2. Dietary Interventions in Concussion
11.3. Clinical Judgment: Nutrition and Concussion
11.3.1. Nutrition Advice for Patients
- Consume a mix of the core macronutrients on a daily basis, and ideally, in each meal: carbohydrates, proteins, healthy fats, and fiber.
- Get the bulk of your calories from unprocessed vegetables and fruits. Try to eat a wide variety of different-colored vegetables. This is sometimes called the “eat a rainbow” approach.
- Minimize processed foods.
- Stay well hydrated. Avoid sweetened beverages, including fruit juices.
12. Relaxation and Behavioral Activation Module
12.1. Deep-Breathing Training
12.2. Behavioral Activation Therapy/Pleasant Event Scheduling
12.2.1. Comment on Nature-Based Behavioral Activation
13. Synthesis and Conclusions
13.1. Comment on Potential Iatrogenic Factors
13.2. Next Steps
- Posttraumatic headache interventions [566].
13.3. Weaknesses and Strengths
- While several of the components of the treatment program have been the subject of treatment research exploration in populations recovering from concussion, the overall framework has not yet been evaluated in research trials but is based on neuroanatomic principles and clinical experiences that are suggestive of value. While this is to be somewhat expected based on the current state of concussion research in general, it remains a significant limitation.
- The model requires most clinicians to broaden their scope of practice and upskill in some areas. Developing skills in behavioral sleep interventions, exercise interventions, fatigue management, nutrition interventions, behavioral activation strategies, and relaxation protocols is achievable and within the capacity of most clinicians. However, it does require time and effort on the part of the clinician to organize resources, understand the material, and develop a plan for implementation with patients. Some clinicians are excited by the prospect of learning new skills and some are not, preferring to focus their continuing education efforts on narrower specialties. Both are legitimate choices. It is our impression that the initial foray into intervention with patients with persisting complaints requires clinicians with a broader rather than a narrower skill base.
- In protracted cases (such as symptoms present for 6 months or more), unless the treating clinician has the opportunity to meet with the patient regularly at first (ideally weekly, at a minimum fortnightly), the intervention described in this paper will likely be less effective. Some clinical practices are overwhelmed, and individual clinicians may struggle to shift systemic practices in the clinics where they work to allow for such regular contact. In cases where intervention cannot be this frequent, we would discourage the attempt at the program described in this paper. An inadequate, ineffectual dose has the potential to be demoralizing to a patient who is invested in complying with recommended interventions but is making little progress. In settings where frequent visits are prohibitive, clinicians can consider educating and engaging other clinicians in the treatment process, for example, by providing a written summary of modules to a psychological therapist that the patient is already seeing regularly to determine if that clinician is open to integrating some of the modules into that setting.
- It can be implemented by a wide variety of rehabilitation professionals with only modest training and supervision needs.
- It is relatively lean and cost-effective in terms of (a) a low need for specialized equipment and (b) clinical intervention time. Most patients can be served by one or two clinicians to implement this program rather than an entire multidisciplinary team (see comments regarding iatrogenic risks above).
- There is a clear rationale for the modules based on the neurophysiology of concussion and human physiology in general. Sharing this rationale with patients tends to increase engagement and compliance.
- It is respectful of both the limitations and strengths in the research and provides a balanced approach based on the available evidence.
- The strategies in the program (while particularly relevant for concussion patients) are beneficial for all humans across the developmental lifespan, and this presents opportunities to involve significant others in the treatment process in an effort to create a family culture that values these “lifestyle interventions.”
- The interventions are beneficial for multiple other health and emotional conditions and represent a set of behaviors that, in most cases, would benefit patients if they were continued for life, even after concussion symptoms resolve.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daugherty, J.; DePadilla, L.; Sarmiento, K.; Breiding, M.J. Self-Reported Lifetime Concussion Among Adults: Comparison of 3 Different Survey Questions. J. Head Trauma Rehabil. 2020, 35, E136–E143. [Google Scholar] [CrossRef] [PubMed]
- Whiteneck, G.G.; Cuthbert, J.P.; Corrigan, J.D.; Bogner, J.A. Prevalence of Self-Reported Lifetime History of Traumatic Brain Injury and Associated Disability: A Statewide Population-Based Survey. J. Head Trauma Rehabil. 2016, 31, E55–E62. [Google Scholar] [CrossRef] [PubMed]
- Veliz, P.; McCabe, S.E.; Eckner, J.T.; Schulenberg, J.E. Trends in the Prevalence of Concussion Reported by US Adolescents, 2016–2020. JAMA 2021, 325, 1789–1791. [Google Scholar] [CrossRef]
- Voormolen, D.C.; Haagsma, J.A.; Polinder, S.; Maas, A.I.R.; Steyerberg, E.W.; Vulekovic, P.; Sewalt, C.A.; Gravesteijn, B.Y.; Covic, A.; Andelic, N.; et al. Post-Concussion Symptoms in Complicated vs. Uncomplicated Mild Traumatic Brain Injury Patients at Three and Six Months Post-Injury: Results from the CENTER-TBI Study. J. Clin. Med. 2019, 8, 1921. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.W.; Theadom, A.; Snell, D.L.; Williams, M.N. Network analysis applied to post-concussion symptoms in two mild traumatic brain injury samples. Front. Neurol. 2023, 14, 1226367. [Google Scholar] [CrossRef] [PubMed]
- Pertab, J.L.; Merkley, T.L.; Cramond, A.J.; Cramond, K.; Paxton, H.; Wu, T. Concussion and the autonomic nervous system: An introduction to the field and the results of a systematic review. NeuroRehabilitation 2018, 42, 397–427. [Google Scholar] [CrossRef]
- Keatley, E.; Bechtold, K.; Psoter, K.; Peters, M.E.; Everett, A.; Rao, V.; Van Meter, T.E.; Falk, H.; Korley, F.K.; Roy, D. Longitudinal Trajectories of Post-Concussive Symptoms Following Mild Traumatic Brain Injury. Brain Inj. 2023, 37, 737–745. [Google Scholar] [CrossRef]
- Fried, E.; Balla, U.; Catalogna, M.; Kozer, E.; Oren-Amit, A.; Hadanny, A.; Efrati, S. Persistent post-concussive syndrome in children after mild traumatic brain injury is prevalent and vastly underdiagnosed. Sci. Rep. 2022, 12, 4364. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, C.L.; Barber, J.; Patterson, J.; Johnson, A.M.; Parsey, C.; Scott, B.; Fann, J.R.; Temkin, N.R. Comparison of Clinical Outcomes 1 and 5 Years Post-Injury Following Combat Concussion. Neurology 2021, 96, e387–e398. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Parag, V.; Dowell, T.; McPherson, K.; Starkey, N.; Barker-Collo, S.; Jones, K.; Ameratunga, S.; Feigin, V.L.; Group, B.R. Persistent problems 1 year after mild traumatic brain injury: A longitudinal population study in New Zealand. Br. J. Gen. Pract. 2016, 66, e16–e23. [Google Scholar] [CrossRef]
- Cairns, K.; Beaulieu-Bonneau, S.; Jomphe, V.; Lamontagne, M.E.; de Guise, E.; Moore, L.; Savard, J.; Sirois, M.J.; Swaine, B.; Ouellet, M.C. Four-Year Trajectories of Symptoms and Quality of Life in Individuals Hospitalized After Mild Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2024, in press. [Google Scholar] [CrossRef]
- Lefevre-Dognin, C.; Cogne, M.; Perdrieau, V.; Granger, A.; Heslot, C.; Azouvi, P. Definition and epidemiology of mild traumatic brain injury. Neurochirurgie 2021, 67, 218–221. [Google Scholar] [CrossRef]
- Harmon, K.G.; Drezner, J.A.; Gammons, M.; Guskiewicz, K.M.; Halstead, M.; Herring, S.A.; Kutcher, J.S.; Pana, A.; Putukian, M.; Roberts, W.O. American Medical Society for Sports Medicine position statement: Concussion in sport. Br. J. Sports Med. 2013, 47, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.C.M.; Kosofsky, B.E. Autonomic dysfunction following mild traumatic brain injury. Curr. Opin. Neurol. 2019, 32, 802–807. [Google Scholar] [CrossRef]
- Purkayastha, S.; Stokes, M.; Bell, K.R. Autonomic nervous system dysfunction in mild traumatic brain injury: A review of related pathophysiology and symptoms. Brain Inj. 2019, 33, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Pelo, R.; Suttman, E.; Fino, P.C.; McFarland, M.M.; Dibble, L.E.; Cortez, M.M. Autonomic dysfunction and exercise intolerance in concussion: A scoping review. Clin. Auton. Res. 2023, 33, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.J.; Batycky, J.; Campbell, C.; Schneider, K.; Smirl, J.; Debert, C.T. Autonomic dysfunction in adults following mild traumatic brain injury: A systematic review. NeuroRehabilitation 2022, 50, 3–32. [Google Scholar] [CrossRef]
- Wesolowski, E.; Ahmed, Z.; Di Pietro, V. History of concussion and lowered heart rate variability at rest beyond symptom recovery: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1285937. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.; Hogg-Johnson, S. Autonomic nervous system dysfunction in pediatric sport-related concussion: A systematic review. J. Can. Chiropr. Assoc. 2023, 67, 246–268. [Google Scholar] [PubMed]
- Talbert, L.D.; Kaelberer, Z.; Gleave, E.; Driggs, A.; Driggs, A.S.; Baldwin, S.A.; Steffen, P.R.; Larson, M.J. A Systematic Review of the Relationship Between Traumatic Brain Injury and Disruptions in Heart Rate Variability. Appl. Psychophysiol. Biofeedback 2024, 49, 523–540. [Google Scholar] [CrossRef]
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr. Physiol. 2016, 6, 1239–1278. [Google Scholar] [CrossRef] [PubMed]
- Kenney, M.J.; Ganta, C.K. Autonomic nervous system and immune system interactions. Compr. Physiol. 2014, 4, 1177–1200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Tsigos, C.; Kyrou, I.; Kassi, E.; Chrousos, G.P. Stress: Endocrine Physiology and Pathophysiology. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [CrossRef] [PubMed]
- El Baassiri, M.G.; Raouf, Z.; Badin, S.; Escobosa, A.; Sodhi, C.P.; Nasr, I.W. Dysregulated brain-gut axis in the setting of traumatic brain injury: Review of mechanisms and anti-inflammatory pharmacotherapies. J. Neuroinflammation 2024, 21, 124. [Google Scholar] [CrossRef]
- Weil, Z.M.; White, B.; Whitehead, B.; Karelina, K. The role of the stress system in recovery after traumatic brain injury: A tribute to Bruce S. McEwen. Neurobiol. Stress 2022, 19, 100467. [Google Scholar] [CrossRef]
- Yamakawa, G.R.; Brady, R.D.; Sun, M.; McDonald, S.J.; Shultz, S.R.; Mychasiuk, R. The interaction of the circadian and immune system: Desynchrony as a pathological outcome to traumatic brain injury. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100058. [Google Scholar] [CrossRef]
- Mahajan, C.; Prabhakar, H.; Bilotta, F. Endocrine Dysfunction After Traumatic Brain Injury: An Ignored Clinical Syndrome? Neurocritical Care 2023, 39, 714–723. [Google Scholar] [CrossRef]
- Verboon, L.N.; Patel, H.C.; Greenhalgh, A.D. The Immune System’s Role in the Consequences of Mild Traumatic Brain Injury (Concussion). Front. Immunol. 2021, 12, 620698. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef]
- Fleming, M.A., 2nd; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D. Primer on the Autonomic Nervous System, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2004; p. xxvii. 459p. [Google Scholar]
- Burtscher, J.; Niedermeier, M.; Hufner, K.; van den Burg, E.; Kopp, M.; Stoop, R.; Burtscher, M.; Gatterer, H.; Millet, G.P. The interplay of hypoxic and mental stress: Implications for anxiety and depressive disorders. Neurosci. Biobehav. Rev. 2022, 138, 104718. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Na, K.S.; Kim, Y.K. Associations between Melatonin, Neuroinflammation, and Brain Alterations in Depression. Int. J. Mol. Sci. 2021, 23, 305. [Google Scholar] [CrossRef]
- Hyun, U.; Sohn, J.W. Autonomic control of energy balance and glucose homeostasis. Exp. Mol. Med. 2022, 54, 370–376. [Google Scholar] [CrossRef]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef] [PubMed]
- Marumo, C.; Nakano, T. Early phase of pupil dilation is mediated by the peripheral parasympathetic pathway. J. Neurophysiol. 2021, 126, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Carrick, F.R.; Azzolino, S.F.; Hunfalvay, M.; Pagnacco, G.; Oggero, E.; D’Arcy, R.C.N.; Abdulrahman, M.; Sugaya, K. The Pupillary Light Reflex as a Biomarker of Concussion. Life 2021, 11, 1104. [Google Scholar] [CrossRef] [PubMed]
- Temme, L.; Bleiberg, J.; Reeves, D.; Still, D.L.; Levinson, D.; Browning, R. Uncovering latent deficits due to mild traumatic brain injury by using normobaric hypoxia stress. Front. Neurol. 2013, 4, 41. [Google Scholar] [CrossRef]
- Hanna-Pladdy, B.; Berry, Z.M.; Bennett, T.; Phillips, H.L.; Gouvier, W.D. Stress as a diagnostic challenge for postconcussive symptoms: Sequelae of mild traumatic brain injury or physiological stress response. Clin. Neuropsychol. 2001, 15, 289–304. [Google Scholar] [CrossRef]
- Glenn, D.E.; Acheson, D.T.; Geyer, M.A.; Nievergelt, C.M.; Baker, D.G.; Risbrough, V.B.; Team, M.-I. Fear learning alterations after traumatic brain injury and their role in development of posttraumatic stress symptoms. Depress. Anxiety 2017, 34, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.W.; Fernandes, M.A. Long-term cognitive and affective consequences of mild traumatic brain injury: Comparison with older adults. Brain Inj. 2024, 38, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.N.; Johnson, B.D.; Horn, E.C.; Leddy, J.J.; Wilber, C.G.; Reed, E.L.; O’Leary, M.; Bloomfield, A.; Decezaro, L.L.; Willer, B.S. Blunted Cardiac Parasympathetic Activation in Student Athletes with a Remote History of Concussion: A Pilot Study. Front. Neurol. 2020, 11, 547126. [Google Scholar] [CrossRef]
- Ewing, R.; McCarthy, D.; Gronwall, D.; Wrightson, P. Persisting effects of minor head injury observable during hypoxic stress. J. Clin. Exp. Neuropsychol. 1980, 2, 147–155. [Google Scholar] [CrossRef]
- Worley, M.L.; O’Leary, M.C.; Sackett, J.R.; Schlader, Z.J.; Willer, B.; Leddy, J.J.; Johnson, B.D. Preliminary Evidence of Orthostatic Intolerance and Altered Cerebral Vascular Control Following Sport-Related Concussion. Front. Neurol. 2021, 12, 620757. [Google Scholar] [CrossRef]
- McPherson, J.I.; Nazir, M.S.Z.; Willer, B.S.; Leddy, J.J.; Haider, M.N. Does Physiologic Post-Concussion Disorder Cause Persistent Post-Traumatic Headache? Curr. Pain Headache Rep. 2023, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Torre, P.; Antonello, R.M.; Manganaro, D.; Vilotti, C.; Pizzolato, G. Risk factors for vascular dementia: Hypotension as a key point. Vasc. Health Risk Manag. 2008, 4, 395–402. [Google Scholar] [CrossRef]
- Tan, C.O.; Grashow, R.; Thorpe, R., Jr.; Miller, K.K.; Nathan, D.M.; Izzy, S.; Radmanesh, F.; Kim, J.H.; Weisskopf, M.G.; Taylor, H.A., Jr.; et al. Concussion burden and later-life cardiovascular risk factors in former professional American-style football players. Ann. Clin. Transl. Neurol. 2024, 11, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Grashow, R.; Tan, C.O.; Izzy, S.; Taylor, H.A., Jr.; Weisskopf, M.G.; Wasfy, M.M.; Whittington, A.J.; Speizer, F.; Zafonte, R.; Baggish, A.L. Association Between Concussion Burden During Professional American-Style Football and Postcareer Hypertension. Circulation 2023, 147, 1112–1114. [Google Scholar] [CrossRef]
- Kolliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediat. Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ashwal, S.; Siebold, L.; Krueger, A.C.; Wilson, C.G. Post-traumatic Neuroinflammation: Relevance to Pediatrics. Pediatr. Neurol. 2021, 122, 50–58. [Google Scholar] [CrossRef]
- Gorji, A. Neuroinflammation: The Pathogenic Mechanism of Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 5744. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Morley, W.A.; Seneff, S. Diminished brain resilience syndrome: A modern day neurological pathology of increased susceptibility to mild brain trauma, concussion, and downstream neurodegeneration. Surg. Neurol. Int. 2014, 5, 97. [Google Scholar] [CrossRef]
- McEwen, B.S. In pursuit of resilience: Stress, epigenetics, and brain plasticity. Ann. N. Y. Acad. Sci. 2016, 1373, 56–64. [Google Scholar] [CrossRef]
- Schimmel, S.J.; Acosta, S.; Lozano, D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017, 3, 135–142. [Google Scholar] [CrossRef]
- Chaban, V.; Clarke, G.J.B.; Skandsen, T.; Islam, R.; Einarsen, C.E.; Vik, A.; Damas, J.K.; Mollnes, T.E.; Haberg, A.K.; Pischke, S.E. Systemic Inflammation Persists the First Year after Mild Traumatic Brain Injury: Results from the Prospective Trondheim Mild Traumatic Brain Injury Study. J. Neurotrauma 2020, 37, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.D.; Broshek, D.K.; Newman, B.T.; Druzgal, T.J.; Kundu, B.K.; Resch, J.E. Concussion: Beyond the Cascade. Cells 2023, 12, 2128. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Du, Y.; Sweeney, S.E.; O’Toole, R.; Thomas, C.L.; Zandi, A.G.; Shinehouse, L.K.; Brosnan, M.K.; Nam, H.; Burke, M.E.; et al. Imaging Brain Injury in Former National Football League Players. JAMA Netw. Open 2023, 6, e2340580. [Google Scholar] [CrossRef] [PubMed]
- Gard, A.; Vedung, F.; Piehl, F.; Khademi, M.; Wernersson, M.P.; Rorsman, I.; Tegner, Y.; Pessah-Rasmussen, H.; Ruscher, K.; Marklund, N. Cerebrospinal fluid levels of neuroinflammatory biomarkers are increased in athletes with persistent post-concussive symptoms following sports-related concussion. J. Neuroinflammation 2023, 20, 189. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Alnaji, O.; Malik, M.; Gambale, T.; Farrokhyar, F.; Rathbone, M.P. Inflammatory cytokines associated with mild traumatic brain injury and clinical outcomes: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1123407. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; He, Y.; Hu, Z.; Gu, Y.; Li, Y.; Ye, Y.; Hu, J. Neurological Symptoms and Their Associations with Inflammatory Biomarkers in the Chronic Phase Following Traumatic Brain Injuries. Front. Psychiatry 2022, 13, 895852. [Google Scholar] [CrossRef]
- Rathbone, A.T.; Tharmaradinam, S.; Jiang, S.; Rathbone, M.P.; Kumbhare, D.A. A review of the neuro- and systemic inflammatory responses in post concussion symptoms: Introduction of the “post-inflammatory brain syndrome” PIBS. Brain Behav. Immun. 2015, 46, 1–16. [Google Scholar] [CrossRef]
- Malik, S.; Alnaji, O.; Malik, M.; Gambale, T.; Rathbone, M.P. Correlation between Mild Traumatic Brain Injury-Induced Inflammatory Cytokines and Emotional Symptom Traits: A Systematic Review. Brain Sci. 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Visser, K.; Koggel, M.; Blaauw, J.; van der Horn, H.J.; Jacobs, B.; van der Naalt, J. Blood-based biomarkers of inflammation in mild traumatic brain injury: A systematic review. Neurosci. Biobehav. Rev. 2022, 132, 154–168. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Herrero Babiloni, A.; Baril, A.A.; Charlebois-Plante, C.; Jodoin, M.; Sanchez, E.; De Baets, L.; Arbour, C.; Lavigne, G.J.; Gosselin, N.; De Beaumont, L. The Putative Role of Neuroinflammation in the Interaction between Traumatic Brain Injuries, Sleep, Pain and Other Neuropsychiatric Outcomes: A State-of-the-Art Review. J. Clin. Med. 2023, 12, 1793. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- O’Byrne, N.A.; Yuen, F.; Butt, W.Z.; Liu, P.Y. Sleep and Circadian Regulation of Cortisol: A Short Review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Robertson-Dixon, I.; Murphy, M.J.; Crewther, S.G.; Riddell, N. The Influence of Light Wavelength on Human HPA Axis Rhythms: A Systematic Review. Life 2023, 13, 1968. [Google Scholar] [CrossRef]
- Daneva, E.; Makris, K.; Korompeli, A.; Muurlink, O.; Kaklamanos, I.; Fildissis, G.; Vlachos, K.; Myrianthefs, P. Saliva cortisol levels and physiological parameter fluctuations in mild traumatic brain injury patients compared to controls. Int. J. Neurosci. 2023, 133, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, S.; Kallenbach, M.D.; Huber, D.L.; Raff, H.; Johnson, B.D.; Leddy, J.; McCrea, M.A.; Meier, T.B.; Nelson, L.D. Salivary Cortisol Dynamics After Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 2023, 38, E318–E327. [Google Scholar] [CrossRef] [PubMed]
- Villegas, E.; Hartsock, M.J.; Aben, B.; Lenahan, K.N.; Hernandez, T.D.; Spencer, R.L. Association between Altered Cortisol Profiles and Neurobehavioral Impairment after Mild Traumatic Brain Injury in College Students. J. Neurotrauma 2022, 39, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, N.K.; Geer, E.B.; Greenwald, B.D. The impact of traumatic brain injury on pituitary function. Endocrinol. Metab. Clin. N. Am. 2013, 42, 565–583. [Google Scholar] [CrossRef]
- West, T.A.; Sharp, S. Neuroendocrine dysfunction following mild TBI: When to screen for it. J. Fam. Pract. 2014, 63, 11–16. [Google Scholar] [PubMed]
- Hiller-Sturmhofel, S.; Bartke, A. The endocrine system: An overview. Alcohol Health Res. World 1998, 22, 153–164. [Google Scholar]
- Yu, J. Endocrine disorders and the neurologic manifestations. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 184–190. [Google Scholar] [CrossRef]
- Bondanelli, M.; De Marinis, L.; Ambrosio, M.R.; Monesi, M.; Valle, D.; Zatelli, M.C.; Fusco, A.; Bianchi, A.; Farneti, M.; degli Uberti, E.C. Occurrence of pituitary dysfunction following traumatic brain injury. J. Neurotrauma 2004, 21, 685–696. [Google Scholar] [CrossRef]
- Wilkinson, C.W.; Pagulayan, K.F.; Petrie, E.C.; Mayer, C.L.; Colasurdo, E.A.; Shofer, J.B.; Hart, K.L.; Hoff, D.; Tarabochia, M.A.; Peskind, E.R. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Front. Neurol. 2012, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, F.; Unluhizarci, K.; Kelestimur, F. Pituitary function in subjects with mild traumatic brain injury: A review of literature and proposal of a screening strategy. Pituitary 2010, 13, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, S.; Talarico, S.; Bruno, L.; Nicoletti, F.B.; Ceccotti, C.; Belfiore, A. Growth hormone deficiency and hypopituitarism in adults after complicated mild traumatic brain injury. Endocrine 2017, 58, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.K.; Powell, J.R.; Cools, M.J.; Walton, S.R.; Barnett, R.R.; Delellis, S.M.; Goldberg, R.L.; Kane, S.F.; Means, G.E.; Zamora, C.A.; et al. Relationship between Anterior Pituitary Volume and IGF-1 Serum Levels in Soldiers with Mild Traumatic Brain Injury History. Med. Sci. Sports Exerc. 2022, 54, 1364–1370. [Google Scholar] [CrossRef]
- Eggertsdottir Claessen, L.O.; Kristjansdottir, H.; Jonsdottir, M.K.; Lund, S.H.; Unnsteinsdottir Kristensen, I.; Sigurjonsdottir, H.A. Pituitary dysfunction following mild traumatic brain injury in female athletes. Endocr. Connect. 2024, 13, e230363. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.; Urban, R.; Durham, W.; Dillon, E.L.; Randolph, K.M.; Danesi, C.; Gilkison, C.; Karmonik, C.; Zgaljardic, D.J.; Masel, B.; et al. Growth Hormone Alters Brain Morphometry, Connectivity, and Behavior in Subjects with Fatigue after Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Gilis-Januszewska, A.; Kluczynski, L.; Hubalewska-Dydejczyk, A. Traumatic brain injuries induced pituitary dysfunction: A call for algorithms. Endocr. Connect. 2020, 9, R112–R123. [Google Scholar] [CrossRef] [PubMed]
- Bondanelli, M.; Ambrosio, M.R.; Zatelli, M.C.; De Marinis, L.; degli Uberti, E.C. Hypopituitarism after traumatic brain injury. Eur. J. Endocrinol. 2005, 152, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Bilski, T.; Dieudonne, B.; Saeed, S. Hypopituitarism After Traumatic Brain Injury. Cureus 2019, 11, e4163. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.J.; Kruger, N.; Le, Q.B.; Fung, T.S.; Kline, G.A.; Debert, C.T. Growth hormone deficiency testing and treatment following mild traumatic brain injury. Sci. Rep. 2021, 11, 8534. [Google Scholar] [CrossRef]
- Palacios, E.M.; Yuh, E.L.; Mac Donald, C.L.; Bourla, I.; Wren-Jarvis, J.; Sun, X.; Vassar, M.J.; Diaz-Arrastia, R.; Giacino, J.T.; Okonkwo, D.O.; et al. Diffusion Tensor Imaging Reveals Elevated Diffusivity of White Matter Microstructure that Is Independently Associated with Long-Term Outcome after Mild Traumatic Brain Injury: A TRACK-TBI Study. J. Neurotrauma 2022, 39, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Irimia, A.; Ngo, V.; Chaudhari, N.N.; Zhang, F.; Joshi, S.H.; Penkova, A.N.; O’Donnell, L.J.; Sheikh-Bahaei, N.; Zheng, X.; Chui, H.C. White matter degradation near cerebral microbleeds is associated with cognitive change after mild traumatic brain injury. Neurobiol. Aging 2022, 120, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Chang, X.; Bai, L.; Wang, Y.; Dong, D.; Gan, S.; Wang, S.; Li, X.; Yang, X.; Sun, Y.; et al. A Longitudinal Study of White Matter Functional Network in Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2686–2697. [Google Scholar] [CrossRef]
- Lima Santos, J.P.; Kontos, A.P.; Mailliard, S.; Eagle, S.R.; Holland, C.L.; Suss, S.J., Jr.; Abdul-Waalee, H.; Stiffler, R.S.; Bitzer, H.B.; Blaney, N.A.; et al. White Matter Abnormalities Associated with Prolonged Recovery in Adolescents Following Concussion. Front. Neurol. 2021, 12, 681467. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gan, S.; Yang, X.; Li, T.; Xiong, F.; Jia, X.; Sun, Y.; Liu, J.; Zhang, M.; Bai, L. Decoupling of Structural and Functional Connectivity in Hubs and Cognitive Impairment After Mild Traumatic Brain Injury. Brain Connect. 2021, 11, 745–758. [Google Scholar] [CrossRef]
- Arciniega, H.; Shires, J.; Furlong, S.; Kilgore-Gomez, A.; Cerreta, A.; Murray, N.G.; Berryhill, M.E. Impaired visual working memory and reduced connectivity in undergraduates with a history of mild traumatic brain injury. Sci. Rep. 2021, 11, 2789. [Google Scholar] [CrossRef]
- King, R.; Grohs, M.N.; Kirton, A.; Lebel, C.; Esser, M.J.; Barlow, K.M. Microstructural neuroimaging of white matter tracts in persistent post-concussion syndrome: A prospective controlled cohort study. NeuroImage Clin. 2019, 23, 101842. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Byun, D.H. Hidden Truth in Cerebral Concussion-Traumatic Axonal Injury: A Narrative Mini-Review. Healthcare 2022, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, S.; Teixeira-Dias, M.; Medford, N.; Nicholson, T.; Okai, D.; Shotbolt, P.; Deeley, Q. Predictive Utility of Diffusion MRI After Mild Traumatic Brain Injury in Civilian Populations: A Systematic Review. J. Neuropsychiatry Clin. Neurosci. 2024, 36, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ware, A.L.; Yeates, K.O.; Tang, K.; Shukla, A.; Onicas, A.I.; Guo, S.; Goodrich-Hunsaker, N.; Abdeen, N.; Beauchamp, M.H.; Beaulieu, C.; et al. Longitudinal white matter microstructural changes in pediatric mild traumatic brain injury: An A-CAP study. Hum. Brain Mapp. 2022, 43, 3809–3823. [Google Scholar] [CrossRef] [PubMed]
- Eisele, A.; Hill-Strathy, M.; Michels, L.; Rauen, K. Magnetic Resonance Spectroscopy following Mild Traumatic Brain Injury: A Systematic Review and Meta-Analysis on the Potential to Detect Posttraumatic Neurodegeneration. Neurodegener. Dis. 2020, 20, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The new neurometabolic cascade of concussion. Neurosurgery 2014, 75 (Suppl. S4), S24–S33. [Google Scholar] [CrossRef]
- Howell, D.R.; Southard, J. The Molecular Pathophysiology of Concussion. Clin. Sports Med. 2021, 40, 39–51. [Google Scholar] [CrossRef]
- Olivera, A.; Lejbman, N.; Jeromin, A.; French, L.M.; Kim, H.S.; Cashion, A.; Mysliwiec, V.; Diaz-Arrastia, R.; Gill, J. Peripheral Total Tau in Military Personnel Who Sustain Traumatic Brain Injuries During Deployment. JAMA Neurol. 2015, 72, 1109–1116. [Google Scholar] [CrossRef]
- Dean, P.J.A.; Sato, J.R.; Vieira, G.; McNamara, A.; Sterr, A. Long-term structural changes after mTBI and their relation to post-concussion symptoms. Brain Inj. 2015, 29, 1211–1218. [Google Scholar] [CrossRef]
- Bigler, E.D. Volumetric MRI Findings in Mild Traumatic Brain Injury (mTBI) and Neuropsychological Outcome. Neuropsychol. Rev. 2023, 33, 5–41. [Google Scholar] [CrossRef]
- Hurtubise, J.M.; Gorbet, D.J.; Hynes, L.; Macpherson, A.K.; Sergio, L.E. Cortical and cerebellar structural correlates of cognitive-motor integration performance in females with and without persistent concussion symptoms. Brain Inj. 2023, 37, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.J.; Salat, D.H.; Milberg, W.P.; McGlinchey, R.E.; Fortier, C.B. Poor sleep and decreased cortical thickness in veterans with mild traumatic brain injury and post-traumatic stress disorder. Mil. Med. Res. 2024, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.B.; Savitz, J.; Espana, L.Y.; Goeckner, B.D.; Kent Teague, T.; van der Horn, H.J.; Tugan Muftuler, L.; Mayer, A.R.; Brett, B.L. Association of concussion history with psychiatric symptoms, limbic system structure, and kynurenine pathway metabolites in healthy, collegiate-aged athletes. Brain Behav. Immun. 2024, 123, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kierans, A.; Kenul, D.; Ge, Y.; Rath, J.; Reaume, J.; Grossman, R.I.; Lui, Y.W. Mild traumatic brain injury: Longitudinal regional brain volume changes. Radiology 2013, 267, 880–890. [Google Scholar] [CrossRef]
- Prichep, L.S.; McCrea, M.; Barr, W.; Powell, M.; Chabot, R.J. Time course of clinical and electrophysiological recovery after sport-related concussion. J. Head Trauma Rehabil. 2013, 28, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.M.; Urban, J.E.; Vaughan, C.; DeSimone, J.C.; Wagner, B.; Espeland, M.A.; Powers, A.K.; Whitlow, C.T.; Stitzel, J.D.; Maldjian, J.A. MEG measured delta waves increase in adolescents after concussion. Brain Behav. 2022, 12, e2720. [Google Scholar] [CrossRef] [PubMed]
- Kerasidis, H.; Simmons, J. Quantitative EEG Analysis in Clinical Practice: Concussion Injury. Clin. EEG Neurosci. 2021, 52, 114–118. [Google Scholar] [CrossRef]
- Gosselin, N.; Theriault, M.; Leclerc, S.; Montplaisir, J.; Lassonde, M. Neurophysiological anomalies in symptomatic and asymptomatic concussed athletes. Neurosurgery 2006, 58, 1151–1161; discussion 1151–1161. [Google Scholar] [CrossRef]
- Barlow, K.M.; Iyer, K.; Yan, T.; Scurfield, A.; Carlson, H.; Wang, Y. Cerebral Blood Flow Predicts Recovery in Children with Persistent Post-Concussion Symptoms after Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2275–2283. [Google Scholar] [CrossRef]
- Xiong, F.; Li, T.; Pan, Y.; Liu, Y.; Zhang, J.; Bai, L. Arterial spin labeling magnetic resonance evaluates changes of cerebral blood flow in patients with mild traumatic brain injury. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47, 1016–1024. [Google Scholar] [CrossRef]
- Churchill, N.W.; Hutchison, M.G.; Graham, S.J.; Schweizer, T.A. Symptom correlates of cerebral blood flow following acute concussion. Neuroimage Clin. 2017, 16, 234–239. [Google Scholar] [CrossRef]
- Barlow, K.M.; Marcil, L.D.; Dewey, D.; Carlson, H.L.; MacMaster, F.P.; Brooks, B.L.; Lebel, R.M. Cerebral Perfusion Changes in Post-Concussion Syndrome: A Prospective Controlled Cohort Study. J. Neurotrauma 2017, 34, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.B.; Bellgowan, P.S.; Singh, R.; Kuplicki, R.; Polanski, D.W.; Mayer, A.R. Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 2015, 72, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Hemachandran, N.; Meena, S.; Kumar, A.; Sharma, R.; Gupta, D.; Gamanagatti, S. Utility of admission perfusion CT for the prediction of suboptimal outcome following uncomplicated minor traumatic brain injury. Emerg. Radiol. 2021, 28, 541–548. [Google Scholar] [CrossRef]
- Papadaki, E.; Kavroulakis, E.; Manolitsi, K.; Makrakis, D.; Papastefanakis, E.; Tsagaraki, P.; Papadopoulou, S.; Zampetakis, A.; Malliou, M.; Vakis, A.; et al. Cerebral perfusion disturbances in chronic mild traumatic brain injury correlate with psychoemotional outcomes. Brain Imaging Behav. 2021, 15, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Weigand, A.J.; Bangen, K.J.; Merritt, V.C.; Bondi, M.W.; Delano-Wood, L. Repetitive mTBI is associated with age-related reductions in cerebral blood flow but not cortical thickness. J. Cereb. Blood Flow Metab. 2021, 41, 431–444. [Google Scholar] [CrossRef]
- Albalawi, T.; Hamner, J.W.; Lapointe, M.; Meehan, W.P.R.; Tan, C.O. The Relationship between Cerebral Vasoreactivity and Post-Concussive Symptom Severity. J. Neurotrauma 2017, 34, 2700–2705. [Google Scholar] [CrossRef]
- Stephens, J.A.; Liu, P.; Lu, H.; Suskauer, S.J. Cerebral Blood Flow after Mild Traumatic Brain Injury: Associations between Symptoms and Post-Injury Perfusion. J. Neurotrauma 2018, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Tarumi, T.; Tomoto, T.; McColloster, M.; Le, T.; Dieppa, M.; Diaz-Arrastia, R.; Bell, K.; Madden, C.; Cullum, C.M.; et al. Impaired cerebral blood flow regulation in chronic traumatic brain injury. Brain Res. 2020, 1743, 146924. [Google Scholar] [CrossRef] [PubMed]
- Koziarz, A.; Koziarz, F.; Shen, R.; Gopee-Ramanan, P.; Black, S.E.; Worsley, D.; Chan, I.Y.M.; Streiner, D.L.; Zukotynski, K.A. Diagnostic Accuracy of SPECT for Mild Traumatic Brain Injury: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2024, 49, 938–947. [Google Scholar] [CrossRef]
- Raji, C.A.; Tarzwell, R.; Pavel, D.; Schneider, H.; Uszler, M.; Thornton, J.; van Lierop, M.; Cohen, P.; Amen, D.G.; Henderson, T. Clinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: A systematic review. PLoS ONE 2014, 9, e91088. [Google Scholar] [CrossRef] [PubMed]
- Neill, M.G.; Burma, J.S.; Miutz, L.N.; Kennedy, C.M.; Penner, L.C.; Newel, K.T.; Smirl, J.D. Transcranial Doppler Ultrasound and Concussion-Supplemental Symptoms with Physiology: A Systematic Review. J. Neurotrauma 2024, 41, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Mortaheb, S.; Filippini, M.M.; Kaux, J.F.; Annen, J.; Lejeune, N.; Martens, G.; Calderon, M.A.F.; Laureys, S.; Thibaut, A. Neurophysiological Biomarkers of Persistent Post-concussive Symptoms: A Scoping Review. Front. Neurol. 2021, 12, 687197. [Google Scholar] [CrossRef]
- Wang, Y.; Bartels, H.M.; Nelson, L.D. A Systematic Review of ASL Perfusion MRI in Mild TBI. Neuropsychol. Rev. 2023, 33, 160–191. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, Y.; Yuryshinetz, I.; Zabenko, Y.; Pivneva, T. Mild traumatic brain injury as a pathological process. Heliyon 2023, 9, e18342. [Google Scholar] [CrossRef]
- Aljabri, A.; Halawani, A.; Ashqar, A.; Alageely, O.; Alhazzani, A. The Efficacy of Vestibular Rehabilitation Therapy for Mild Traumatic Brain Injury: A Systematic Review and Meta-analysis. J. Head Trauma Rehabil. 2024, 39, E59–E69. [Google Scholar] [CrossRef] [PubMed]
- Heslot, C.; Azouvi, P.; Perdrieau, V.; Granger, A.; Lefevre-Dognin, C.; Cogne, M. A Systematic Review of Treatments of Post-Concussion Symptoms. J. Clin. Med. 2022, 11, 6224. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.M.; Pauhl, A.N.; Christie, A.D. The Role of Active Rehabilitation in Concussion Management: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2021, 53, 1835–1845. [Google Scholar] [CrossRef]
- McIntyre, M.; Kempenaar, A.; Amiri, M.; Alavinia, S.M.; Kumbhare, D. The Role of Subsymptom Threshold Aerobic Exercise for Persistent Concussion Symptoms in Patients with Postconcussion Syndrome: A Systematic Review. Am. J. Phys. Med. Rehabil. 2020, 99, 257–264. [Google Scholar] [CrossRef]
- Rytter, H.M.; Graff, H.J.; Henriksen, H.K.; Aaen, N.; Hartvigsen, J.; Hoegh, M.; Nisted, I.; Naess-Schmidt, E.T.; Pedersen, L.L.; Schytz, H.W.; et al. Nonpharmacological Treatment of Persistent Postconcussion Symptoms in Adults: A Systematic Review and Meta-analysis and Guideline Recommendation. JAMA Netw. Open 2021, 4, e2132221. [Google Scholar] [CrossRef] [PubMed]
- Jennings, T.; Islam, M.S. Examining the interdisciplinary approach for treatment of persistent post-concussion symptoms in adults: A systematic review. Brain Impair. 2023, 24, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Comper, P.; Bisschop, S.M.; Carnide, N.; Tricco, A. A systematic review of treatments for mild traumatic brain injury. Brain Inj. 2005, 19, 863–880. [Google Scholar] [CrossRef]
- Thomas, R.E.; Alves, J.; Vaska Mlis, M.M.; Magalhaes, R. Therapy and rehabilitation of mild brain injury/concussion: Systematic review. Restor. Neurol. Neurosci. 2017, 35, 643–666. [Google Scholar] [CrossRef] [PubMed]
- Hanalioglu, D.; Hanalioglu, S.; Arango, J.I.; Adelson, P.D. Current evidence for pharmacological management of pediatric concussion: A systematic review. Childs Nerv. Syst. 2023, 39, 1831–1849. [Google Scholar] [CrossRef] [PubMed]
- Dobney, D.M.; Miller, M.B.; Tufts, E. Non-pharmacological rehabilitation interventions for concussion in children: A scoping review. Disabil. Rehabil. 2019, 41, 727–739. [Google Scholar] [CrossRef] [PubMed]
- De Luigi, A.J.; Bell, K.R.; Bramhall, J.P.; Choe, M.; Dec, K.; Finnoff, J.T.; Halstead, M.; Herring, S.A.; Matuszak, J.; Raksin, P.B.; et al. Consensus statement: An evidence-based review of exercise, rehabilitation, rest, and return to activity protocols for the treatment of concussion and mild traumatic brain injury. PM R 2023, 15, 1605–1642. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Musgrave, C.; Sandler, J.; Bradley, B.; Jones, J.R.A. Early intervention treatment in the first 2 weeks following concussion in adults: A systematic review of randomised controlled trials. Phys. Ther. Sport 2024, 65, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.J.; Critchley, M.L.; Anderson, V.; Davis, G.A.; Debert, C.T.; Feddermann-Demont, N.; Gagnon, I.; Guskiewicz, K.M.; Hayden, K.A.; Herring, S.; et al. Targeted interventions and their effect on recovery in children, adolescents and adults who have sustained a sport-related concussion: A systematic review. Br. J. Sports Med. 2023, 57, 771–779. [Google Scholar] [CrossRef]
- Pertab, J.L. Evidence-Based Rehabilitation in Typical Concussive Brain Injury: Results of a Systematic Review. In Concussion and Traumatic Encephalopathy: Causes, Diagnosis and Management; Victoroff, J., Bigler, E.D., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 780–799. [Google Scholar]
- Sharp, D.J.; Jenkins, P.O. Concussion is confusing us all. Pract. Neurol. 2015, 15, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Gronseth, G.S.; Cox, J.; Gloss, D.; Merillat, S.; Dittman, J.; Armstrong, M.J.; Getchius, T.S.D. Clinical Practice Guideline Process Manual, 2017th ed.; The American Academy of Neurology: Minneapolis, MN, USA, 2017. [Google Scholar]
- Halstead, M.E. Pharmacologic Therapies for Pediatric Concussions. Sports Health 2016, 8, 50–52. [Google Scholar] [CrossRef]
- Feinberg, C.; Carr, C.; Zemek, R.; Yeates, K.O.; Master, C.; Schneider, K.; Bell, M.J.; Wisniewski, S.; Mannix, R. Association of Pharmacological Interventions with Symptom Burden Reduction in Patients with Mild Traumatic Brain Injury: A Systematic Review. JAMA Neurol. 2021, 78, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Stache, S.; Howell, D.; Meehan, W.P., 3rd. Concussion Management Practice Patterns Among Sports Medicine Physicians. Clin. J. Sport Med. 2016, 26, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.M.; Klein, T.A.; Panther, S.G.; Moore, M.; Abshire, D.; Graham, J. Nurse practitioners’ recommendations for pharmacotherapy in the management of adolescent concussion. J. Am. Assoc. Nurse Pract. 2018, 30, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Mannix, R.; Zemek, R.; Yeates, K.O.; Arbogast, K.; Atabaki, S.; Badawy, M.; Beauchamp, M.H.; Beer, D.; Bin, S.; Burstein, B.; et al. Practice Patterns in Pharmacological and Non-Pharmacological Therapies for Children with Mild Traumatic Brain Injury: A Survey of 15 Canadian and United States Centers. J. Neurotrauma 2019, 36, 2886–2894. [Google Scholar] [CrossRef]
- Kinnaman, K.A.; Mannix, R.C.; Comstock, R.D.; Meehan, W.P., 3rd. Management strategies and medication use for treating paediatric patients with concussions. Acta Paediatr. 2013, 102, e424–e428. [Google Scholar] [CrossRef]
- Jones, J.C.; O’Brien, M.J. Medical Therapies for Concussion. Clin. Sports Med. 2021, 40, 123–131. [Google Scholar] [CrossRef]
- Becker, D.E. Basic and clinical pharmacology of autonomic drugs. Anesth. Prog. 2012, 59, 159–168; quiz 169. [Google Scholar] [CrossRef] [PubMed]
- Koschke, M.; Boettger, M.K.; Schulz, S.; Berger, S.; Terhaar, J.; Voss, A.; Yeragani, V.K.; Bar, K.J. Autonomy of autonomic dysfunction in major depression. Psychosom. Med. 2009, 71, 852–860. [Google Scholar] [CrossRef]
- Pleuvry, B.J. Drugs affecting the autonomic nervous system. Anaesth. Intensive Care Med. 2008, 9, 84–87. [Google Scholar] [CrossRef]
- Larson, E.B.; Zollman, F.S. The effect of sleep medications on cognitive recovery from traumatic brain injury. J. Head Trauma Rehabil. 2010, 25, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Heyer, G.L.; Idris, S.A. Does analgesic overuse contribute to chronic post-traumatic headaches in adolescent concussion patients? Pediatr. Neurol. 2014, 50, 464–468. [Google Scholar] [CrossRef]
- Jammoul, M.; Jammoul, D.; Wang, K.K.; Kobeissy, F.; Depalma, R.G. Traumatic Brain Injury and Opioids: Twin Plagues of the Twenty-First Century. Biol. Psychiatry 2024, 95, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.N.; Leddy, J.J.; Du, W.; Macfarlane, A.J.; Viera, K.B.; Willer, B.S. Practical Management: Brief Physical Examination for Sport-Related Concussion in the Outpatient Setting. Clin. J. Sport Med. 2020, 30, 513–517. [Google Scholar] [CrossRef]
- McCormick, K.; Kolar, B. Research Letter: Rate of BPPV in Patients Diagnosed with Concussion. J. Head Trauma Rehabil. 2023, 38, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhou, G.; Kawai, K.; O’Brien, M.; Shearer, A.E.; Brodsky, J.R. Benign Paroxysmal Positional Vertigo in Children and Adolescents with Concussion. Sports Health 2021, 13, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Baker, J.G.; Merchant, A.; Picano, J.; Gaile, D.; Matuszak, J.; Willer, B. Brain or strain? Symptoms alone do not distinguish physiologic concussion from cervical/vestibular injury. Clin. J. Sport Med. 2015, 25, 237–242. [Google Scholar] [CrossRef]
- Nampiaparampil, D.E. Prevalence of chronic pain after traumatic brain injury: A systematic review. JAMA 2008, 300, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Pickens, S.; Barta, Z.; Rice, M.; Dagher, M.; Lebens, R.; Nguyen, T.V.; Cummings, B.J.; Cahill, C.M. Neuroinflammation drives sex-dependent effects on pain and negative affect in a murine model of repeated mild traumatic brain injury. Pain 2024, 165, 848–865. [Google Scholar] [CrossRef]
- Leung, A. Addressing chronic persistent headaches after MTBI as a neuropathic pain state. J. Headache Pain 2020, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Bosak, N.; Bielefeld, J.; Cong, O.; Granovsky, Y.; Kahn, I.; Yarnitsky, D.; Apkarian, A.V. Structural brain connectivity predicts early acute pain after mild traumatic brain injury. Pain 2023, 164, 1312–1320. [Google Scholar] [CrossRef]
- Sahbaie, P.; Irvine, K.A.; Liang, D.Y.; Shi, X.; Clark, J.D. Mild Traumatic Brain Injury Causes Nociceptive Sensitization through Spinal Chemokine Upregulation. Sci. Rep. 2019, 9, 19500. [Google Scholar] [CrossRef]
- Ye, J.J.; Lee, K.T.; Lin, J.S.; Chuang, C.C. Observing continuous change in heart rate variability and photoplethysmography-derived parameters during the process of pain production/relief with thermal stimuli. J. Pain Res. 2017, 10, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Hohenschurz-Schmidt, D.J.; Calcagnini, G.; Dipasquale, O.; Jackson, J.B.; Medina, S.; O’Daly, O.; O’Muircheartaigh, J.; de Lara Rubio, A.; Williams, S.C.R.; McMahon, S.B.; et al. Linking Pain Sensation to the Autonomic Nervous System: The Role of the Anterior Cingulate and Periaqueductal Gray Resting-State Networks. Front. Neurosci. 2020, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.R.; Tuckett, R.P.; Song, C.W. Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. J. Pain 2008, 9, 122–145. [Google Scholar] [CrossRef]
- Zouikr, I.; Karshikoff, B. Lifetime Modulation of the Pain System via Neuroimmune and Neuroendocrine Interactions. Front. Immunol. 2017, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.M.; Vernon, H.; Leddy, J.J.; Baldwin, B.A. The role of the cervical spine in post-concussion syndrome. Physician Sportsmed. 2015, 43, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Langevin, P.; Fait, P. Cervical Spine Involvement in Mild Traumatic Brain Injury: A Review. J. Sports Med. (Hindawi Publ. Corp.) 2016, 2016, 1590161. [Google Scholar] [CrossRef] [PubMed]
- Sheldrake, E.; Al-Hakeem, H.; Lam, B.; Goldstein, B.I.; Wheeler, A.L.; Burke, M.; Dunkley, B.T.; Reed, N.; Scratch, S.E. Mental Health Outcomes Across the Lifespan in Individuals with Persistent Post-Concussion Symptoms: A Scoping Review. Front. Neurol. 2022, 13, 850590. [Google Scholar] [CrossRef]
- Delmonico, R.L.; Theodore, B.R.; Sandel, M.E.; Armstrong, M.A.; Camicia, M. Prevalence of depression and anxiety disorders following mild traumatic brain injury. PM R 2022, 14, 753–763. [Google Scholar] [CrossRef]
- Lambert, M.; Sheldrake, E.; Deneault, A.A.; Wheeler, A.; Burke, M.; Scratch, S. Depressive Symptoms in Individuals with Persistent Postconcussion Symptoms: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2248453. [Google Scholar] [CrossRef] [PubMed]
- Hellewell, S.C.; Beaton, C.S.; Welton, T.; Grieve, S.M. Characterizing the Risk of Depression Following Mild Traumatic Brain Injury: A Meta-Analysis of the Literature Comparing Chronic mTBI to Non-mTBI Populations. Front. Neurol. 2020, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Cotton, A.S.; Brickman, K.R.; Lewis, T.J.; Wall, J.T.; Tamburrino, M.B.; Bauer, W.R.; Law, K.; McLean, S.A.; et al. Early Changes in Cortical Emotion Processing Circuits after Mild Traumatic Brain Injury from Motor Vehicle Collision. J. Neurotrauma 2017, 34, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.B.; Savitz, J. The Kynurenine Pathway in Traumatic Brain Injury: Implications for Psychiatric Outcomes. Biol. Psychiatry 2022, 91, 449–458. [Google Scholar] [CrossRef]
- Visser, K.; Ciubotariu, D.; de Koning, M.E.; Jacobs, B.; van Faassen, M.; van der Ley, C.; Mayer, A.R.; Meier, T.B.; Bourgonje, A.R.; Kema, I.P.; et al. Exploring the kynurenine pathway in mild traumatic brain injury: A longitudinal study. J. Neurochem. 2024, 168, 2710–2721. [Google Scholar] [CrossRef] [PubMed]
- Broshek, D.K.; De Marco, A.P.; Freeman, J.R. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015, 29, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Sandel, N.; Reynolds, E.; Cohen, P.E.; Gillie, B.L.; Kontos, A.P. Anxiety and Mood Clinical Profile following Sport-related Concussion: From Risk Factors to Treatment. Sport Exerc. Perform. Psychol. 2017, 6, 304–323. [Google Scholar] [CrossRef]
- Donnell, A.J.; Kim, M.S.; Silva, M.A.; Vanderploeg, R.D. Incidence of postconcussion symptoms in psychiatric diagnostic groups, mild traumatic brain injury, and comorbid conditions. Clin. Neuropsychol. 2012, 26, 1092–1101. [Google Scholar] [CrossRef]
- Sarris, J.; O’Neil, A.; Coulson, C.E.; Schweitzer, I.; Berk, M. Lifestyle medicine for depression. BMC Psychiatry 2014, 14, 107. [Google Scholar] [CrossRef]
- Morales-Torres, R.; Carrasco-Gubernatis, C.; Grasso-Cladera, A.; Cosmelli, D.; Parada, F.J.; Palacios-Garcia, I. Psychobiotic Effects on Anxiety Are Modulated by Lifestyle Behaviors: A Randomized Placebo-Controlled Trial on Healthy Adults. Nutrients 2023, 15, 1706. [Google Scholar] [CrossRef]
- Sharma, I.; Marwale, A.V.; Sidana, R.; Gupta, I.D. Lifestyle modification for mental health and well-being. Indian J. Psychiatry 2024, 66, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Manger, S.H.; Blencowe, M.; Murray, G.; Ho, F.Y.; Lawn, S.; Blumenthal, J.A.; Schuch, F.; Stubbs, B.; Ruusunen, A.; et al. Clinical guidelines for the use of lifestyle-based mental health care in major depressive disorder: World Federation of Societies for Biological Psychiatry (WFSBP) and Australasian Society of Lifestyle Medicine (ASLM) taskforce. World J. Biol. Psychiatry 2023, 24, 333–386. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R. Lifestyle and mental health. Am. Psychol. 2011, 66, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Solmi, M.; Wootton, R.E.; Vancampfort, D.; Schuch, F.B.; Hoare, E.; Gilbody, S.; Torous, J.; Teasdale, S.B.; Jackson, S.E.; et al. A meta-review of “lifestyle psychiatry”: The role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020, 19, 360–380. [Google Scholar] [CrossRef]
- Jaqua, E.; Biddy, E.; Moore, C.; Browne, G. The Impact of the Six Pillars of Lifestyle Medicine on Brain Health. Cureus 2023, 15, e34605. [Google Scholar] [CrossRef]
- Hsu, I.; Saha, S.; Korthuis, P.T.; Sharp, V.; Cohn, J.; Moore, R.D.; Beach, M.C. Providing support to patients in emotional encounters: A new perspective on missed empathic opportunities. Patient Educ. Couns. 2012, 88, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Fradgley, E.A.; Clinton-McHarg, T.; Hall, A.; Paul, C.L. Perceived importance of emotional support provided by health care professionals and social networks: Should we broaden our focus for the delivery of supportive care? Asia Pac. J. Clin. Oncol. 2023, 19, 681–689. [Google Scholar] [CrossRef]
- Stein, M.B.; Jain, S.; Giacino, J.T.; Levin, H.; Dikmen, S.; Nelson, L.D.; Vassar, M.J.; Okonkwo, D.O.; Diaz-Arrastia, R.; Robertson, C.S.; et al. Risk of Posttraumatic Stress Disorder and Major Depression in Civilian Patients After Mild Traumatic Brain Injury: A TRACK-TBI Study. JAMA Psychiatry 2019, 76, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Moulds, M.; Guthrie, R.; Nixon, R.D. Treating acute stress disorder following mild traumatic brain injury. Am. J. Psychiatry 2003, 160, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Cheavens, J.S.; Whitted, W.M. Hope therapy. Curr. Opin. Psychol. 2023, 49, 101509. [Google Scholar] [CrossRef] [PubMed]
- Snell, D.L.; Faulkner, J.W.; Williman, J.A.; Silverberg, N.D.; Theadom, A.; Surgenor, L.J.; Hackney, J.; Siegert, R.J. Fear avoidance and return to work after mild traumatic brain injury. Brain Inj. 2023, 37, 541–550. [Google Scholar] [CrossRef]
- Buzzanca-Fried, K.E.; Snyder, A.R.; Bauer, R.M.; Morgan-Daniel, J.; de Corcho, C.P.; Addeo, R.; Lahey, S.M.; Houck, Z.; Beneciuk, J.M. Psychological Constructs From the Fear Avoidance Model and Beyond as Predictors for Persisting Symptoms After Concussion: An Integrative Review. Arch. Phys. Med. Rehabil. 2024, 105, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.D.; Panenka, W.J.; Iverson, G.L. Fear Avoidance and Clinical Outcomes from Mild Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1864–1873. [Google Scholar] [CrossRef]
- Ferguson, R.J.; Mittenberg, W.; Barone, D.F.; Schneider, B. Postconcussion syndrome following sports-related head injury: Expectation as etiology. Neuropsychology 1999, 13, 582–589. [Google Scholar] [CrossRef]
- Waldron-Perrine, B.; Tree, H.A.; Spencer, R.J.; Suhr, J.; Bieliauskas, L. Informational literature influences symptom expression following mild head injury: An analog study. Brain Inj. 2015, 29, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The Neurometabolic Cascade of Concussion. J. Athl. Train. 2001, 36, 228–235. [Google Scholar] [CrossRef]
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The Molecular Pathophysiology of Concussive Brain Injury—An Update. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 373–393. [Google Scholar] [CrossRef]
- Sigurdardottir, S.; Andelic, N.; Roe, C.; Jerstad, T.; Schanke, A.K. Post-concussion symptoms after traumatic brain injury at 3 and 12 months post-injury: A prospective study. Brain Inj. 2009, 23, 489–497. [Google Scholar] [CrossRef]
- Sullivan, K.A.; Edmed, S.L.; Allan, A.C.; Karlsson, L.J.; Smith, S.S. Characterizing self-reported sleep disturbance after mild traumatic brain injury. J. Neurotrauma 2015, 32, 474–486. [Google Scholar] [CrossRef]
- Theadom, A.; Cropley, M.; Parmar, P.; Barker-Collo, S.; Starkey, N.; Jones, K.; Feigin, V.L.; Group, B.R. Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. 2015, 16, 926–932. [Google Scholar] [CrossRef]
- Bramley, H.; Henson, A.; Lewis, M.M.; Kong, L.; Stetter, C.; Silvis, M. Sleep Disturbance Following Concussion Is a Risk Factor for a Prolonged Recovery. Clin. Pediatr. 2017, 56, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.; Sveen, U.; Alvsaker, K.; Bautz-Holter, E. Post-concussion symptoms after mild traumatic brain injury: Influence of demographic factors and injury severity in a 1-year cohort study. Disabil. Rehabil. 2009, 31, 1235–1243. [Google Scholar] [CrossRef]
- Chan, L.G.; Feinstein, A. Persistent Sleep Disturbances Independently Predict Poorer Functional and Social Outcomes 1 Year After Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 2015, 30, E67–E75. [Google Scholar] [CrossRef]
- Chaput, G.; Giguere, J.F.; Chauny, J.M.; Denis, R.; Lavigne, G. Relationship among subjective sleep complaints, headaches, and mood alterations following a mild traumatic brain injury. Sleep Med. 2009, 10, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Mollayeva, T.; Shapiro, C.M.; Mollayeva, S.; Cassidy, J.D.; Colantonio, A. Modeling community integration in workers with delayed recovery from mild traumatic brain injury. BMC Neurol. 2015, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Towns, S.J.; Silva, M.A.; Belanger, H.G. Subjective sleep quality and postconcussion symptoms following mild traumatic brain injury. Brain Inj. 2015, 29, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Magliato, S.N.; Wingerson, M.J.; Seehusen, C.N.; Smulligan, K.L.; Simon, S.L.; Wilson, J.C.; Howell, D.R. Sleep Problems After Concussion Are Associated with Poor Balance and Persistent Postconcussion Symptoms. J. Child Neurol. 2023, 38, 198–205. [Google Scholar] [CrossRef]
- Kostyun, R.O.; Milewski, M.D.; Hafeez, I. Sleep disturbance and neurocognitive function during the recovery from a sport-related concussion in adolescents. Am. J. Sports Med. 2015, 43, 633–640. [Google Scholar] [CrossRef]
- Beetar, J.T.; Guilmette, T.J.; Sparadeo, F.R. Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch. Phys. Med. Rehabil. 1996, 77, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Clinchot, D.M.; Bogner, J.; Mysiw, W.J.; Fugate, L.; Corrigan, J. Defining sleep disturbance after brain injury. Am. J. Phys. Med. Rehabil. 1998, 77, 291–295. [Google Scholar] [CrossRef]
- Mahmood, O.; Rapport, L.J.; Hanks, R.A.; Fichtenberg, N.L. Neuropsychological performance and sleep disturbance following traumatic brain injury. J. Head Trauma Rehabil. 2004, 19, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.; Khoury, S.; Chauny, J.M.; Desautels, A. Pain and sleep in post-concussion/mild traumatic brain injury. Pain 2015, 156 (Suppl. S1), S75–S85. [Google Scholar] [CrossRef] [PubMed]
- Ayalon, L.; Borodkin, K.; Dishon, L.; Kanety, H.; Dagan, Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology 2007, 68, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.C.; Savard, J.; Morin, C.M. Insomnia following traumatic brain injury: A review. Neurorehabilit. Neural Repair 2004, 18, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Smith, K.E.; Nguyen, L.; Turner, R.C.; Logsdon, A.F.; Jackson, G.J.; Huber, J.D.; Rosen, C.L.; Miller, D.B. Sleep disruption and the sequelae associated with traumatic brain injury. Neurosci. Biobehav. Rev. 2015, 55, 68–77. [Google Scholar] [CrossRef]
- Ouellet, M.C.; Beaulieu-Bonneau, S.; Morin, C.M. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015, 14, 746–757. [Google Scholar] [CrossRef]
- Mollayeva, T.; Colantonio, A.; Cassidy, J.D.; Vernich, L.; Moineddin, R.; Shapiro, C.M. Sleep stage distribution in persons with mild traumatic brain injury: A polysomnographic study according to American Academy of Sleep Medicine standards. Sleep Med. 2017, 34, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.R.; Lazic, S.E.; Ogilvie, R.D. Polysomnographic and quantitative EEG analysis of subjects with long-term insomnia complaints associated with mild traumatic brain injury. Clin. Neurophysiol. 2008, 119, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases—A review. Sleep Med. 2021, 77, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.F.; Ortiz, J.B.; Wonnacott, S.; Williams, R.J.; Rowe, R.K. The Bidirectional Relationship Between Sleep and Inflammation Links Traumatic Brain Injury and Alzheimer’s Disease. Front. Neurosci. 2020, 14, 894. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, N.; Yu, P.K.; Siegel, N.S. Sleep physiology, pathophysiology, and sleep hygiene. Prog. Cardiovasc. Dis. 2023, 77, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Farina, B.; Dittoni, S.; Colicchio, S.; Testani, E.; Losurdo, A.; Gnoni, V.; Di Blasi, C.; Brunetti, R.; Contardi, A.; Mazza, S.; et al. Heart rate and heart rate variability modification in chronic insomnia patients. Behav. Sleep Med. 2014, 12, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Greenlund, I.M.; Carter, J.R. Sympathetic neural responses to sleep disorders and insufficiencies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H337–H349. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Zhan, G.; Fenik, P.; Panossian, L.; Wang, M.M.; Reid, S.; Lai, D.; Davis, J.G.; Baur, J.A.; et al. Extended wakefulness: Compromised metabolics in and degeneration of locus ceruleus neurons. J. Neurosci. 2014, 34, 4418–4431. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Zhang, H.C.; Zuo, Z.F.; Liu, Y.X. Heterogeneous organization of Locus coeruleus: An intrinsic mechanism for functional complexity. Physiol. Behav. 2023, 268, 114231. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Pereira, I.; Llorca-Torralba, M.; Bravo, L.; Camarena-Delgado, C.; Soriano-Mas, C.; Berrocoso, E. The Role of the Locus Coeruleus in Pain and Associated Stress-Related Disorders. Biol. Psychiatry 2022, 91, 786–797. [Google Scholar] [CrossRef]
- Samuels, E.R.; Szabadi, E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part I: Principles of functional organisation. Curr. Neuropharmacol. 2008, 6, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Kureshi, S.; Stowe, C.; Francis, J.; Djalilian, H. Circadian therapy interventions for glymphatic dysfunction in concussions injuries: A narrative review. Sci. Prog. 2023, 106, 368504231189536. [Google Scholar] [CrossRef] [PubMed]
- Donahue, C.C.; Resch, J.E. Concussion and the Sleeping Brain. Sports Med. Open 2024, 10, 68. [Google Scholar] [CrossRef]
- Stocker, R.P.J.; Khan, H.; Henry, L.; Germain, A. Effects of Sleep Loss on Subjective Complaints and Objective Neurocognitive Performance as Measured by the Immediate Post-Concussion Assessment and Cognitive Testing. Arch. Clin. Neuropsychol. 2017, 32, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Moran, R.N.; Ingargiola, A. Self-reported prior night’s sleep quantity on baseline symptom factors and computerized neurocognitive testing in high school athletes. Appl. Neuropsychol. Child 2022, 11, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Riegler, K.E.; Guty, E.T.; Thomas, G.A.; Arnett, P.A. Sleep Deprived or Concussed? The Acute Impact of Self-Reported Insufficient Sleep in College Athletes. J. Int. Neuropsychol. Soc. 2021, 27, 35–46. [Google Scholar] [CrossRef]
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 2011, 30, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Sikoglu, E.M.; Liso Navarro, A.A.; Czerniak, S.M.; McCafferty, J.; Eisenstock, J.; Stevenson, J.H.; King, J.A.; Moore, C.M. Effects of Recent Concussion on Brain Bioenergetics: A Phosphorus-31 Magnetic Resonance Spectroscopy Study. Cogn. Behav. Neurol. 2015, 28, 181–187. [Google Scholar] [CrossRef]
- Hammeke, T.A.; McCrea, M.; Coats, S.M.; Verber, M.D.; Durgerian, S.; Flora, K.; Olsen, G.S.; Leo, P.D.; Gennarelli, T.A.; Rao, S.M. Acute and subacute changes in neural activation during the recovery from sport-related concussion. J. Int. Neuropsychol. Soc. 2013, 19, 863–872. [Google Scholar] [CrossRef]
- Mayer, A.R.; Hanlon, F.M.; Dodd, A.B.; Ling, J.M.; Klimaj, S.D.; Meier, T.B. A functional magnetic resonance imaging study of cognitive control and neurosensory deficits in mild traumatic brain injury. Hum. Brain Mapp. 2015, 36, 4394–4406. [Google Scholar] [CrossRef]
- Mayer, A.R.; Toulouse, T.; Klimaj, S.; Ling, J.M.; Pena, A.; Bellgowan, P.S. Investigating the properties of the hemodynamic response function after mild traumatic brain injury. J. Neurotrauma 2014, 31, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, R.E.; Winzeck, S.; Luppi, A.I.; Kelleher-Unger, I.R.; Spindler, L.R.B.; Wilson, J.T.L.; Newcombe, V.F.J.; Coles, J.P.; CENTER-TBI MRI Substudy Participants and Investigators; Menon, D.K.; et al. Acute thalamic connectivity precedes chronic post-concussive symptoms in mild traumatic brain injury. Brain 2023, 146, 3484–3499. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Wilson, C.M.; Brabazon, F.; von Leden, R.; Jurgens, J.S.; Oakes, T.R.; Selwyn, R.G. FDG-PET imaging in mild traumatic brain injury: A critical review. Front. Neuroenergetics 2014, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Komura, A.; Kawasaki, T.; Yamada, Y.; Uzuyama, S.; Asano, Y.; Shinoda, J. Cerebral Glucose Metabolism in Patients with Chronic Mental and Cognitive Sequelae after a Single Blunt Mild Traumatic Brain Injury without Visible Brain Lesions. J. Neurotrauma 2019, 36, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Li, Y.H.; Lu, W.; Huang, S.H.; Li, M.J.; Xiao, L.Z.; Liu, J. Positron emission tomography imaging for the assessment of mild traumatic brain injury and chronic traumatic encephalopathy: Recent advances in radiotracers. Neural Regen. Res. 2022, 17, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Fahmy, L.M.; Davoodi-Bojd, E.; Zhang, L.; Ding, G.; Hu, J.; Zhang, Z.; Chopp, M.; Jiang, Q. Waste Clearance in the Brain. Front. Neuroanat. 2021, 15, 665803. [Google Scholar] [CrossRef]
- Lejbman, N.; Olivera, A.; Heinzelmann, M.; Feng, R.; Yun, S.; Kim, H.S.; Gill, J. Active duty service members who sustain a traumatic brain injury have chronically elevated peripheral concentrations of Abeta40 and lower ratios of Abeta42/40. Brain Inj. 2016, 30, 1436–1441. [Google Scholar] [CrossRef]
- Gill, J.; Mustapic, M.; Diaz-Arrastia, R.; Lange, R.; Gulyani, S.; Diehl, T.; Motamedi, V.; Osier, N.; Stern, R.A.; Kapogiannis, D. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 2018, 32, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Nedergaard, M. Neuroscience. Garbage truck of the brain. Science 2013, 340, 1529–1530. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Voumvourakis, K.I.; Sideri, E.; Papadimitropoulos, G.N.; Tsantzali, I.; Hewlett, P.; Kitsos, D.; Stefanou, M.; Bonakis, A.; Giannopoulos, S.; Tsivgoulis, G.; et al. The Dynamic Relationship between the Glymphatic System, Aging, Memory, and Sleep. Biomedicines 2023, 11, 2092. [Google Scholar] [CrossRef] [PubMed]
- Sullan, M.J.; Asken, B.M.; Jaffee, M.S.; DeKosky, S.T.; Bauer, R.M. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci. Biobehav. Rev. 2018, 84, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.; Raghavan, P.; Jiang, L.; Roys, S.; Tchoquessi, R.L.N.; Chen, H.; Wickwire, E.M.; Parikh, G.Y.; Schwartzbauer, G.T.; Grattan, L.M.; et al. Longitudinal Assessment of Glymphatic Changes Following Mild Traumatic Brain Injury: Insights from PVS burden and DTI-ALPS Imaging. medRxiv 2024. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Dworak, M.; McCarley, R.W.; Kim, T.; Kalinchuk, A.V.; Basheer, R. Sleep and brain energy levels: ATP changes during sleep. J. Neurosci. 2010, 30, 9007–9016. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Espie, C.A.; Altena, E.; Arnardottir, E.S.; Baglioni, C.; Bassetti, C.L.A.; Bastien, C.; Berzina, N.; Bjorvatn, B.; Dikeos, D.; et al. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J. Sleep Res. 2023, 32, e14035. [Google Scholar] [CrossRef]
- Trauer, J.M.; Qian, M.Y.; Doyle, J.S.; Rajaratnam, S.M.; Cunnington, D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 163, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.Y.; Chan, J.W.Y.; Li, S.X.; Wing, Y.K. Non-pharmacological Approaches for Management of Insomnia. Neurotherapeutics 2021, 18, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.L. Insomnia. Ann. Intern. Med. 2021, 174, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Hertenstein, E.; Trinca, E.; Wunderlin, M.; Schneider, C.L.; Zust, M.A.; Feher, K.D.; Su, T.; Straten, A.V.; Berger, T.; Baglioni, C.; et al. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 62, 101597. [Google Scholar] [CrossRef]
- Feng, G.; Han, M.; Li, X.; Geng, L.; Miao, Y. The Clinical Effectiveness of Cognitive Behavioral Therapy for Patients with Insomnia and Depression: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2020, 2020, 8071821. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Gehrman, P.; Perlis, M.; Umscheid, C.A. Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Fam. Pract. 2012, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Espie, C.A.; Riemann, D. Cognitive-behavioural therapy for insomnia (CBT-I) across the lifespan. In Guidelines and Clinical Protocols for Health Professionals; Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Eddinger, J.D.; Carney, C. Overcoming Insomnia: A Cognitive-Behavioral Therapy Approach, Therapist Guide (Treatments That Work), 2nd ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Furukawa, Y.; Sakata, M.; Yamamoto, R.; Nakajima, S.; Kikuchi, S.; Inoue, M.; Ito, M.; Noma, H.; Takashina, H.N.; Funada, S.; et al. Components and Delivery Formats of Cognitive Behavioral Therapy for Chronic Insomnia in Adults: A Systematic Review and Component Network Meta-Analysis. JAMA Psychiatry 2024, 81, 357–365. [Google Scholar] [CrossRef]
- Edinger, J.D.; Arnedt, J.T.; Bertisch, S.M.; Carney, C.E.; Harrington, J.J.; Lichstein, K.L.; Sateia, M.J.; Troxel, W.M.; Zhou, E.S.; Kazmi, U.; et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2021, 17, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Muench, A.; Perlis, M.L.; Vargas, I. Cognitive Behavioral Therapy for Insomnia (CBT-I): A Primer. Klin. Spec. Psihol. 2022, 11, 123–137. [Google Scholar] [CrossRef]
- Rossman, J. Cognitive-Behavioral Therapy for Insomnia: An Effective and Underutilized Treatment for Insomnia. Am. J. Lifestyle Med. 2019, 13, 544–547. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Xia, J.; Zhou, X.; Chen, N.; Chen, Z.; Fan, Q.; Wang, H.; Ding, P.; Du, Q. Effects of Cognitive Behavioral Therapy on Pain and Sleep in Adults with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Neural Plast. 2021, 2021, 6552246. [Google Scholar] [CrossRef] [PubMed]
- Ruff, R.L.; Riechers, R.G., 2nd; Wang, X.F.; Piero, T.; Ruff, S.S. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J. Rehabil. Res. Dev. 2012, 49, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Malarkey, M.E.; Fu, A.J.; Mannan, N.; Shaw, O.M.; Haight, T.J.; Cota, M.R.; Jahed, N.C.; Werner, J.K.; Brody, D.L. Internet-Guided Cognitive Behavioral Therapy for Insomnia Among Patients with Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2420090. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.; Rippee, M.; D’Silva, L.; Radel, J.; Eakman, A.M.; Beltramo, A.; Drerup, M.; Siengsukon, C. Cognitive Behavioral Therapy for Insomnia Improves Sleep Outcomes in Individuals with Concussion: A Preliminary Randomized Wait-List Control Study. J. Head Trauma Rehabil. 2024, 39, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Tomfohr-Madsen, L.; Madsen, J.W.; Bonneville, D.; Virani, S.; Plourde, V.; Barlow, K.M.; Yeates, K.O.; Brooks, B.L. A Pilot Randomized Controlled Trial of Cognitive-Behavioral Therapy for Insomnia in Adolescents with Persistent Postconcussion Symptoms. J. Head Trauma Rehabil. 2020, 35, E103–E112. [Google Scholar] [CrossRef] [PubMed]
- Lah, S.; Phillips, N.L.; Palermo, T.M.; Bartlett, D.; Epps, A.; Teng, A.; Brookes, N.; Parry, L.; Naismith, S.L. A feasibility and acceptability study of cognitive behavioural treatment for insomnia in adolescents with traumatic brain injury: A-B with follow up design, randomized baseline, and replication across participants. Neuropsychol. Rehabil. 2021, 31, 345–368. [Google Scholar] [CrossRef]
- Ludwig, R.; Vaduvathiriyan, P.; Siengsukon, C. Does cognitive-behavioural therapy improve sleep outcomes in individuals with traumatic brain injury: A scoping review. Brain Inj. 2020, 34, 1569–1578. [Google Scholar] [CrossRef]
- Bogdanov, S.; Naismith, S.; Lah, S. Sleep outcomes following sleep-hygiene-related interventions for individuals with traumatic brain injury: A systematic review. Brain Inj. 2017, 31, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.C.; Morin, C.M. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: A single-case experimental design. Arch. Phys. Med. Rehabil. 2007, 88, 1581–1592. [Google Scholar] [CrossRef]
- Morrow, E.L.; Mattis-Roesch, H.; Walsh, K.; Duff, M.C. Measurement of Sleep in Chronic Traumatic Brain Injury: Relationship Between Self-report and Actigraphy. J. Head Trauma Rehabil. 2024, 39, E132–E140. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.P.; Miro, E.; Sanchez, A.I.; Diaz-Piedra, C.; Caliz, R.; Vlaeyen, J.W.; Buela-Casal, G. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: A randomized controlled trial. J. Behav. Med. 2014, 37, 683–697. [Google Scholar] [CrossRef]
- Nishinoue, N.; Takano, T.; Kaku, A.; Eto, R.; Kato, N.; Ono, Y.; Tanaka, K. Effects of sleep hygiene education and behavioral therapy on sleep quality of white-collar workers: A randomized controlled trial. Ind. Health 2012, 50, 123–131. [Google Scholar] [CrossRef]
- Nakamura, Y.; Lipschitz, D.L.; Landward, R.; Kuhn, R.; West, G. Two sessions of sleep-focused mind-body bridging improve self-reported symptoms of sleep and PTSD in veterans: A pilot randomized controlled trial. J. Psychosom. Res. 2011, 70, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Schmidt-Nowara, W.; Jessop, C.A.; Ahearn, J. Sleep restriction therapy and hypnotic withdrawal versus sleep hygiene education in hypnotic using patients with insomnia. J. Clin. Sleep Med. 2010, 6, 169–175. [Google Scholar] [CrossRef]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Tahkamo, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Hoyt, L.T.; Zeiders, K.H.; Ehrlich, K.B.; Adam, E.K. Positive upshots of cortisol in everyday life. Emotion 2016, 16, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Munch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]
- Dijk, D.J.; Lockley, S.W. Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. (1985) 2002, 92, 852–862. [Google Scholar] [CrossRef]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef]
- Serin, Y.; Acar Tek, N. Effect of Circadian Rhythm on Metabolic Processes and the Regulation of Energy Balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Monti, K.; Conkright, M.W.; Eagle, S.R.; Lawrence, D.W.; Dretsch, L.M. The role of nutrition in mild traumatic brain injury rehabilitation for service members and veterans. NeuroRehabilitation 2024, 55, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Vanuk, J.R.; Smith, R.; Dailey, N.S.; Killgore, W.D.S. Blue-Light Therapy following Mild Traumatic Brain Injury: Effects on White Matter Water Diffusion in the Brain. Front. Neurol. 2017, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Raikes, A.C.; Dailey, N.S.; Forbeck, B.; Alkozei, A.; Killgore, W.D.S. Daily Morning Blue Light Therapy for Post-mTBI Sleep Disruption: Effects on Brain Structure and Function. Front. Neurol. 2021, 12, 625431. [Google Scholar] [CrossRef]
- Raikes, A.C.; Killgore, W.D. Potential for the development of light therapies in mild traumatic brain injury. Concussion 2018, 3, CNC57. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Vanuk, J.R.; Shane, B.R.; Weber, M.; Bajaj, S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol. Dis. 2020, 134, 104679. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.; Bischof, A.; Rumpf, H.J. Motivational Interviewing: An Evidence-Based Approach for Use in Medical Practice. Dtsch. Arztebl. Int. 2021, 118, 109–115. [Google Scholar] [CrossRef]
- Bakalidou, D.; Krommydas, G.; Abdimioti, T.; Theodorou, P.; Doskas, T.; Fillopoulos, E. The Dimensionality of the Multidimensional Fatigue Inventory (MFI-20) Derived From Healthy Adults and Patient Subpopulations: A Challenge for Clinicians. Cureus 2022, 14, e26344. [Google Scholar] [CrossRef]
- Stulemeijer, M.; van der Werf, S.; Bleijenberg, G.; Biert, J.; Brauer, J.; Vos, P.E. Recovery from mild traumatic brain injury: A focus on fatigue. J. Neurol. 2006, 253, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.B.; Gordon, W.; Gumber, S. What is post TBI fatigue? NeuroRehabilitation 2013, 32, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, R.S. Functional Magnetic Resonance Imaging of Cognitive Control following Traumatic Brain Injury. Front. Neurol. 2017, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Pardini, J.E.; Pardini, D.A.; Becker, J.T.; Dunfee, K.L.; Eddy, W.F.; Lovell, M.R.; Welling, J.S. Postconcussive symptoms are associated with compensatory cortical recruitment during a working memory task. Neurosurgery 2010, 67, 1020–1027; discussion 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.R.; Stephenson, D.D.; Wertz, C.J.; Dodd, A.B.; Shaff, N.A.; Ling, J.M.; Park, G.; Oglesbee, S.J.; Wasserott, B.C.; Meier, T.B.; et al. Proactive inhibition deficits with normal perfusion after pediatric mild traumatic brain injury. Hum. Brain Mapp. 2019, 40, 5370–5381. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W.; Sparling, M.B.; Flashman, L.A.; Guerin, S.J.; Mamourian, A.C.; Saykin, A.J. Differential working memory load effects after mild traumatic brain injury. Neuroimage 2001, 14, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W.; Saykin, A.J.; Flashman, L.A.; Sparling, M.B.; Johnson, S.C.; Guerin, S.J.; Mamourian, A.C.; Weaver, J.B.; Yanofsky, N. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology 1999, 53, 1300–1308. [Google Scholar] [CrossRef]
- Klimas, N.G.; Broderick, G.; Fletcher, M.A. Biomarkers for chronic fatigue. Brain Behav. Immun. 2012, 26, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tajima, S.; Mizuno, K.; Ishii, A.; Konishi, Y.; Miike, T.; Watanabe, Y. Frontier studies on fatigue, autonomic nerve dysfunction, and sleep-rhythm disorder. J. Physiol. Sci. 2015, 65, 483–498. [Google Scholar] [CrossRef]
- Karshikoff, B.; Sundelin, T.; Lasselin, J. Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Front. Immunol. 2017, 8, 21. [Google Scholar] [CrossRef]
- Parkin, G.M.; Clarke, C.; Takagi, M.; Hearps, S.; Babl, F.E.; Davis, G.A.; Anderson, V.; Ignjatovic, V. Plasma Tumor Necrosis Factor Alpha Is a Predictor of Persisting Symptoms Post-Concussion in Children. J. Neurotrauma 2019, 36, 1768–1775. [Google Scholar] [CrossRef]
- Powell, D.J.; Liossi, C.; Moss-Morris, R.; Schlotz, W. Unstimulated cortisol secretory activity in everyday life and its relationship with fatigue and chronic fatigue syndrome: A systematic review and subset meta-analysis. Psychoneuroendocrinology 2013, 38, 2405–2422. [Google Scholar] [CrossRef]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- Pednekar, D.D.; Amin, M.R.; Azgomi, H.F.; Aschbacher, K.; Crofford, L.J.; Faghih, R.T. Characterization of Cortisol Dysregulation in Fibromyalgia and Chronic Fatigue Syndromes: A State-Space Approach. IEEE Trans. Biomed. Eng. 2020, 67, 3163–3172. [Google Scholar] [CrossRef]
- Kumari, M.; Badrick, E.; Chandola, T.; Adam, E.K.; Stafford, M.; Marmot, M.G.; Kirschbaum, C.; Kivimaki, M. Cortisol secretion and fatigue: Associations in a community based cohort. Psychoneuroendocrinology 2009, 34, 1476–1485. [Google Scholar] [CrossRef]
- McEwen, B.S. What Is the Confusion with Cortisol? Chronic Stress 2019, 3, 2470547019833647. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.; Pahlen, S.; Kremen, W.S.; McGue, M.; Dahl Aslan, A.; Nygaard, M.; Christensen, K.; Reynolds, C.A. Does sleep duration moderate genetic and environmental contributions to cognitive performance? Sleep 2022, 45, zsac140. [Google Scholar] [CrossRef]
- Ding, G.; Li, J.; Lian, Z. Both short and long sleep durations are associated with cognitive impairment among community-dwelling Chinese older adults. Medicine 2020, 99, e19667. [Google Scholar] [CrossRef] [PubMed]
- van Oostrom, S.H.; Nooyens, A.C.J.; van Boxtel, M.P.J.; Verschuren, W.M.M. Long sleep duration is associated with lower cognitive function among middle-age adults—The Doetinchem Cohort Study. Sleep Med. 2018, 41, 78–85. [Google Scholar] [CrossRef]
- Joustra, M.L.; Zijlema, W.L.; Rosmalen, J.G.M.; Janssens, K.A.M. Physical Activity and Sleep in Chronic Fatigue Syndrome and Fibromyalgia Syndrome: Associations with Symptom Severity in the General Population Cohort LifeLines. Pain Res. Manag. 2018, 2018, 5801510. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, R.; Chen, S.; Tan, C. Effects of head-down bed rest on the executive functions and emotional response. PLoS ONE 2012, 7, e52160. [Google Scholar] [CrossRef]
- Sarabon, N.; Mekjavic, I.B.; Eiken, O.; Babic, J. The Effect of Bed Rest and Hypoxic Environment on Postural Balance and Trunk Automatic (Re)Actions in Young Healthy Males. Front. Physiol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Harper, C.M.; Lyles, Y.M. Physiology and complications of bed rest. J. Am. Geriatr. Soc. 1988, 36, 1047–1054. [Google Scholar] [CrossRef]
- Mendt, S.; Brauns, K.; Friedl-Werner, A.; Belavy, D.L.; Steinach, M.; Schlabs, T.; Werner, A.; Gunga, H.C.; Stahn, A.C. Long-Term Bed Rest Delays the Circadian Phase of Core Body Temperature. Front. Physiol. 2021, 12, 658707. [Google Scholar] [CrossRef]
- Convertino, V.A.; Bloomfield, S.A.; Greenleaf, J.E. An overview of the issues: Physiological effects of bed rest and restricted physical activity. Med. Sci. Sports Exerc. 1997, 29, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Obeid, J.; DeMatteo, C.; Noseworthy, M.D.; Timmons, B.W. New Insights Into Accelerometer-Measured Habitual Physical Activity and Sedentary Time During Early Recovery in Pediatric Concussion. Pediatr. Exerc. Sci. 2024, 36, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kreher, J.B.; Schwartz, J.B. Overtraining syndrome: A practical guide. Sports Health 2012, 4, 128–138. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Bergeron, M.F.; Lee, E.C.; Mershon, J.E.; Armstrong, E.M. Overtraining Syndrome as a Complex Systems Phenomenon. Front. Netw. Physiol. 2021, 1, 794392. [Google Scholar] [CrossRef]
- Chung, Y.; Hsiao, Y.T.; Huang, W.C. Physiological and Psychological Effects of Treadmill Overtraining Implementation. Biology 2021, 10, 515. [Google Scholar] [CrossRef]
- Khammissa, R.A.G.; Nemutandani, S.; Feller, G.; Lemmer, J.; Feller, L. Burnout phenomenon: Neurophysiological factors, clinical features, and aspects of management. J. Int. Med. Res. 2022, 50, 3000605221106428. [Google Scholar] [CrossRef] [PubMed]
- Steffey, M.A.; Griffon, D.J.; Risselada, M.; Buote, N.J.; Scharf, V.F.; Zamprogno, H.; Winter, A.L. A narrative review of the physiology and health effects of burnout associated with veterinarian-pertinent occupational stressors. Front. Vet. Sci. 2023, 10, 1184525. [Google Scholar] [CrossRef] [PubMed]
- Sjors Dahlman, A.; Jonsdottir, I.H.; Hansson, C. The hypothalamo-pituitary-adrenal axis and the autonomic nervous system in burnout. Handb. Clin. Neurol. 2021, 182, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kanthak, M.K.; Stalder, T.; Hill, L.K.; Thayer, J.F.; Penz, M.; Kirschbaum, C. Autonomic dysregulation in burnout and depression: Evidence for the central role of exhaustion. Scand. J. Work. Environ. Health 2017, 43, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.M.; Damian, A.C.; Neagu, C. Association between burnout and immunological and endocrine alterations. Rom. J. Morphol. Embryol. 2021, 62, 13–18. [Google Scholar] [CrossRef] [PubMed]
- de Vente, W.; van Amsterdam, J.G.; Olff, M.; Kamphuis, J.H.; Emmelkamp, P.M. Burnout Is Associated with Reduced Parasympathetic Activity and Reduced HPA Axis Responsiveness, Predominantly in Males. Biomed Res. Int. 2015, 2015, 431725. [Google Scholar] [CrossRef]
- Hilton, J. On Rest and Pain: A Course of Lectures on the Influence of Mechanical and Physiological Rest in the Treatment of Accidents and Surgical Diseases and the Diagnostic Value of Pain, 2nd ed.; William Wood & Company: New York, NY, USA, 1867. [Google Scholar]
- Weil, Z.M.; Ivey, J.T.; Karelina, K. Putting the Mind to Rest: A Historical Foundation for Rest as a Treatment for Traumatic Brain Injury. J. Neurotrauma 2023, 40, 1286–1296. [Google Scholar] [CrossRef]
- De Kruijk, J.R.; Twijnstra, A.; Meerhoff, S.; Leffers, P. Management of mild traumatic brain injury: Lack of consensus in Europe. Brain Inj. 2001, 15, 117–123. [Google Scholar] [CrossRef] [PubMed]
- DiFazio, M.; Silverberg, N.D.; Kirkwood, M.W.; Bernier, R.; Iverson, G.L. Prolonged Activity Restriction After Concussion: Are We Worsening Outcomes? Clin. Pediatr. 2016, 55, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.D.; Iverson, G.L. Is rest after concussion “the best medicine?”: Recommendations for activity resumption following concussion in athletes, civilians, and military service members. J. Head Trauma Rehabil. 2013, 28, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A.; Chang, D.G. Return to Learn: A review of cognitive rest versus rehabilitation after sports concussion. NeuroRehabilitation 2015, 37, 235–244. [Google Scholar] [CrossRef] [PubMed]
- McLeod, T.C.; Lewis, J.H.; Whelihan, K.; Bacon, C.E. Rest and Return to Activity After Sport-Related Concussion: A Systematic Review of the Literature. J. Athl. Train. 2017, 52, 262–287. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.J.; Leddy, J.J.; Guskiewicz, K.M.; Seifert, T.; McCrea, M.; Silverberg, N.D.; Feddermann-Demont, N.; Iverson, G.L.; Hayden, A.; Makdissi, M. Rest and treatment/rehabilitation following sport-related concussion: A systematic review. Br. J. Sports Med. 2017, 51, 930–934. [Google Scholar] [CrossRef]
- Leddy, J.J.; Wilber, C.G.; Willer, B.S. Active recovery from concussion. Curr. Opin. Neurol. 2018, 31, 681–686. [Google Scholar] [CrossRef]
- Leddy, J.J.; Burma, J.S.; Toomey, C.M.; Hayden, A.; Davis, G.A.; Babl, F.E.; Gagnon, I.; Giza, C.C.; Kurowski, B.G.; Silverberg, N.D.; et al. Rest and exercise early after sport-related concussion: A systematic review and meta-analysis. Br. J. Sports Med. 2023, 57, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Kontos, A.P.; Eagle, S.R.; Braithwaite, R.; Preszler, J.; Manderino, L.; Turner, R.L.; Jennings, S.; Trbovich, A.; Hickey, R.W.; Collins, M.W.; et al. The Effects of Rest on Concussion Symptom Resolution and Recovery Time: A Meta-analytic Review and Subgroup Analysis of 4329 Patients. Am. J. Sports Med. 2023, 51, 3893–3903. [Google Scholar] [CrossRef]
- Craton, N.; Leslie, O. Is rest the best intervention for concussion? Lessons learned from the whiplash model. Curr. Sports Med. Rep. 2014, 13, 201–204. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Otamendi, T. Advice to Rest for More Than 2 Days After Mild Traumatic Brain Injury Is Associated with Delayed Return to Productivity: A Case-Control Study. Front. Neurol. 2019, 10, 362. [Google Scholar] [CrossRef]
- Majerske, C.W.; Mihalik, J.P.; Ren, D.; Collins, M.W.; Reddy, C.C.; Lovell, M.R.; Wagner, A.K. Concussion in sports: Postconcussive activity levels, symptoms, and neurocognitive performance. J. Athl. Train. 2008, 43, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.J.; Kowalski, K.; Fung, T.S.; Joyce, J.M.; Yeates, K.O.; Debert, C.T. Characterizing Physical Activity and Sedentary Behavior in Adults with Persistent Postconcussive Symptoms After Mild Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2021, 102, 1918–1925.e1911. [Google Scholar] [CrossRef] [PubMed]
- Macnow, T.; Curran, T.; Tolliday, C.; Martin, K.; McCarthy, M.; Ayturk, D.; Babu, K.M.; Mannix, R. Effect of Screen Time on Recovery From Concussion: A Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 1124–1131. [Google Scholar] [CrossRef]
- Cairncross, M.; Yeates, K.O.; Tang, K.; Madigan, S.; Beauchamp, M.H.; Craig, W.; Doan, Q.; Zemek, R.; Kowalski, K.; Silverberg, N.D. Early Postinjury Screen Time and Concussion Recovery. Pediatrics 2022, 150, e2022056835. [Google Scholar] [CrossRef]
- Palm, S.; Ronnback, L.; Johansson, B. Long-term mental fatigue after traumatic brain injury and impact on employment status. J. Rehabil. Med. 2017, 49, 228–233. [Google Scholar] [CrossRef]
- Levine, J.; Greenwald, B.D. Fatigue in Parkinson disease, stroke, and traumatic brain injury. Phys. Med. Rehabil. Clin. N. Am. 2009, 20, 347–361. [Google Scholar] [CrossRef]
- Ali, A.; Morfin, J.; Mills, J.; Pasipanodya, E.C.; Maas, Y.J.; Huang, E.; Dirlikov, B.; Englander, J.; Zedlitz, A. Fatigue After Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2022, 37, E249–E257. [Google Scholar] [CrossRef]
- Shuman-Paretsky, M.; Gumber, S.; Dams-O’Connor, K. Interventions for Posttraumatic Brain Injury Fatigue: An Updated Review. Interv. Posttraumatic Brain Inj. Fatigue Updat. Rev. 2017, 5, 12–21. [Google Scholar] [CrossRef]
- Cantor, J.B.; Ashman, T.; Bushnik, T.; Cai, X.; Farrell-Carnahan, L.; Gumber, S.; Hart, T.; Rosenthal, J.; Dijkers, M.P. Systematic review of interventions for fatigue after traumatic brain injury: A NIDRR traumatic brain injury model systems study. J. Head Trauma Rehabil. 2014, 29, 490–497. [Google Scholar] [CrossRef]
- Raina, K.D.; Morse, J.Q.; Chisholm, D.; Leibold, M.L.; Shen, J.; Whyte, E. Feasibility of a Cognitive Behavioral Intervention to Manage Fatigue in Individuals with Traumatic Brain Injury: A Pilot Study. J. Head Trauma Rehabil. 2016, 31, E41–E49. [Google Scholar] [CrossRef]
- Raina, K.D.; Morse, J.Q.; Chisholm, D.; Whyte, E.M.; Terhorst, L. An Internet-Based Self-Management Intervention to Reduce Fatigue Among People with Traumatic Brain Injury: A Pilot Randomized Controlled Trial. Am. J. Occup. Ther. 2022, 76, 7604205100. [Google Scholar] [CrossRef] [PubMed]
- Adra, N.; Reddy, M.; Attarian, H.; Sahni, A.S. Autonomic dysfunction in idiopathic hypersomnia: An overlooked association and potential management. J. Clin. Sleep Med. 2022, 18, 963–965. [Google Scholar] [CrossRef]
- Nevsimalova, S.; Blazejova, K.; Illnerova, H.; Hajek, I.; Vankova, J.; Pretl, M.; Sonka, K. A contribution to pathophysiology of idiopathic hypersomnia. Suppl. Clin. Neurophysiol. 2000, 53, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Wichi, R.B.; De Angelis, K.; Jones, L.; Irigoyen, M.C. A brief review of chronic exercise intervention to prevent autonomic nervous system changes during the aging process. Clinics 2009, 64, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, R.; Sasawaki, Y.; Shiotani, H. The influence of short-term sedentary behavior on circadian rhythm of heart rate and heart rate variability. Chronobiol. Int. 2019, 36, 374–380. [Google Scholar] [CrossRef]
- Pinto, A.J.; Bergouignan, A.; Dempsey, P.C.; Roschel, H.; Owen, N.; Gualano, B.; Dunstan, D.W. Physiology of sedentary behavior. Physiol. Rev. 2023, 103, 2561–2622. [Google Scholar] [CrossRef] [PubMed]
- Chauntry, A.J.; Bishop, N.C.; Hamer, M.; Kingsnorth, A.P.; Chen, Y.L.; Paine, N.J. Sedentary behaviour is associated with heightened cardiovascular, inflammatory and cortisol reactivity to acute psychological stress. Psychoneuroendocrinology 2022, 141, 105756. [Google Scholar] [CrossRef]
- Potter, S.; Brown, R.G. Cognitive behavioural therapy and persistent post-concussional symptoms: Integrating conceptual issues and practical aspects in treatment. Neuropsychol. Rehabil. 2012, 22, 1–25. [Google Scholar] [CrossRef]
- Wrightson, P.; Gronwall, D. Mild Head Injury: A Guide to Management; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Hicks, E.J.; Larkins, B.M.; Purdy, S.C. Fatigue management by speech-language pathologists for adults with traumatic brain injury. Int. J. Speech Lang. Pathol. 2011, 13, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Pellerine, L.P.; Miller, K.; Frayne, R.J.; O’Brien, M.W. Characterizing objective and self-report habitual physical activity and sedentary time in outpatients with an acquired brain injury. Sports Med. Health Sci. 2024, 6, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.H.; Anderson, V.; Ewing-Cobbs, L.; Haarbauer-Krupa, J.; McKinlay, A.; Wade, S.L.; Suskauer, S.J. Early Childhood Concussion. Pediatrics 2024, 154, e2023065484. [Google Scholar] [CrossRef]
- Dupont, D.; Beaudoin, C.; Desire, N.; Tran, M.; Gagnon, I.; Beauchamp, M.H. Report of Early Childhood Traumatic Injury Observations & Symptoms: Preliminary Validation of an Observational Measure of Postconcussive Symptoms. J. Head Trauma Rehabil. 2022, 37, E102–E112. [Google Scholar] [CrossRef]
- Bezherano, I.; Haider, M.N.; Willer, B.S.; Leddy, J.J. Practical Management: Prescribing Subsymptom Threshold Aerobic Exercise for Sport-Related Concussion in the Outpatient Setting. Clin. J. Sport Med. 2021, 31, 465–468. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, C.; Hegazi, I.; Hu, W. Understanding Patient Expectations of Health Care: A Qualitative Study. J. Patient Exp. 2020, 7, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.J.; Fitzgerald, M.; Peiffer, J.J.; Scott, B.R.; Rainey-Smith, S.R.; Sohrabi, H.R.; Brown, B.M. The impact of exercise, sleep, and diet on neurocognitive recovery from mild traumatic brain injury in older adults: A narrative review. Ageing Res. Rev. 2021, 68, 101322. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Bernardo, T.C.; Marques-Aleixo, I.; Beleza, J.; Oliveira, P.J.; Ascensao, A.; Magalhaes, J. Physical Exercise and Brain Mitochondrial Fitness: The Possible Role Against Alzheimer’s Disease. Brain Pathol. 2016, 26, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Lin, M.; Gao, J.; Xu, S.; Huang, L.; Zhu, J.; Huang, J.; Tao, J.; Chen, L. The impact of physical activity on blood inflammatory cytokines and neuroprotective factors in individuals with mild cognitive impairment: A systematic review and meta-analysis of randomized-controlled trials. Aging Clin. Exp. Res. 2022, 34, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- De Nys, L.; Anderson, K.; Ofosu, E.F.; Ryde, G.C.; Connelly, J.; Whittaker, A.C. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 143, 105843. [Google Scholar] [CrossRef] [PubMed]
- Besnier, F.; Labrunee, M.; Pathak, A.; Pavy-Le Traon, A.; Gales, C.; Senard, J.M.; Guiraud, T. Exercise training-induced modification in autonomic nervous system: An update for cardiac patients. Ann. Phys. Rehabil. Med. 2017, 60, 27–35. [Google Scholar] [CrossRef]
- Routledge, F.S.; Campbell, T.S.; McFetridge-Durdle, J.A.; Bacon, S.L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Hasan, H.; Habashy, K.J.; Fakih, W.; Abdelhady, S.; Ahmad, F.; Zibara, K.; Eid, A.H.; El-Yazbi, A.F.; Kobeissy, F.H. Western diet aggravates neuronal insult in post-traumatic brain injury: Proposed pathways for interplay. EBioMedicine 2020, 57, 102829. [Google Scholar] [CrossRef]
- Snowden, T.; Morrison, J.; Boerstra, M.; Eyolfson, E.; Acosta, C.; Grafe, E.; Reid, H.; Brand, J.; Galati, M.; Gargaro, J.; et al. Brain changes: Aerobic exercise for traumatic brain injury rehabilitation. Front. Hum. Neurosci. 2023, 17, 1307507. [Google Scholar] [CrossRef]
- Archer, T.; Svensson, K.; Alricsson, M. Physical exercise ameliorates deficits induced by traumatic brain injury. Acta Neurol. Scand. 2012, 125, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Cordingley, D.M.; Marquez, I.; Buchwald, S.C.L.; Zeiler, F.A. Response of Central Nervous System Biomolecules and Systemic Biomarkers to Aerobic Exercise Following Concussion: A Scoping Review of Human and Animal Research. Neurotrauma Rep. 2024, 5, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.O.; Meehan, W.P., 3rd; Iverson, G.L.; Taylor, J.A. Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology 2014, 83, 1665–1672. [Google Scholar] [CrossRef]
- Meng, D.; Ai, S.; Spanos, M.; Shi, X.; Li, G.; Cretoiu, D.; Zhou, Q.; Xiao, J. Exercise and microbiome: From big data to therapy. Comput. Struct. Biotechnol. J. 2023, 21, 5434–5445. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, C.; Wang, D.; Li, H.; Zhang, S.; He, Y.; Wang, P. Optimal exercise dose and type for improving sleep quality: A systematic review and network meta-analysis of RCTs. Front. Psychol. 2024, 15, 1466277. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Leger, D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef]
- Howell, D.R.; Wingerson, M.J.; Smulligan, K.L.; Magliato, S.; Simon, S.; Wilson, J.C. Exercising More Than 150 min/wk After Concussion Is Associated with Sleep Quality Improvements. J. Head Trauma Rehabil. 2024, 39, E216–E224. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.; Hellewell, S.C.; Cowen, G.; Ring, A.; Jefferson, A.; Chih, H.; Gozt, A.K.; Buhagiar, F.; Thomas, E.; Papini, M.; et al. Symptoms Associated with Exercise Intolerance and Resting Heart Rate Following Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 2024, 39, E381–E392. [Google Scholar] [CrossRef]
- Ziaks, L.; Tucker, J.; Koc, T.; Schaefer, A.; Hanson, K. Identifying Trends of Dysautonomia Signs and Symptoms Associated with Protracted Concussion Recovery during the Buffalo Concussion Treadmill Test: A Retrospective Study. Brain Impair. 2022, 25, IB22030. [Google Scholar] [CrossRef]
- Leddy, J.J.; Cox, J.L.; Baker, J.G.; Wack, D.S.; Pendergast, D.R.; Zivadinov, R.; Willer, B. Exercise treatment for postconcussion syndrome: A pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. J. Head Trauma Rehabil. 2013, 28, 241–249. [Google Scholar] [CrossRef]
- Clausen, M.; Pendergast, D.R.; Willer, B.; Leddy, J. Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes. J. Head Trauma Rehabil. 2016, 31, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wade, S.L.; Quatman-Yates, C.; Hugentobler, J.A.; Gubanich, P.J.; Kurowski, B.G. Structural Connectivity Related to Persistent Symptoms After Mild TBI in Adolescents and Response to Aerobic Training: Preliminary Investigation. J. Head Trauma Rehabil. 2017, 32, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Haider, M.N.; Ellis, M.; Willer, B.S. Exercise is Medicine for Concussion. Curr. Sports Med. Rep. 2018, 17, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Balke, B.; Ware, R.W. An experimental study of physical fitness of Air Force personnel. United States Armed Forces Med. J. 1959, 10, 675–688. [Google Scholar]
- Haider, M.N.; Johnson, S.L.; Mannix, R.; Macfarlane, A.J.; Constantino, D.; Johnson, B.D.; Willer, B.; Leddy, J. The Buffalo Concussion Bike Test for Concussion Assessment in Adolescents. Sports Health 2019, 11, 492–497. [Google Scholar] [CrossRef]
- Baker, J.G.; Freitas, M.S.; Leddy, J.J.; Kozlowski, K.F.; Willer, B.S. Return to full functioning after graded exercise assessment and progressive exercise treatment of postconcussion syndrome. Rehabil. Res. Pract. 2012, 2012, 705309. [Google Scholar] [CrossRef] [PubMed]
- Florez, M.; Roberge, E.; Ostrowski, J. Aerobic Exercise as an Intervention for Mild Traumatic Brain Injury: A Critically Appraised Topic. J. Sport Rehabil. 2024, 33, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Gao, B.; Wang, Z.; Yang, Y.; Chen, Z.; Yu, L.; Wang, Z. Therapeutic Effect of Aerobic Exercise for Adolescents After Mild Traumatic Brain Injury and Sport-Related Concussion: A Meta-Analysis from Randomized Controlled Trials. World Neurosurg. 2021, 146, e22–e29. [Google Scholar] [CrossRef]
- Howell, D.R.; Taylor, J.A.; Tan, C.O.; Orr, R.; Meehan, W.P., 3rd. The Role of Aerobic Exercise in Reducing Persistent Sport-related Concussion Symptoms. Med. Sci. Sports Exerc. 2019, 51, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Callahan, C.E.; Stoner, L.; Zieff, G.H.; Register-Mihalik, J.K. The Additive Benefits of Aerobic Exercise and Cognitive Training Postconcussion: Current Clinical Concepts. J. Athl. Train. 2023, 58, 602–610. [Google Scholar] [CrossRef]
- Grool, A.M.; Aglipay, M.; Momoli, F.; Meehan, W.P., 3rd; Freedman, S.B.; Yeates, K.O.; Gravel, J.; Gagnon, I.; Boutis, K.; Meeuwisse, W.; et al. Association Between Early Participation in Physical Activity Following Acute Concussion and Persistent Postconcussive Symptoms in Children and Adolescents. JAMA 2016, 316, 2504–2514. [Google Scholar] [CrossRef]
- Mercier, L.J.; McIntosh, S.J.; Boucher, C.; Joyce, J.M.; Batycky, J.; Galarneau, J.M.; Esser, M.J.; Schneider, K.J.; Dukelow, S.P.; Harris, A.D.; et al. Effect of Aerobic Exercise on Symptom Burden and Quality of Life in Adults with Persisting Post-concussive Symptoms: The ACTBI Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, A.A.; Sicard, V.; Bijelic, V.; Barrowman, N.; Borghese, M.M.; Kuzik, N.; Tremblay, M.S.; Yeates, K.O.; Davis, A.L.; Sangha, G.; et al. Optimal Volume of Moderate-to-Vigorous Physical Activity Postconcussion in Children and Adolescents. JAMA Netw. Open 2024, 7, e2356458. [Google Scholar] [CrossRef]
- Varner, C.E.; Thompson, C.; de Wit, K.; Borgundvaag, B.; Houston, R.; McLeod, S. A randomized trial comparing prescribed light exercise to standard management for emergency department patients with acute mild traumatic brain injury. Acad. Emerg. Med. 2021, 28, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Gauvin-Lepage, J.; Friedman, D.; Grilli, L.; Sufrategui, M.; De Matteo, C.; Iverson, G.L.; Gagnon, I. Effectiveness of an Exercise-Based Active Rehabilitation Intervention for Youth Who Are Slow to Recover After Concussion. Clin. J. Sport Med. 2020, 30, 423–432. [Google Scholar] [CrossRef]
- Stumph, J.; Young, J.; Singichetti, B.; Yi, H.; Valasek, A.; Bowman, E.; MacDonald, J.; Yang, J.; Fischer, A. Effect of Exercise Recommendation on Adolescents with Concussion. J. Child Neurol. 2020, 35, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.L.; Hing, W.; Simas, V.; Climstein, M.; Coombes, J.S.; Jayasinghe, R.; Byrnes, J.; Furness, J. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: A systematic review and meta-analysis. Open Access J. Sports Med. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Houghton, D.; Trenell, M.I. High-intensity interval training: A review of its impact on glucose control and cardiometabolic health. Diabetologia 2017, 60, 7–23. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, W.; Thirupathi, A. Sprint Interval Training Improves Brain-Derived Neurotropic Factor-Induced Benefits in Brain Health-A Possible Molecular Signaling Intervention. Biology 2024, 13, 562. [Google Scholar] [CrossRef]
- Wu, Y.N.; Stark, C.; Gravel, J.; White, M.; Avery, J.; Enis, T.; Cantu, R.C. Effects of Interval-Training Exercise on People Who Have Had Persistent Post-Concussive Symptoms for Less Than One Year: A Pilot Study. J. Neurotrauma 2021, 38, 573–581. [Google Scholar] [CrossRef]
- Haider, M.N.; Cole, W.R.; Willer, B.S.; McCulloch, K.; Horn, E.C.; Bertz, P.E.; Ramsey, C.; Leddy, J.J. Early targeted heart rate exercise is safe and May hasten return-to-duty in service members with acute concussion, a preliminary study. Brain Inj. 2024, 38, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Javra, R.; Burma, J.S.; Johnson, N.E.; Smirl, J.D. Feasibility of superimposed supine cycling and lower body negative pressure as an effective means of prolonging exercise tolerance in individuals experiencing persisting post-concussive symptoms: Preliminary results. Exp. Physiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.A.; Hills, A.P.; Iverson, G.L. Graded Combined Aerobic Resistance Exercise (CARE) to Prevent or Treat the Persistent Post-concussion Syndrome. Curr. Neurol. Neurosci. Rep. 2018, 18, 75. [Google Scholar] [CrossRef]
- Sas, A.R.; Popovich, M.J.; Gillenkirk, A.; Greer, C.; Grant, J.; Almeida, A.; Ichesco, I.K.; Lorincz, M.T.; Eckner, J.T. Orthostatic Vital Signs After Sport-Related Concussion: A Cohort Study. Am. J. Sports Med. 2024, 52, 2902–2910. [Google Scholar] [CrossRef]
- Kokorelis, C.; Slomine, B.; Rowe, P.C.; Suskauer, S. Screening for Orthostatic Intolerance in Symptomatic Children Presenting for Concussion Care. Clin. Pediatr. 2020, 59, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Heyer, G.L.; Fischer, A.; Wilson, J.; MacDonald, J.; Cribbs, S.; Ravindran, R.; Pommering, T.L.; Cuff, S. Orthostatic Intolerance and Autonomic Dysfunction in Youth with Persistent Postconcussion Symptoms: A Head-Upright Tilt Table Study. Clin. J. Sport Med. 2016, 26, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.; Sheridan, C.A.; Kang, K.; Brown, A.; Baham, M.; Asarnow, R.; Giza, C.C.; Choe, M.C. Post-Concussive Orthostatic Tachycardia is Distinct from Postural Orthostatic Tachycardia Syndrome (POTS) in Children and Adolescents. Child Neurol. Open 2022, 9, 2329048X221082753. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.C.; Haman, K.; Garland, E.M.; Raj, V.; Dupont, W.D.; Biaggioni, I.; Robertson, D.; Raj, S.R. Cognitive dysfunction in postural tachycardia syndrome. Clin. Sci. 2015, 128, 39–45. [Google Scholar] [CrossRef]
- Garland, E.M.; Celedonio, J.E.; Raj, S.R. Postural Tachycardia Syndrome: Beyond Orthostatic Intolerance. Curr. Neurol. Neurosci. Rep. 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.N.; Patel, K.S.; Willer, B.S.; Videira, V.; Wilber, C.G.; Mayer, A.R.; Master, C.L.; Mariotti, B.L.; Wertz, C.; Storey, E.P.; et al. Symptoms upon postural change and orthostatic hypotension in adolescents with concussion. Brain Inj. 2021, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Cochrane, G.D.; Johnson, J.; Hebson, C.L.; Kazamel, M. Orthostatic intolerance in post-concussion patients. Physician Sportsmed. 2022, 50, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Levine, B.D. Exercise and non-pharmacological treatment of POTS. Auton. Neurosci. 2018, 215, 20–27. [Google Scholar] [CrossRef]
- Miranda, N.A.; Boris, J.R.; Kouvel, K.M.; Stiles, L. Activity and Exercise Intolerance After Concussion: Identification and Management of Postural Orthostatic Tachycardia Syndrome. J. Neurol. Phys. Ther. 2018, 42, 163–171. [Google Scholar] [CrossRef]
- Peebles, K.C.; Jacobs, C.; Makaroff, L.; Pacey, V. The use and effectiveness of exercise for managing postural orthostatic tachycardia syndrome in young adults with joint hypermobility and related conditions: A scoping review. Auton. Neurosci. 2024, 252, 103156. [Google Scholar] [CrossRef]
- Ziaks, L.; Johnson, K.; Schiltz, K.; Pelo, R.; Lamotte, G.; Dal Molin, C.; Chung, T.; Cortez, M.M. Adaptive Approaches to Exercise Rehabilitation for Postural Tachycardia Syndrome and Related Autonomic Disorders. Arch. Rehabil. Res. Clin. Transl. 2024, 6, 100366. [Google Scholar] [CrossRef]
- Snyder, A.; Sheridan, C.; Tanner, A.; Bickart, K.; Sullan, M.; Craske, M.; Choe, M.; Babikian, T.; Giza, C.; Asarnow, R. Cardiorespiratory Functioning in Youth with Persistent Post-Concussion Symptoms: A Pilot Study. J. Clin. Med. 2021, 10, 561. [Google Scholar] [CrossRef]
- Ludvigsson, M.L.; Peterson, G.; O’Leary, S.; Dedering, A.; Peolsson, A. The effect of neck-specific exercise with, or without a behavioral approach, on pain, disability, and self-efficacy in chronic whiplash-associated disorders: A randomized clinical trial. Clin. J. Pain 2015, 31, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Seferiadis, A.; Rosenfeld, M.; Gunnarsson, R. A review of treatment interventions in whiplash-associated disorders. Eur. Spine J. 2004, 13, 387–397. [Google Scholar] [CrossRef]
- Chrcanovic, B.; Larsson, J.; Malmstrom, E.M.; Westergren, H.; Haggman-Henrikson, B. Exercise therapy for whiplash-associated disorders: A systematic review and meta-analysis. Scand. J. Pain 2022, 22, 232–261. [Google Scholar] [CrossRef]
- Schneider, K.J.; Meeuwisse, W.H.; Nettel-Aguirre, A.; Barlow, K.; Boyd, L.; Kang, J.; Emery, C.A. Cervicovestibular rehabilitation in sport-related concussion: A randomised controlled trial. Br. J. Sports Med. 2014, 48, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Ferro Moura Franco, K.; Lenoir, D.; Dos Santos Franco, Y.R.; Jandre Reis, F.J.; Nunes Cabral, C.M.; Meeus, M. Prescription of exercises for the treatment of chronic pain along the continuum of nociplastic pain: A systematic review with meta-analysis. Eur. J. Pain 2021, 25, 51–70. [Google Scholar] [CrossRef]
- Register-Mihalik, J.K.; Guskiewicz, K.M.; Marshall, S.W.; McCulloch, K.L.; Mihalik, J.P.; Mrazik, M.; Murphy, I.; Naidu, D.; Ranapurwala, S.I.; Schneider, K.J.; et al. Symptom Exacerbation and Adverse Events during a Randomized Trial of Early-stage Concussion Rehabilitation. J. Athl. Train. 2024, 59, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Lishchynsky, J.T.; Rutschmann, T.D.; Toomey, C.M.; Palacios-Derflingher, L.; Yeates, K.O.; Emery, C.A.; Schneider, K.J. The Association Between Moderate and Vigorous Physical Activity and Time to Medical Clearance to Return to Play Following Sport-Related Concussion in Youth Ice Hockey Players. Front. Neurol. 2019, 10, 588. [Google Scholar] [CrossRef]
- Wingerson, M.J.; Hunt, D.L.; Wilson, J.C.; Mannix, R.C.; Meehan, W.P.; Howell, D.R. Factors Associated with Symptom Resolution after Aerobic Exercise Intervention in Adolescent and Young Adults with Concussion. Med. Sci. Sports Exerc. 2024, 56, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.G.; Lininger, M.R.; Oldham, J.R.; Smetana, R.M.; Kelshaw, P.M.; Beidler, E.; Campbell, T.R.; Walton, S.R.; Munce, T.A.; Larson, M.J.; et al. Physical activity and recovery following concussion in collegiate athletes: A LIMBIC MATARS Consortium Investigation. Brain Inj. 2024, Feb 7, 1–8. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef]
- Xu, Z.; Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 2015, 113 (Suppl. S1), S1–S5. [Google Scholar] [CrossRef]
- Shoubridge, A.P.; Choo, J.M.; Martin, A.M.; Keating, D.J.; Wong, M.L.; Licinio, J.; Rogers, G.B. The gut microbiome and mental health: Advances in research and emerging priorities. Mol. Psychiatry 2022, 27, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.; Mayr, H.; De Vlieger, N.; Abbott, K.; Starck, C.; Fayet-Moore, F.; Marshall, S. Should We ’Eat a Rainbow’? An Umbrella Review of the Health Effects of Colorful Bioactive Pigments in Fruits and Vegetables. Molecules 2022, 27, 4061. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Song, R.; Liu, Y.; Wu, Z.; Zhang, X. Effects of ultra-processed foods on the microbiota-gut-brain axis: The bread-and-butter issue. Food Res. Int. 2023, 167, 112730. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Santos, L.E.; Ferreira, S.T. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front. Neurosci. 2019, 13, 265. [Google Scholar] [CrossRef]
- Oyovwi, M.O.; Udi, O.A. The Gut-Brain Axis and Neuroinflammation in Traumatic Brain Injury. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Celorrio, M.; Friess, S.H. Gut-brain axis in traumatic brain injury: Impact on neuroinflammation. Neural Regen. Res. 2022, 17, 1007–1008. [Google Scholar] [CrossRef]
- Panther, E.J.; Dodd, W.; Clark, A.; Lucke-Wold, B. Gastrointestinal Microbiome and Neurologic Injury. Biomedicines 2022, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Munley, J.A.; Kirkpatrick, S.L.; Gillies, G.S.; Bible, L.E.; Efron, P.A.; Nagpal, R.; Mohr, A.M. The Intestinal Microbiome after Traumatic Injury. Microorganisms 2023, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Soriano, S.; Curry, K.; Sadrameli, S.S.; Wang, Q.; Nute, M.; Reeves, E.; Kabir, R.; Wiese, J.; Criswell, A.; Schodrof, S.; et al. Alterations to the gut microbiome after sport-related concussion in a collegiate football players cohort: A pilot study. Brain Behav. Immun. Health 2022, 21, 100438. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Sun, S.; Nemes, J.; Brenner, L.A.; Hoisington, A.; Skotak, M.; LaValle, C.R.; Ge, Y.; Carr, W.; et al. Association of Blast Exposure in Military Breaching with Intestinal Permeability Blood Biomarkers Associated with Leaky Gut. Int. J. Mol. Sci. 2024, 25, 3549. [Google Scholar] [CrossRef] [PubMed]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montero, C.; Fraile-Martinez, O.; Gomez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; Garcia-Honduvilla, N.; Asunsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Logsdon, A.F.; Nguyen, L.; Eltanahay, A.; Turner, R.C.; Bonasso, P.; Knotts, C.; Moeck, A.; Maroon, J.C.; Bailes, J.E.; et al. Supplements, nutrition, and alternative therapies for the treatment of traumatic brain injury. Nutr. Neurosci. 2018, 21, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Ibeh, S.; Bablale, I.; Nwaiwu, J.; Reslan, M.; Mohamed, W.; Goli, M.; Mechref, Y.; Kobeissy, F. The Western Diet Puzzle: Connecting Metabolic Dysfunction to Cognitive and Neurological Consequences. In Nutrition and Psychiatric Disorders; Mohamed, W., Kobeissy, F., Eds.; Nutritional Neurosciences; Springer: Singapore, 2024. [Google Scholar]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Kwan, M.; Woo, J. Healthy Diet for Healthy Aging. Nutrients 2021, 13, 4310. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet and Lifestyle Drive The Pandemic Of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Ghezzi, A.C.; Cambri, L.T. Higher blood glucose impairs cardiac autonomic modulation in fasting and after carbohydrate overload in adults. Appl. Physiol. Nutr. Metab. 2021, 46, 221–228. [Google Scholar] [CrossRef]
- Shively, C.A.; Frye, B.M.; Negrey, J.D.; Johnson, C.S.C.; Sutphen, C.L.; Molina, A.J.A.; Yadav, H.; Snyder-Mackler, N.; Register, T.C. The interactive effects of psychosocial stress and diet composition on health in primates. Neurosci. Biobehav. Rev. 2023, 152, 105320. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Madry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Laugero, K.D.; Keim, N.L. A Diet Pattern Characterized by Sugar-Sweetened Beverages Is Associated with Lower Decision-Making Performance in the Iowa Gambling Task, Elevated Stress Exposure, and Altered Autonomic Nervous System Reactivity in Men and Women. Nutrients 2023, 15, 3930. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Appt, S.E.; Chen, H.; Day, S.M.; Frye, B.M.; Shaltout, H.A.; Silverstein-Metzler, M.G.; Snyder-Mackler, N.; Uberseder, B.; Vitolins, M.Z.; et al. Mediterranean diet, stress resilience, and aging in nonhuman primates. Neurobiol. Stress 2020, 13, 100254. [Google Scholar] [CrossRef] [PubMed]
- Mazza, E.; Troiano, E.; Ferro, Y.; Lisso, F.; Tosi, M.; Turco, E.; Pujia, R.; Montalcini, T. Obesity, Dietary Patterns, and Hormonal Balance Modulation: Gender-Specific Impacts. Nutrients 2024, 16, 1629. [Google Scholar] [CrossRef]
- Hiebert, J.B.; Shen, Q.; Thimmesch, A.R.; Pierce, J.D. Traumatic brain injury and mitochondrial dysfunction. Am. J. Med. Sci. 2015, 350, 132–138. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Joseph, B.; Spry, M.; Vekaria, H.J.; Saatman, K.E.; Sullivan, P.G. Acute Mitochondrial Impairment Underlies Prolonged Cellular Dysfunction after Repeated Mild Traumatic Brain Injuries. J. Neurotrauma 2019, 36, 1252–1263. [Google Scholar] [CrossRef]
- Hakiminia, B.; Alikiaii, B.; Khorvash, F.; Mousavi, S. Oxidative stress and mitochondrial dysfunction following traumatic brain injury: From mechanistic view to targeted therapeutic opportunities. Fundam. Clin. Pharmacol. 2022, 36, 612–662. [Google Scholar] [CrossRef] [PubMed]
- Walrand, S.; Gaulmin, R.; Aubin, R.; Sapin, V.; Coste, A.; Abbot, M. Nutritional factors in sport-related concussion. Neurochirurgie 2021, 67, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Patterson, Z.R.; Holahan, M.R. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front. Cell. Neurosci. 2012, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Camandola, S.; Mattson, M.P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017, 36, 1474–1492. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpi-Sarda, M.; Corella, D.; Castaner, O.; Lamuela-Raventos, R.M.; Salas-Salvado, J.; Martinez-Gonzalez, M.A.; Ros, E.; Estruch, R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvencion con DIeta MEDiterranea (PREDIMED) Randomized Controlled Trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, C.; Lopez-Enriquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 23–34. [Google Scholar] [CrossRef]
- De Alzaa, F.; Guillaume, C.; Ravetti, L. Evaluation of Chemical and Physical Changes in Different Commercial Oils during Heating. Acta Sci. Nutr. Health 2018, 2, 2–11. [Google Scholar]
- Nagpal, R.; Shively, C.A.; Register, T.C.; Craft, S.; Yadav, H. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Res 2019, 8, 699. [Google Scholar] [CrossRef]
- Patel, P.R.; Armistead-Jehle, P.; Eltman, N.R.; Heath, K.M.; Cifu, D.X.; Swanson, R.L. Brain Injury: How Dietary Patterns Impact Long-Term Outcomes. Curr. Phys. Med. Rehabil. Rep. 2023, 11, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, C.; Dickerson Mayes, K.; Jarvis, R.C., 3rd; Carr, C.; Mannix, R. Nutritional Supplement and Dietary Interventions as a Prophylaxis or Treatment of Sub-Concussive Repetitive Head Impact and Mild Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2023, 40, 1557–1566. [Google Scholar] [CrossRef]
- Ryan, T.; Nagle, S.; Daly, E.; Pearce, A.J.; Ryan, L. A Potential Role Exists for Nutritional Interventions in the Chronic Phase of Mild Traumatic Brain Injury, Concussion and Sports-Related Concussion: A Systematic Review. Nutrients 2023, 15, 3726. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.W.; Sharma, B. A review of the neuroprotective role of vitamin D in traumatic brain injury with implications for supplementation post-concussion. Brain Inj. 2016, 30, 960–968. [Google Scholar] [CrossRef] [PubMed]
- van Erp, I.A.M.; Michailidou, I.; van Essen, T.A.; van der Jagt, M.; Moojen, W.; Peul, W.C.; Baas, F.; Fluiter, K. Tackling Neuroinflammation After Traumatic Brain Injury: Complement Inhibition as a Therapy for Secondary Injury. Neurotherapeutics 2023, 20, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Randolph, J.J.; Lacritz, L.H.; Colvin, M.K.; Espe-Pfeifer, P.; Carter, K.R.; Arnett, P.A.; Fox-Fuller, J.; Aduen, P.A.; Cullum, C.M.; Sperling, S.A. Integrating Lifestyle Factor Science into Neuropsychological Practice: A National Academy of Neuropsychology Education Paper. Arch. Clin. Neuropsychol. 2024, 39, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Rippee, M.A.; Chen, J.; Taylor, M.K. The Ketogenic Diet in the Treatment of Post-concussion Syndrome-A Feasibility Study. Front. Nutr. 2020, 7, 160. [Google Scholar] [CrossRef]
- Broglio, S.P.; Register-Mihalik, J.K.; Guskiewicz, K.M.; Leddy, J.J.; Merriman, A.; Valovich McLeod, T.C. National Athletic Trainers’ Association Bridge Statement: Management of Sport-Related Concussion. J. Athl. Train. 2024, 59, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Kramer, E.S.; Smith, P.C.; Schmidt, D.; Argyropoulos, C. A closer look at weight loss interventions in primary care: A systematic review and meta-analysis. Front. Med. 2023, 10, 1204849. [Google Scholar] [CrossRef] [PubMed]
- Yuenyongchaiwat, K.; Changsri, K.; Harnmanop, S.; Namdaeng, P.; Aiemthaisong, M.; Pongpanit, K.; Pariyatkaraphan, T. Effects of slow breathing training on hemodynamic changes, cardiac autonomic function and neuroendocrine response in people with high blood pressure: A randomized control trial. J. Bodyw. Mov. Ther. 2024, 37, 136–141. [Google Scholar] [CrossRef]
- Pal, G.K.; Velkumary, S.; Madanmohan. Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J. Med. Res. 2004, 120, 115–121. [Google Scholar] [PubMed]
- Hamasaki, H. Effects of Diaphragmatic Breathing on Health: A Narrative Review. Medicines 2020, 7, 65. [Google Scholar] [CrossRef]
- Charalambous, A.; Giannakopoulou, M.; Bozas, E.; Paikousis, L. A Randomized Controlled Trial for the Effectiveness of Progressive Muscle Relaxation and Guided Imagery as Anxiety Reducing Interventions in Breast and Prostate Cancer Patients Undergoing Chemotherapy. Evid.-Based Complement. Altern. Med. 2015, 2015, 270876. [Google Scholar] [CrossRef] [PubMed]
- Jerath, R.; Edry, J.W.; Barnes, V.A.; Jerath, V. Physiology of long pranayamic breathing: Neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med. Hypotheses 2006, 67, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, J.; Adamek, M.; Gzik-Zroska, B.; Czyzewski, D.; Rydel, M. Network of breathing. Multifunctional role of the diaphragm: A review. Adv. Respir. Med. 2017, 85, 224–232. [Google Scholar] [CrossRef]
- Tyagi, A.; Cohen, M. Yoga and heart rate variability: A comprehensive review of the literature. Int. J. Yoga 2016, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Nadholta, P.; Dahiya, N.; Sharma, A.; Singh, H.; Kumar, S.; Singh, G. Link between Yoga and Heart Rate Variability: Can Yoga Enhance the Cardiac Resonance. Int. J. Yoga 2024, 17, 67–75. [Google Scholar] [CrossRef]
- Natarajan, A. Heart rate variability during mindful breathing meditation. Front. Physiol. 2022, 13, 1017350. [Google Scholar] [CrossRef]
- Gross, D.; Kohlmann, C.W. Increasing Heart Rate Variability through Progressive Muscle Relaxation and Breathing: A 77-Day Pilot Study with Daily Ambulatory Assessment. Int. J. Environ. Res. Public Health 2021, 18, 11357. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.C.; Chou, Y.S.; Tzeng, I.S.; Wei, C.Y.; Su, C.H.; Liu, W.C.; Kung, W.M. Effect of Tai Chi Synergy T1 Exercise on Autonomic Function, Metabolism, and Physical Fitness of Healthy Individuals. Evid. Based Complement. Altern. Med 2018, 2018, 6351938. [Google Scholar] [CrossRef] [PubMed]
- Kirk, U.; Axelsen, J.L. Heart rate variability is enhanced during mindfulness practice: A randomized controlled trial involving a 10-day online-based mindfulness intervention. PLoS ONE 2020, 15, e0243488. [Google Scholar] [CrossRef]
- Tung, Y.; Hsieh, J. The Impacts of Mindfulness on Heart Rate Variability: A Brief Review. Int. J. Pharma Med. Biol. Sci. 2019, 8, 132–137. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Holzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Meehan, Z.M. A Practical Guide to Resonance Frequency Assessment for Heart Rate Variability Biofeedback. Front. Neurosci. 2020, 14, 570400. [Google Scholar] [CrossRef]
- Chaitanya, S.; Datta, A.; Bhandari, B.; Sharma, V.K. Effect of Resonance Breathing on Heart Rate Variability and Cognitive Functions in Young Adults: A Randomised Controlled Study. Cureus 2022, 14, e22187. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.M. Biofeedback self-regulation training to treat post-concussion headache in a special operations support soldier. J. Spec. Oper. Med. 2012, 12, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Lagos, L.; Thompson, J.; Vaschillo, E. A preliminary study: Heart rate variability biofeedback for treatment of postconcussion syndrome. Biofeedback 2013, 41, 136–143. [Google Scholar] [CrossRef]
- Usmani, S.; Balcer, L.; Galetta, S.; Minen, M. Feasibility of Smartphone-Delivered Progressive Muscle Relaxation in Persistent Post-Traumatic Headache Patients. J. Neurotrauma 2021, 38, 94–101. [Google Scholar] [CrossRef]
- Lu, H.C.; Gevirtz, R.; Yang, C.C.; Hauson, A.O. Heart Rate Variability Biofeedback for Mild Traumatic Brain Injury: A Randomized-Controlled Study. Appl. Psychophysiol. Biofeedback 2023, 48, 405–421. [Google Scholar] [CrossRef]
- Wenzel, A. Basic Strategies of Cognitive Behavioral Therapy. Psychiatr. Clin. N. Am. 2017, 40, 597–609. [Google Scholar] [CrossRef]
- Uphoff, E.; Ekers, D.; Robertson, L.; Dawson, S.; Sanger, E.; South, E.; Samaan, Z.; Richards, D.; Meader, N.; Churchill, R. Behavioural activation therapy for depression in adults. Cochrane Database Syst. Rev. 2020, 7, CD013305. [Google Scholar] [CrossRef]
- Richards, D.A.; Ekers, D.; McMillan, D.; Taylor, R.S.; Byford, S.; Warren, F.C.; Barrett, B.; Farrand, P.A.; Gilbody, S.; Kuyken, W.; et al. Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression (COBRA): A randomised, controlled, non-inferiority trial. Lancet 2016, 388, 871–880. [Google Scholar] [CrossRef]
- Hopko, D.R.; Robertson, S.M.C.; Lejuez, C.W. Behavioral activation for anxiety disorders. Behav. Anal. Today 2006, 7, 212–232. [Google Scholar] [CrossRef]
- Stein, A.T.; Tian, L.; Cuthbert, K.; Gorman, K.R.; Best, S.G.; Bjorgvinsson, T.; Beard, C. Patient experiences with group behavioural activation in a partial hospital program. Behav. Cogn. Psychother. 2021, 49, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, J.; Alcon, S.; Hashim, J.; Meehan, W.P., 3rd; Mannix, R. Environmental Enrichment Mitigates Deficits after Repetitive Mild Traumatic Brain Injury. J. Neurotrauma 2017, 34, 2445–2455. [Google Scholar] [CrossRef]
- Kop, W.J.; Synowski, S.J.; Newell, M.E.; Schmidt, L.A.; Waldstein, S.R.; Fox, N.A. Autonomic nervous system reactivity to positive and negative mood induction: The role of acute psychological responses and frontal electrocortical activity. Biol. Psychol. 2011, 86, 230–238. [Google Scholar] [CrossRef]
- Beatton, T.; Chan, H.F.; Dulleck, U.; Ristl, A.; Schaffner, M.; Torgler, B. Positive affect and heart rate variability: A dynamic analysis. Sci. Rep. 2024, 14, 7004. [Google Scholar] [CrossRef]
- Simpson, T.S.; Peterson, R.L.; Patrick, K.E.; Forster, J.E.; McNally, K.A. Concussion Symptom Treatment and Education Program: A Feasibility Study. J. Head Trauma Rehabil. 2021, 36, E79–E88. [Google Scholar] [CrossRef]
- Goldstein, L. The Role of Psychology in Pediatric Concussion. Semin. Pediatr. Neurol. 2019, 30, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Kusec, A.; Methley, A.; Murphy, F.C.; Peers, P.V.; Carmona, E.; Manly, T. Developing behavioural activation for people with acquired brain injury: A qualitative interpretive description study of barriers and facilitators to activity engagement. BMC Psychol. 2023, 11, 207. [Google Scholar] [CrossRef]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Ulrich, R.S. Human responses to vegetation and landscapes. Landsc. Urban Plan. 1986, 13, 29–44. [Google Scholar] [CrossRef]
- Vibholm, A.P.; Christensen, J.R.; Pallesen, H. Nature-based rehabilitation for adults with acquired brain injury: A scoping review. Int. J. Environ. Health Res. 2020, 30, 661–676. [Google Scholar] [CrossRef]
- Corazon, S.S.; Olsen, L.J.; Kaereby, N.; Poulsen, D.V.; Sidenius, U.; Bekke-Hansen, S.; Marschner, L. Nature-Based Therapeutic Intervention for Individuals with Post-Concussion Symptoms. Behav. Sci. 2024, 14, 594. [Google Scholar] [CrossRef] [PubMed]
- Corazon, S.S.; Olsen, L.J.; Olsen, A.; Sidenius, U. Nature-Based Therapy for People Suffering from Post-Concussion Syndrome—A Pilot Study. Health 2019, 11, 1501–1517. [Google Scholar] [CrossRef]
- Aras, S.G.; Runyon, J.R.; Kazman, J.B.; Thayer, J.F.; Sternberg, E.M.; Deuster, P.A. Is Greener Better? Quantifying the Impact of a Nature Walk on Stress Reduction Using HRV and Saliva Cortisol Biomarkers. Int. J. Environ. Res. Public Health 2024, 21, 1491. [Google Scholar] [CrossRef] [PubMed]
- de Brito, J.N.; Pope, Z.C.; Mitchell, N.R.; Schneider, I.E.; Larson, J.M.; Horton, T.H.; Pereira, M.A. The effect of green walking on heart rate variability: A pilot crossover study. Environ. Res. 2020, 185, 109408. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Shen, X.; Shen, Y. Improving immersive experiences in virtual natural setting for public health and environmental design: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2024, 19, e0297986. [Google Scholar] [CrossRef]
- McDonnell, A.S.; Strayer, D.L. Immersion in nature enhances neural indices of executive attention. Sci. Rep. 2024, 14, 1845. [Google Scholar] [CrossRef] [PubMed]
- Trammell, J.P.; Harriger, J.A.; Krumrei-Mancuso, E.J. Walking in nature may improve affect but not cognition. Front. Psychol. 2023, 14, 1258378. [Google Scholar] [CrossRef] [PubMed]
- LoTemplio, S.; Bettmann, J.E.; Scott, E.; Blumenthal, E. Do Mental Health Changes in Nature Co-occur with Changes in Heartrate Variability and Executive Functioning? A Systematic Review. Curr. Environ. Health Rep. 2023, 10, 278–290. [Google Scholar] [CrossRef]
- Thomas, T.; Aggar, C.; Baker, J.; Massey, D.; Thomas, M.; D’Appio, D.; Brymer, E. Social prescribing of nature therapy for adults with mental illness living in the community: A scoping review of peer-reviewed international evidence. Front. Psychol. 2022, 13, 1041675. [Google Scholar] [CrossRef] [PubMed]
- Yeon, P.S.; Jeon, J.Y.; Jung, M.S.; Min, G.M.; Kim, G.Y.; Han, K.M.; Shin, M.J.; Jo, S.H.; Kim, J.G.; Shin, W.S. Effect of Forest Therapy on Depression and Anxiety: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 12685. [Google Scholar] [CrossRef]
- Jimenez, M.P.; DeVille, N.V.; Elliott, E.G.; Schiff, J.E.; Wilt, G.E.; Hart, J.E.; James, P. Associations between Nature Exposure and Health: A Review of the Evidence. Int. J. Environ. Res. Public Health 2021, 18, 4790. [Google Scholar] [CrossRef] [PubMed]
- Mygind, L.; Kjeldsted, E.; Hartmeyer, R.D.; Mygind, E.; Bolling, M.; Bentsen, P. Immersive Nature-Experiences as Health Promotion Interventions for Healthy, Vulnerable, and Sick Populations? A Systematic Review and Appraisal of Controlled Studies. Front. Psychol. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Kayira, J.; Chawla, L.; Rhoades, J.L. Forest Bathing Increases Adolescents’ Mental Well-Being: A Mixed-Methods Study. Int. J. Environ. Res. Public Health 2023, 21, 8. [Google Scholar] [CrossRef]
- Stier-Jarmer, M.; Throner, V.; Kirschneck, M.; Immich, G.; Frisch, D.; Schuh, A. The Psychological and Physical Effects of Forests on Human Health: A Systematic Review of Systematic Reviews and Meta-Analyses. Int. J. Environ. Res. Public Health 2021, 18, 1770. [Google Scholar] [CrossRef]
- Bratman, G.N.; Hamilton, J.P.; Hahn, K.S.; Daily, G.C.; Gross, J.J. Nature experience reduces rumination and subgenual prefrontal cortex activation. Proc. Natl. Acad. Sci. USA 2015, 112, 8567–8572. [Google Scholar] [CrossRef]
- The Management of Concussion-mild Traumatic Brain Injury Working Group. The VA/DoD Clinical Practice Guideline for the Management of Concussion-Mild Traumatic Brain Injury. J. Rehabil. Res. Dev. 2016, 46, 1–60. [Google Scholar]
- Wallace, B.; Lifshitz, J. Traumatic brain injury and vestibulo-ocular function: Current challenges and future prospects. Eye Brain 2016, 8, 153–164. [Google Scholar] [CrossRef]
- Karbasforoushan, H.; Wren-Jarvis, J.; Hwang, A.; Santiago, R.; Raptentsetsang, S.; Cai, L.T.; Xiao, J.; Maruyama, B.A.; Abrams, G.M.; Novakovic-Agopian, T.; et al. Goal-Oriented Attentional Self-Regulation Training in Chronic Mild Traumatic Brain Injury is Linked to Microstructural Plasticity in Prefrontal White Matter. J. Neurotrauma 2024. [Google Scholar] [CrossRef]
- Master, C.L.; Bacal, D.; Grady, M.F.; Hertle, R.; Shah, A.S.; Strominger, M.; Whitecross, S.; Bradford, G.E.; Lum, F.; Donahue, S.P.; et al. Vision and Concussion: Symptoms, Signs, Evaluation, and Treatment. Pediatrics 2022, 150, e2021056047. [Google Scholar] [CrossRef] [PubMed]
- Gallaway, M.; Scheiman, M.; Mitchell, G.L. Vision Therapy for Post-Concussion Vision Disorders. Optom. Vis. Sci. 2017, 94, 68–73. [Google Scholar] [CrossRef]
- Smaakjaer, P.; Wachner, L.G.; Rasmussen, R.S. Vision therapy improves binocular visual dysfunction in patients with mild traumatic brain injury. Neurol. Res. 2022, 44, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.J.S.; Ranalli, P.J. Vision Therapy: Ocular Motor Training in Mild Traumatic Brain Injury. Ann. Neurol. 2020, 88, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.T.; Mahmood, N.; Khan, F.; Waire, E.K.; George, A.; Datta, S. Treatment Options for Posttraumatic Headache: A Current Review of the Literature. Curr. Pain Headache Rep. 2024, 28, 205–210. [Google Scholar] [CrossRef]
- Claessen, L.O.E.; Kristjansdottir, H.; Jonsdottir, M.K.; Lund, S.H.; Kristensen, I.S.U.; Sigurjonsdottir, H.A. Screening for possible hypopituitarism following mild traumatic brain injury: The first all-female study. Who do we need to evaluate further? NeuroRehabilitation 2023, 52, 259–271. [Google Scholar] [CrossRef] [PubMed]

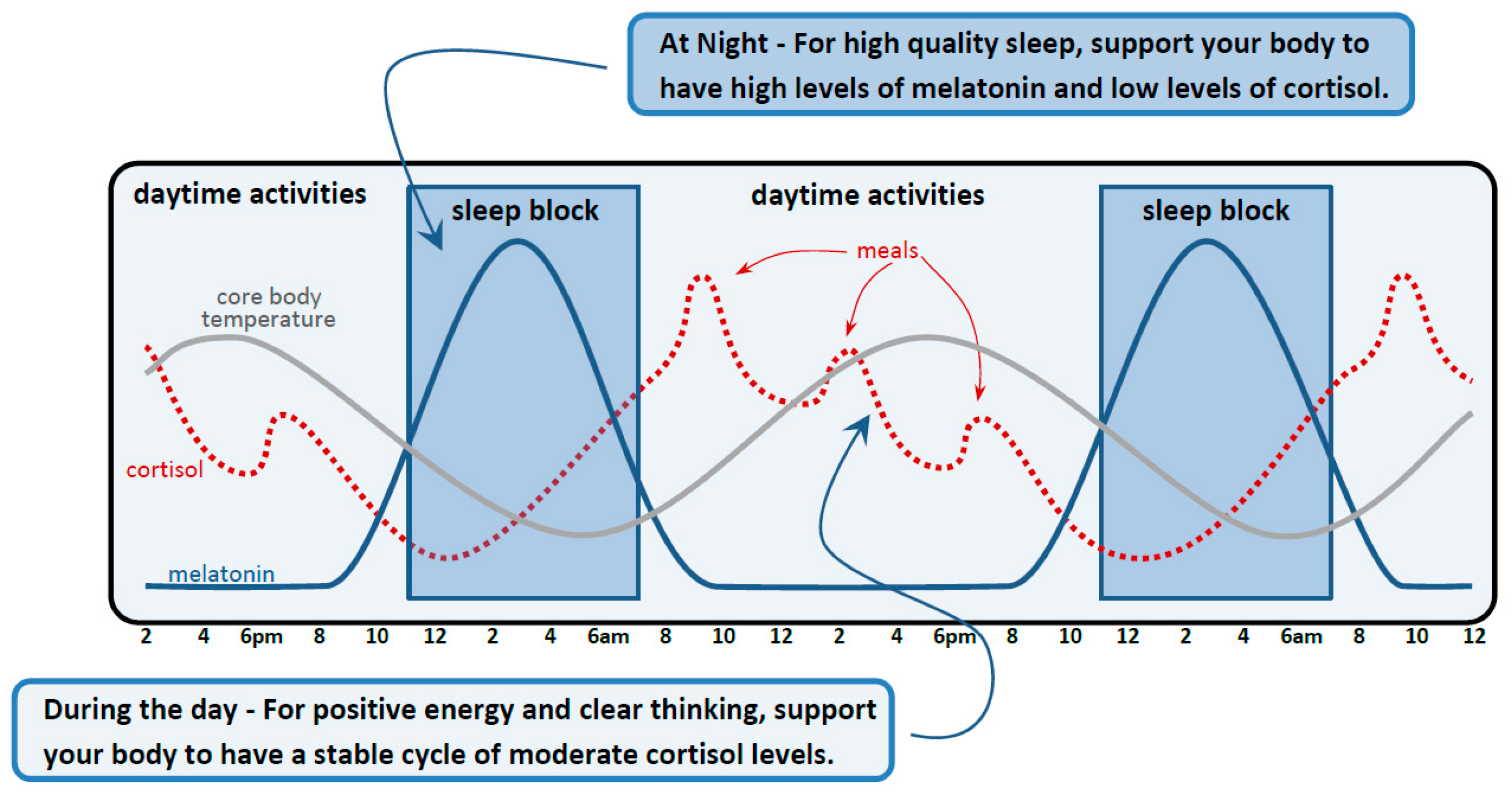
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertab, J.L.; Merkley, T.L.; Winiarski, H.; Cramond, K.M.J.; Cramond, A.J. Concussion and the Autonomic, Immune, and Endocrine Systems: An Introduction to the Field and a Treatment Framework for Persisting Symptoms. J. Pers. Med. 2025, 15, 33. https://doi.org/10.3390/jpm15010033
Pertab JL, Merkley TL, Winiarski H, Cramond KMJ, Cramond AJ. Concussion and the Autonomic, Immune, and Endocrine Systems: An Introduction to the Field and a Treatment Framework for Persisting Symptoms. Journal of Personalized Medicine. 2025; 15(1):33. https://doi.org/10.3390/jpm15010033
Chicago/Turabian StylePertab, Jon L., Tricia L. Merkley, Holly Winiarski, Kelly M. J. Cramond, and Alex J. Cramond. 2025. "Concussion and the Autonomic, Immune, and Endocrine Systems: An Introduction to the Field and a Treatment Framework for Persisting Symptoms" Journal of Personalized Medicine 15, no. 1: 33. https://doi.org/10.3390/jpm15010033
APA StylePertab, J. L., Merkley, T. L., Winiarski, H., Cramond, K. M. J., & Cramond, A. J. (2025). Concussion and the Autonomic, Immune, and Endocrine Systems: An Introduction to the Field and a Treatment Framework for Persisting Symptoms. Journal of Personalized Medicine, 15(1), 33. https://doi.org/10.3390/jpm15010033





