Retrospective Evaluation of the Impact of SLCO1B1 Variation on Statin Effectiveness
Abstract
1. Introduction
2. Materials and Methods
- TT genotype (*1/*1): Normal function phenotype
- TC genotype (*1/*5): Decreased function phenotype
- CC genotype (*5/*5): Poor function phenotype
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| ANOVA | Analysis of variance |
| ASCVD | Atherosclerotic cardiovascular disease |
| BMI | Body mass index |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| CPIC | Clinical Pharmacogenetics Implementation Consortium |
| CTT | Cholesterol Treatment Trialists |
| CVD | Cardiovascular disease |
| eGFR | Estimated glomerular filtration rate |
| EHR | Electronic Health Record |
| HMG-CoA | Hydroxymethylglutaryl-coenzyme A |
| LDL-C | Low-density lipoprotein cholesterol |
| PCSK9i | Proprotein convertase subtilisin/kexin type 9 inhibitors |
| PDC | Proportion of days covered |
| PGx | Pharmacogenomic/pharmacogenomics |
| PharmVar | Pharmacogene Variation Consortium |
| SAMS | Statin-associated muscle symptoms |
| SLCO1B1 | Solute carrier organic anion transporter family member 1B1 |
References
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Young, S.G.; Fong, L.G. Lowering Plasma Cholesterol by Raising LDL Receptors—Revisited. N. Engl. J. Med. 2012, 366, 1154–1155. [Google Scholar] [CrossRef]
- Cooper-DeHoff, R.M.; Niemi, M.; Ramsey, L.B.; Luzum, J.A.; Tarkiainen, E.K.; Straka, R.J.; Gong, L.; Tuteja, S.; Wilke, R.A.; Wadelius, M.; et al. The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 Genotypes and Statin-Associated Musculoskeletal Symptoms. Clin. Pharmacol. Ther. 2022, 111, 1007–1021. [Google Scholar] [CrossRef]
- The SEARCH Collaborative Group. SLCO1B1 Variants and Statin-Induced Myopathy—A Genomewide Study. N. Engl. J. Med. 2008, 359, 789–799. [Google Scholar] [CrossRef]
- Bakar, N.S.; Neely, D.; Avery, P.; Brown, C.; Daly, A.K.; Kamali, F. Genetic and Clinical Factors Are Associated with Statin-Related Myotoxicity of Moderate Severity: A Case–Control Study. Clin. Pharmacol. Ther. 2018, 104, 178–187. [Google Scholar] [CrossRef]
- Carr, D.F.; Francis, B.; Jorgensen, A.L.; Zhang, E.; Chinoy, H.; Heckbert, S.R.; Bis, J.C.; Brody, J.A.; Floyd, J.S.; Psaty, B.M.; et al. Genomewide Association Study of Statin-Induced Myopathy in Patients Recruited Using the UK Clinical Practice Research Datalink. Clin. Pharmacol. Ther. 2019, 106, 1353–1361. [Google Scholar] [CrossRef]
- Hou, Q.; Li, S.; Li, L.; Li, Y.; Sun, X.; Tian, H. Association between SLCO1B1 Gene T521C Polymorphism and Statin-Related Myopathy Risk: A Meta-Analysis of Case-Control Studies. Medicine 2015, 94, e1268. [Google Scholar] [CrossRef]
- Linskey, D.W.; English, J.D.; Perry, D.A.; Ochs-Balcom, H.M.; Ma, C.; Isackson, P.J.; Vladutiu, G.D.; Luzum, J.A. Association of SLCO1B1 c.521T>C (Rs4149056) with Discontinuation of Atorvastatin Due to Statin-Associated Muscle Symptoms. Pharmacogenet. Genom. 2020, 30, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.; Boyd, B.; Jialal, I. Physiology, Cholesterol; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zakria, M.; Hussain, A.; Ahmad, N.; Ahmed, N.; Rauf, M.A.; Siraj, S. The Lipid-Lowering Efficacy of Rosuvastatin Is Associated with Variations in SLCO1B1: A 12-Month Prospective Cohort Study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4708–4717. [Google Scholar] [CrossRef] [PubMed]
- Sivkov, A.; Chernus, N.; Gorenkov, R.; Sivkov, S.; Sivkova, S.; Savina, T. Relationship between Genetic Polymorphism of Drug Transporters and the Efficacy of Rosuvastatin, Atorvastatin and Simvastatin in Patients with Hyperlipidemia. Lipids Health Dis. 2021, 20, 157. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Nguyen, C.T.T.; Mai, T.N.P.; Huong, P.T. Associations between Four Polymorphisms of the SLCO1B1 and Effectiveness of the Statins: A Meta-Analysis. Pharmacogenet. Genom. 2023, 33, 65–78. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and Safety of Cholesterol-Lowering Treatment: Prospective Meta-Analysis of Data from 90,056 Participants in 14 Randomised Trials of Statins. Lancet 2005, 366, 1267–1278, Erratum in Lancet 2005, 366, 1358. [Google Scholar] [PubMed]
- Ramsey, L.B.; Gong, L.; Lee, S.-B.; Wagner, J.B.; Zhou, X.; Sangkuhl, K.; Adams, S.M.; Straka, R.J.; Empey, P.E.; Boone, E.C.; et al. PharmVar GeneFocus: SLCO1B1. Clin. Pharmacol. Ther. 2023, 113, 782–793. [Google Scholar] [CrossRef]
- Flockhart, D.; Thacker, D.; McDonald, C.; Desta, Z.; Division of Clinical Pharmacology, Indiana University School of Medicine. The Flockhart Cytochrome P450 Drug-Drug Interaction Table. 2021. Available online: https://drug-interactions.medicine.iu.edu/main-table (accessed on 15 August 2025).
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef]
- Alrajeh, K.; AlAzzeh, O.; Roman, Y. The Frequency of Major ABCG2, SLCO1B1 and CYP2C9 Variants in Asian, Native Hawaiian and Pacific Islander Women Subgroups: Implications for Personalized Statins Dosing. Pharmacogenomics 2023, 24, 381–398. [Google Scholar] [CrossRef]
- Alrajeh, K.; Roman, Y.M. The Frequency of Rs2231142 in ABCG2 among Asian Subgroups: Implications for Personalized Rosuvastatin Dosing. Pharmacogenomics 2023, 24, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr. Pract. 2017, 23, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, J.E.; Zolk, O.; Fromm, M.F.; Kurkinen, K.J.; Neuvonen, P.J.; Niemi, M. ABCG2 Polymorphism Markedly Affects the Pharmacokinetics of Atorvastatin and Rosuvastatin. Clin. Pharmacol. Ther. 2009, 86, 197–203. [Google Scholar] [CrossRef]
- Chasman, D.I.; Giulianini, F.; MacFadyen, J.; Barratt, B.J.; Nyberg, F.; Ridker, P.M. Genetic Determinants of Statin-Induced Low-Density Lipoprotein Cholesterol Reduction: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial. Circ. Cardiovasc. Genet. 2012, 5, 257–264. [Google Scholar] [CrossRef]
- Fischer, V.; Johanson, L.; Heitz, F.; Tullman, R.; Graham, E.; Baldeck, J.P.; Robinson, W.T. The 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase Inhibitor Fluvastatin: Effect on Human Cytochrome P-450 and Implications for Metabolic Drug Interactions. Drug Metab. Dispos. 1999, 27, 410–416. [Google Scholar] [CrossRef]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [PubMed]
- Kiander, W.; Sinokki, A.; Neuvonen, M.; Kidron, H.; Niemi, M. Comparative Uptake of Statins by Hepatic Organic Anion Transporting Polypeptides. Eur. J. Pharm. Sci. 2025, 209, 107073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lauschke, V.M. Genetic Variability and Population Diversity of the Human SLCO (OATP) Transporter Family. Pharmacol. Res. 2019, 139, 550–559. [Google Scholar] [CrossRef] [PubMed]
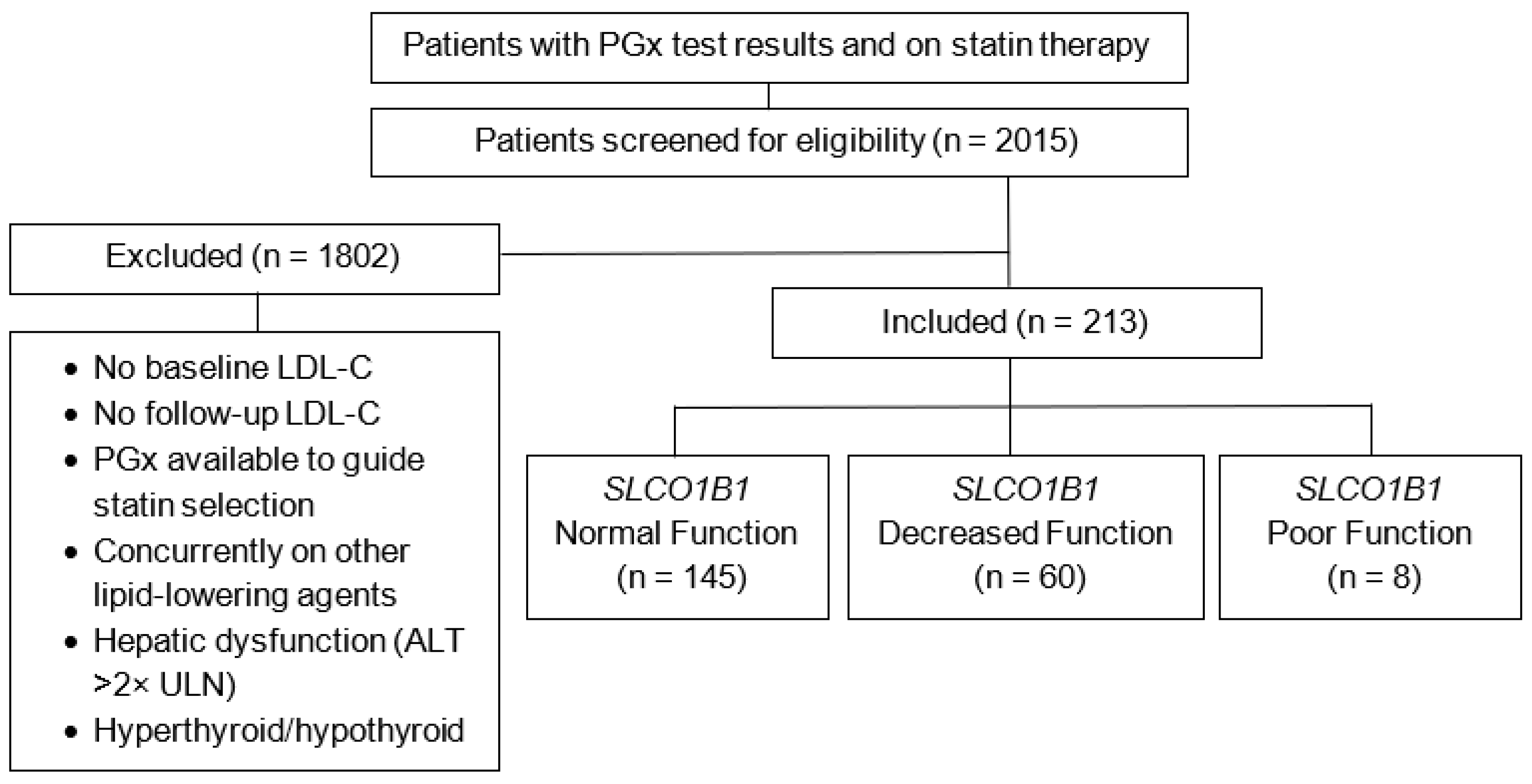
| Included patients, N | 213 |
| SLCO1B1 Functional Status, n (%) | |
| Normal Function (*1/*1) | 145 (68.1) |
| Decreased Function (*1/*5) | 60 (28.2) |
| Poor Function (*5/*5) | 8 (3.76) |
| CYP3A4 Phenotypes, n (%) | |
| Normal Metabolizer (*1/*1, *1/*1A, *1A,*1A) | 191 (89.6) |
| Intermediate Metabolizer (*1/*22, *22/*22) | 21 (9.9) |
| Data Unavailable | 1 (0.5) |
| CYP3A5 Phenotypes, n (%) | |
| Non-expressor (*3/*3, *3/*6, *3/*7, *6/*6,*6/*7, *7/*7) | 185 (86.9) |
| Expressor (*1/*1, *1/*3, *1/*6, *1/*7) | 27 (12.6) |
| Data Unavailable | 1 (0.5) |
| Sex, n (%) | |
| Female | 124 (58.2) |
| Race, n (%) | |
| White | 207 (97.2) |
| Age: Years, mean (±SD) | 58 ± 12.4 |
| BMI: kg/m2, mean (±SD) | 34 ± 16.6 |
| Current Smoker, n (%) | 51 (23.9) |
| Diabetes, n (%) | 50 (23.5) |
| eGFR < 60 mL/min/1.73m2, n (%) | 10 (4.7) |
| Overall Baseline LDL-C: mg/dL, mean (±SD) | 142 ± 35.8 |
| Baseline LDL-C by SLCO1B1 function: mg/dL, mean (±SD) | |
| Normal Function | 141 ± 37.1 |
| Decreased Function | 141 ± 31.7 |
| Poor Function | 153 ± 41.0 |
| Statin Indication, n (%) | |
| Primary Prevention | 187 (87.8) |
| Secondary Prevention | 26 (12.2) |
| Statin Medication, n (%) | |
| Atorvastatin | 124 (58.2) |
| Simvastatin | 32 (15.0) |
| Rosuvastatin | 28 (13.1) |
| Pravastatin | 23 (10.8) |
| Lovastatin | 3 (1.4) |
| Pitavastatin | 2 (0.9) |
| Fluvastatin | 1 (0.5) |
| Statin Intensity, n (%) | |
| High Intensity | 25 (11.7) |
| Moderate Intensity | 155 (72.8) |
| Low Intensity | 33 (15.5) |
| Concurrent Strong CYP3A4 Inducer, n (%) | |
| Carbamazepine | 1 (0.5) |
| Concurrent Moderate CYP3A4 Inhibitor, n (%) | |
| Diltiazem | 3 (1.4) |
| Verapamil | 1 (0.5) |
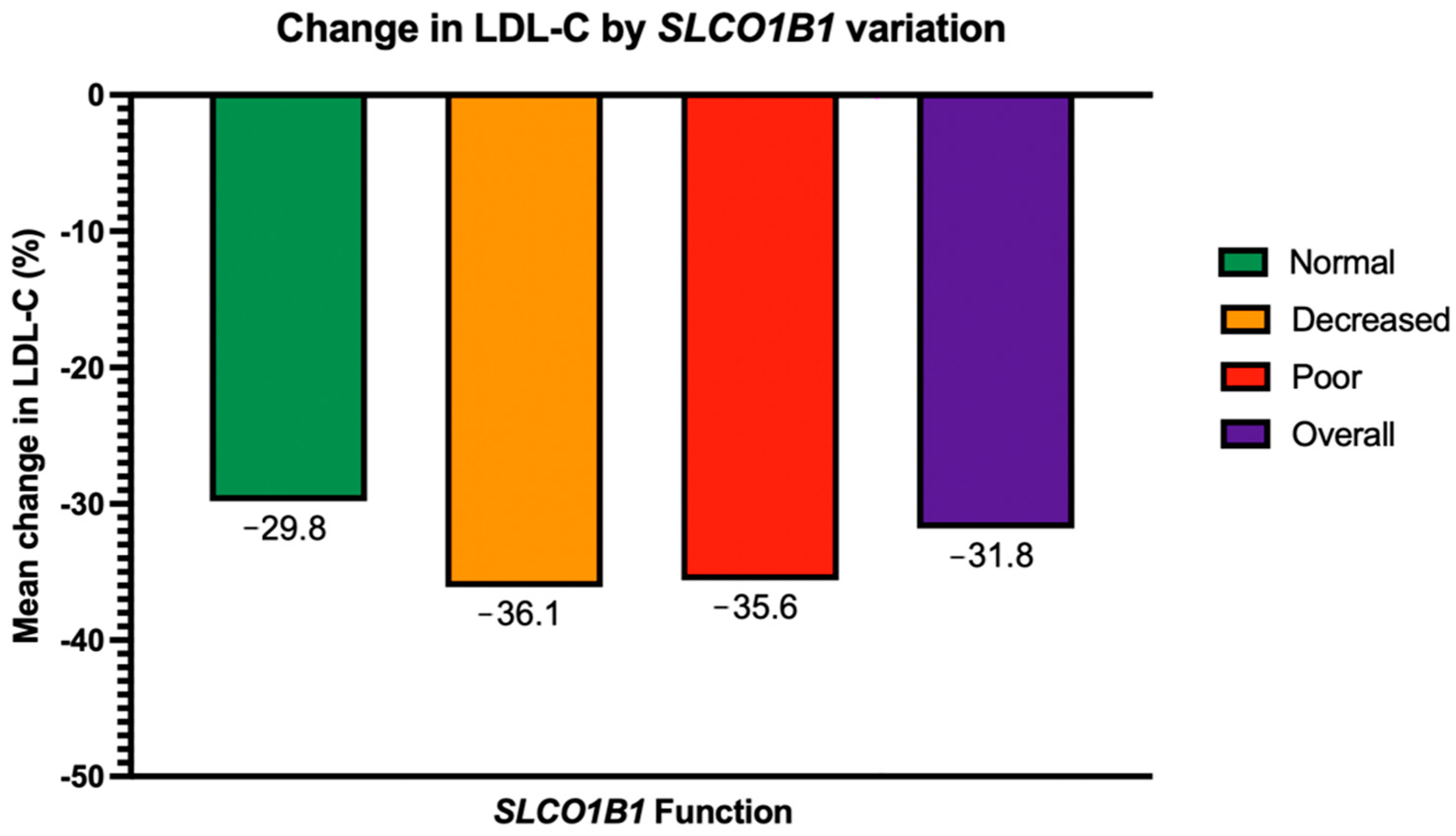
| SLCO1B1 Function | Mean Change in LDL-C (%) | ||||
|---|---|---|---|---|---|
| Overall | p-Value | Statin Intensity: High | Statin Intensity: Moderate | Statin Intensity: Low | |
| Normal (n = 145) | −29.8 | 0.24 | −40 | −31.1 | −15.5 |
| Decreased (n = 60) | −36.1 | −45.1 | −38.9 | −18.9 | |
| Poor (n = 8) | −35.6 | −52.7 | −38.6 | −0.9 | |
| p-value | 0.8 | 0.13 | 0.86 | ||
| Overall Mean Change in LDL-C (%) by Statin Intensity | |||||
| Statin Intensity: High (n = 25) | −42.1 | ||||
| Statin Intensity: Moderate (n = 155) | −33.4 | ||||
| Statin Intensity: Low (n = 33) | −16.2 | ||||
| Overall SAMS Occurrence, n (%) | |||
|---|---|---|---|
| 29 (13.6) | |||
| SLCO1B1 Function | SAMS Occurrence | p-Value | |
| Yes, n (%) | No, n (%) | ||
| Normal | 19 (8.9) | 126 (59.2) | 0.24 |
| Decreased | 10 (4.7) | 50 (23.5) | |
| Poor | 0 (0) | 8 (3.8) | |
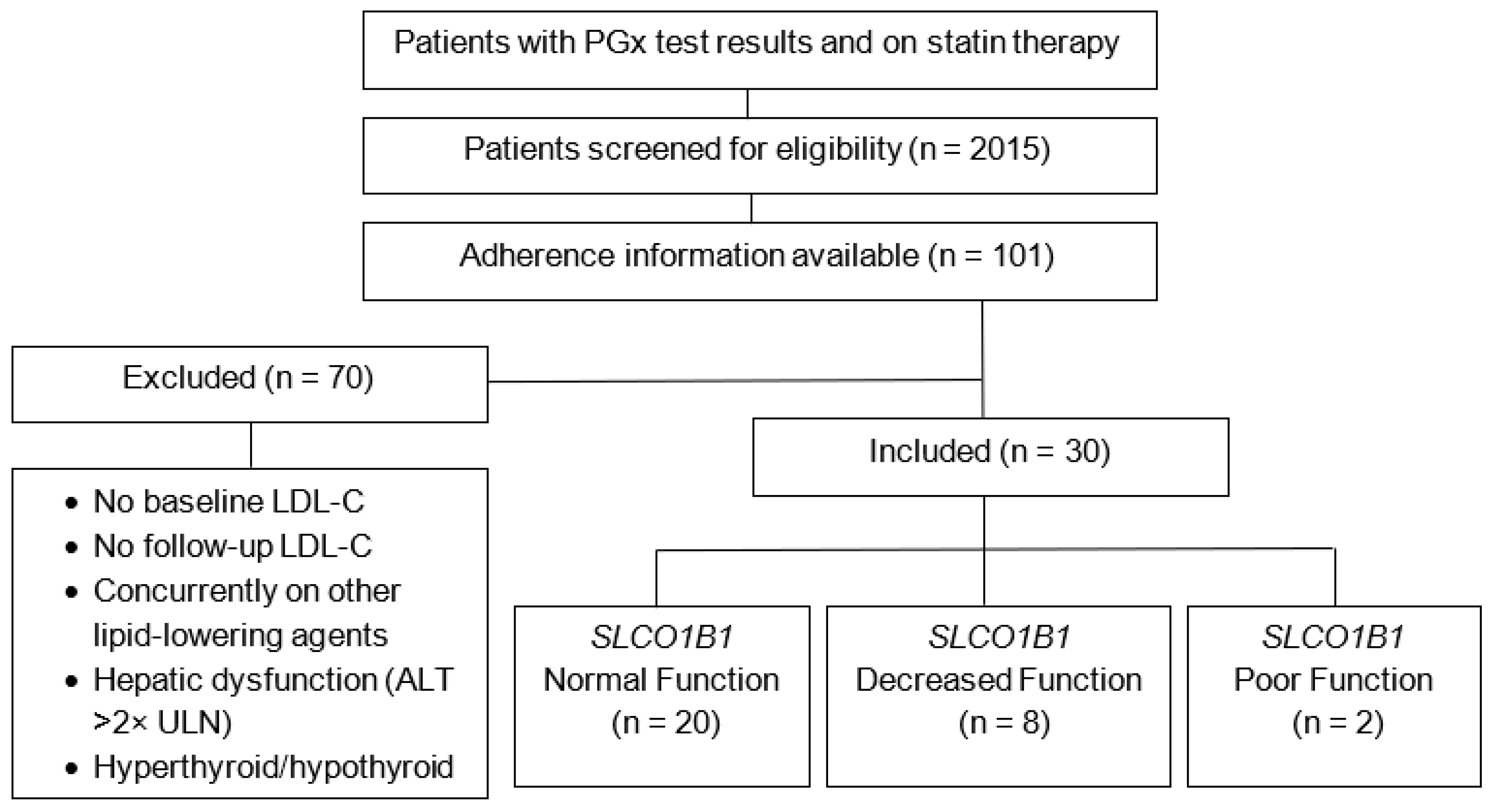
| Overall Adherent, n (%) | |||
|---|---|---|---|
| 22 (73.3) | |||
| SLCO1B1 Function | Adherent, n (%) | Not Adherent, n (%) | p-Value |
| Normal | 15 (50) | 5 (16.7) | 1.00 |
| Decreased | 5 (16.7) | 3 (10) | |
| Poor | 2 (6.6) | 0 (0) | |
| SAMS | Average Change in LDL-C (%) | |
|---|---|---|
| Adherent (n = 22) | Not Adherent (n = 5) | |
| Yes (n = 2) | −2.19 | −9.9 |
| No (n = 28) | −40.5 | −12 |
| p-value | 0.04 | 0.95 |
| SAMS | Adherent (n) | Not Adherent (n) | p-Value |
|---|---|---|---|
| Yes | 1 | 1 | 0.44 |
| No | 21 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed Feldman, M.; Billman, K.; Sennoun, M.; Ng, G.; Hussain, M.; Schlosser, E.G.; Hincapie, A.L.; Allen, J.D. Retrospective Evaluation of the Impact of SLCO1B1 Variation on Statin Effectiveness. J. Pers. Med. 2025, 15, 511. https://doi.org/10.3390/jpm15110511
Ahmed Feldman M, Billman K, Sennoun M, Ng G, Hussain M, Schlosser EG, Hincapie AL, Allen JD. Retrospective Evaluation of the Impact of SLCO1B1 Variation on Statin Effectiveness. Journal of Personalized Medicine. 2025; 15(11):511. https://doi.org/10.3390/jpm15110511
Chicago/Turabian StyleAhmed Feldman, Mayeesha, Kendall Billman, Mounia Sennoun, Gloria Ng, Mariam Hussain, Elizabeth G. Schlosser, Ana L. Hincapie, and Josiah D. Allen. 2025. "Retrospective Evaluation of the Impact of SLCO1B1 Variation on Statin Effectiveness" Journal of Personalized Medicine 15, no. 11: 511. https://doi.org/10.3390/jpm15110511
APA StyleAhmed Feldman, M., Billman, K., Sennoun, M., Ng, G., Hussain, M., Schlosser, E. G., Hincapie, A. L., & Allen, J. D. (2025). Retrospective Evaluation of the Impact of SLCO1B1 Variation on Statin Effectiveness. Journal of Personalized Medicine, 15(11), 511. https://doi.org/10.3390/jpm15110511






