Integrating Novel and Classical Prognostic Factors in Locally Advanced Cervical Cancer: A Machine Learning-Based Predictive Model (ESTHER Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim and Design of the Study
2.2. Staging, Treatment, and Follow-Up
2.3. Evaluated Parameters
2.3.1. Clinical Data
2.3.2. Inflammatory Indices
2.3.3. Body Composition Parameters
2.3.4. Functional Imaging
2.4. Machine Learning Modeling and Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Predictive Model
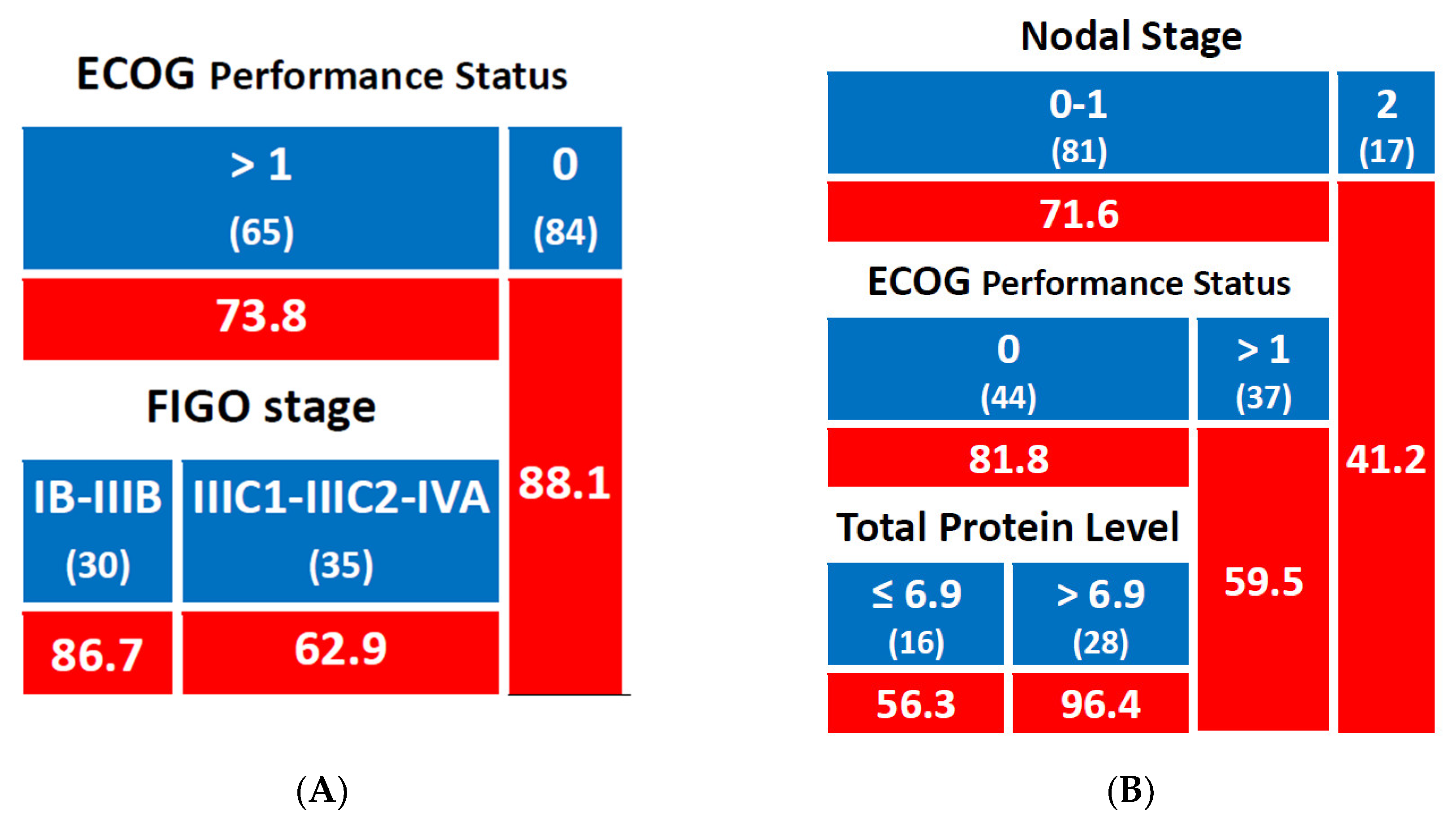
3.2.1. Local Control
3.2.2. Metastasis-Free Survival
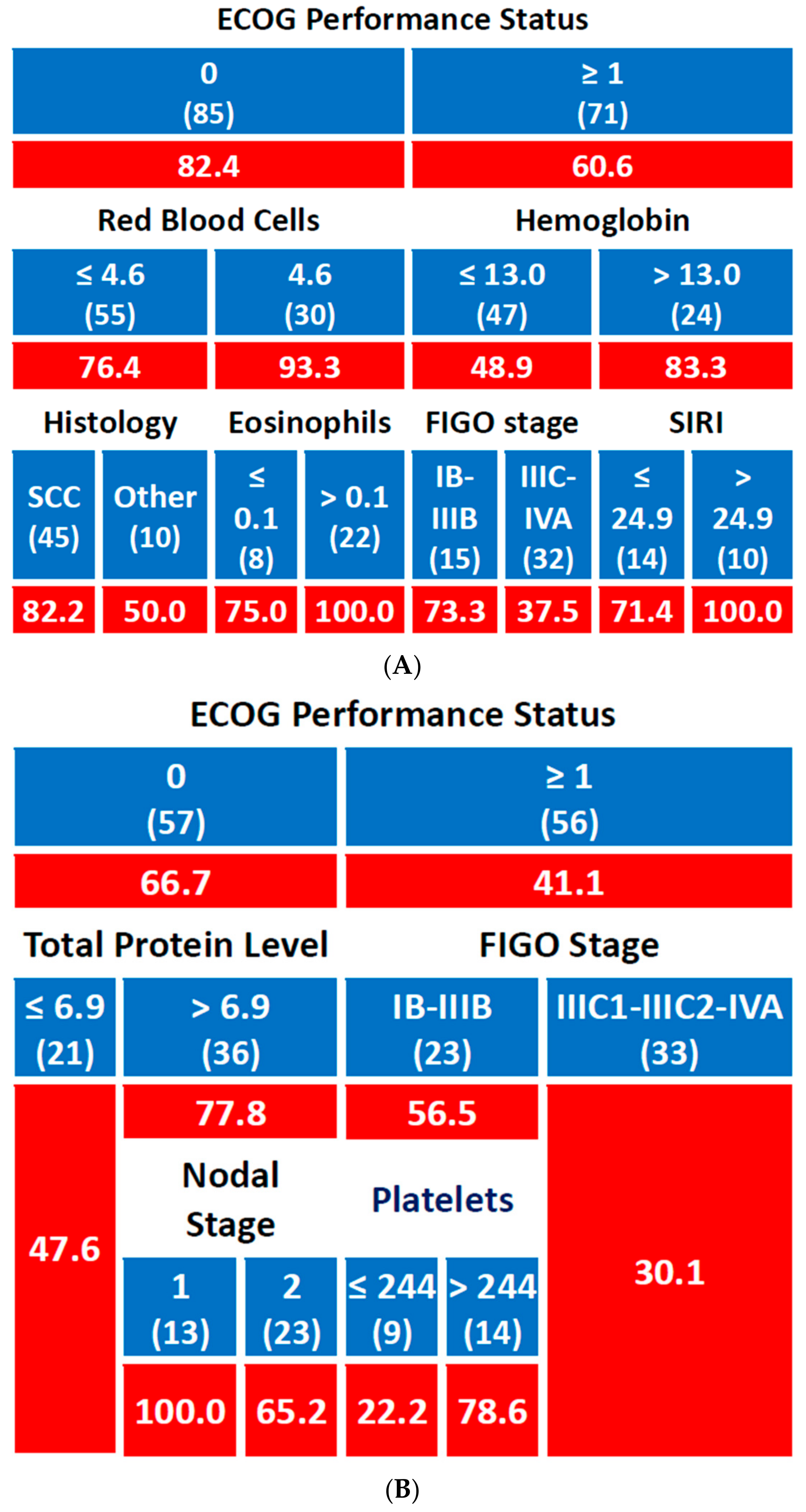
3.2.3. Disease-Free Survival
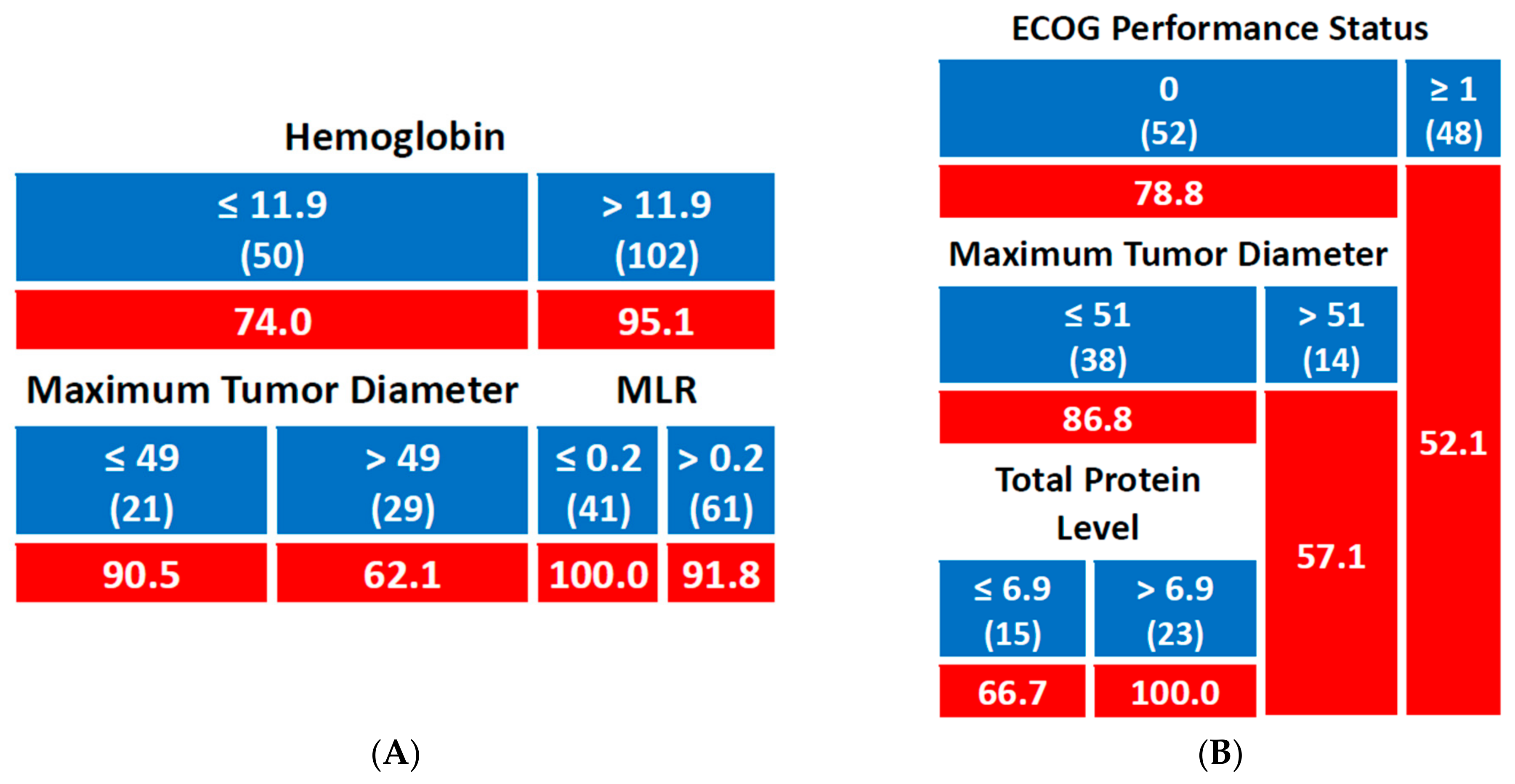
3.2.4. Overall Survival
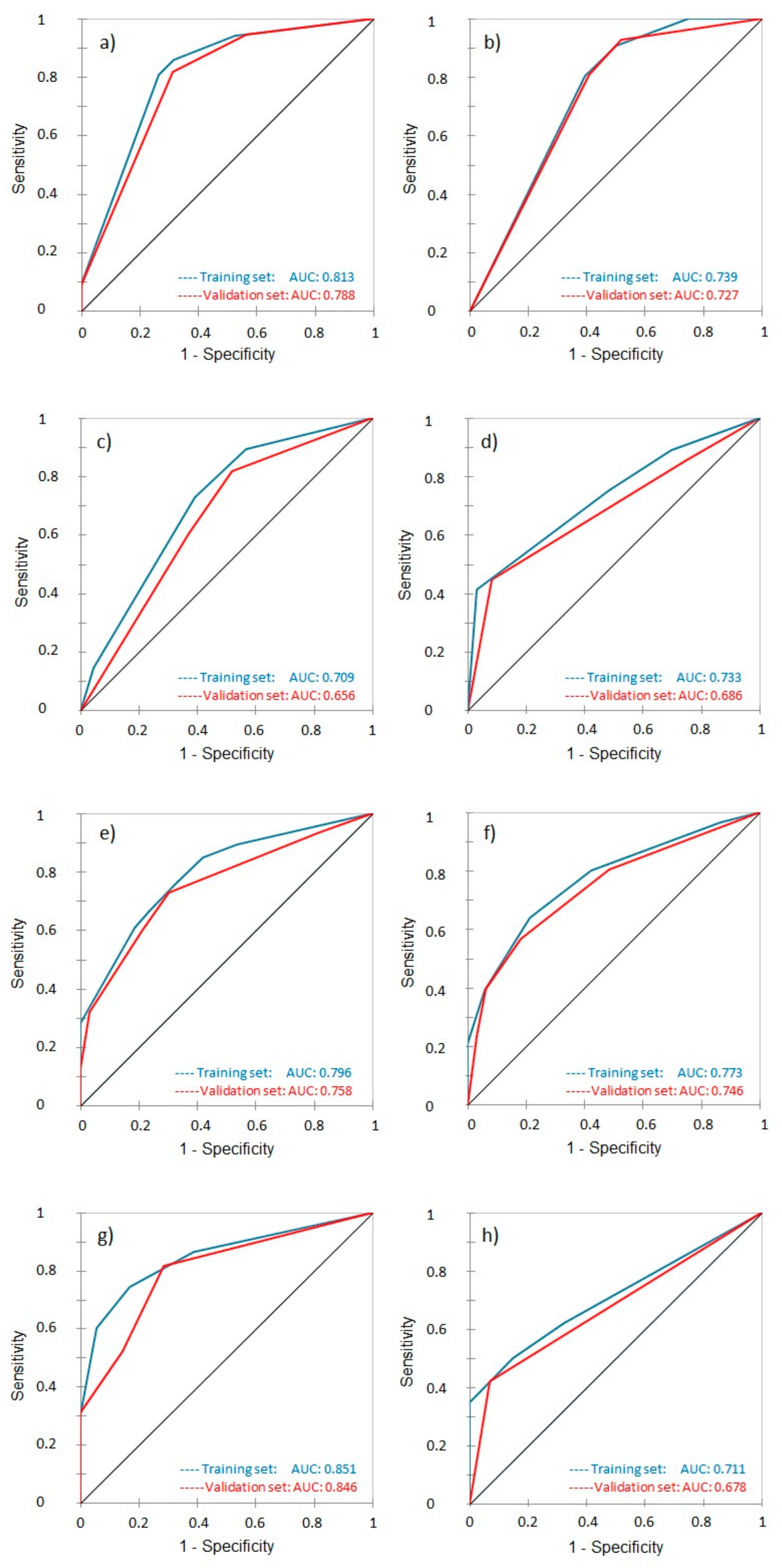
3.3. Receiver Operating Characteristic and Area Under the Curve
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. EMBRACE Collaborative Group. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic Radiation with Concurrent Chemotherapy Compared with Pelvic and Para-Aortic Radiation for High-Risk Cervical Cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.; Jakobsen, A. Reducing Uncertainties About the Effects of Chemoradiotherapy for Cervical Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data From 18 Randomized Trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar]
- Lai, C.H. Management of recurrent cervical cancer. Chang. Gung Med. J. 2004, 27, 711–717. [Google Scholar]
- Atahan, I.L.; Onal, C.; Ozyar, E.; Yiliz, F.; Selek, U.; Kose, F. Long-term outcome and prognostic factors in patients with cervical carcinoma: A retrospective study. Int. J. Gynecol. Cancer 2007, 17, 833–842. [Google Scholar] [CrossRef]
- Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Sobel, E.; Rose, G.S.; Thomas, G.; Carlson, J.W. Association of hemoglobin level with survival in cervical carcinoma patients treated with concurrent cisplatin and radiotherapy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2004, 94, 495–501. [Google Scholar] [CrossRef]
- Grogan, M.; Thomas, G.M.; Melamed, I.; Wong, F.L.; Pearcey, R.G.; Joseph, P.K.; Portelance, L.; Crook, J.; Jones, K.D. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 1999, 86, 1528–1536. [Google Scholar] [CrossRef]
- Thomas, G. The effect of hemoglobin level on radiotherapy outcomes: The Canadian experience. Semin. Oncol. 2001, 28, 60–65. [Google Scholar] [CrossRef]
- Koulis, T.A.; Kornaga, E.N.; Banerjee, R.; Phan, T.; Ghatage, P.; Magliocco, A.M.; Lees-Miller, S.P.; Doll, C.M. Anemia, leukocytosis and thrombocytosis as prognostic factors in patients with cervical cancer treated with radical chemoradiotherapy: A retrospective cohort study. Clin. Transl. Radiat. Oncol. 2017, 12, 51–56. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Šeruga, B.; Ocaña, A.; Tannock, I.F.; Amir, E. Prognostic Role of Platelet to Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Haraga, J.; Nakamura, K.; Omichi, C.; Nishida, T.; Haruma, T.; Kusumoto, T.; Seki, N.; Masuyama, H.; Katayama, N.; Kanazawa, S.; et al. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol. Clin. Oncol. 2016, 5, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.H.; Kim, H.; Kim, T.H.; Kim, M.H.; Kim, B.J.; Ryu, S.Y. Prognostic significance of pretreatment lymphocyte percentage and age at diagnosis in patients with locally advanced cervical cancer treated with definite radiotherapy. Obstet. Gynecol. Sci. 2019, 62, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Seol, K.H. Pretreatment Neutrophil-to-Lymphocyte Ratio Combined with Platelet-to-Lymphocyte Ratio as a Predictor of Survival Outcomes after Definitive Concurrent Chemoradiotherapy for Cervical Cancer. J. Clin. Med. 2021, 10, 2199. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.M.; Chin, Y.J.; Chong, G.O.; Park, S.H.; Lee, Y.H.; Hong, D.G.; Lee, Y.S. Prognostic Value of Hematological Parameters in Locally Advanced Cervical Cancer Patients Treated with Concurrent Chemoradiotherapy. Anticancer. Res. 2020, 40, 451–458. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Choi, C.H.; Kim, H.J.; Kim, T.J.; Lee, J.W.; Lee, J.H.; Bae, D.S.; Kim, B.G. Pretreatment neutrophil: Lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer. Res. 2012, 32, 1555–1561. [Google Scholar]
- Li, Y.X.; Chang, J.Y.; He, M.Y.; Wang, H.R.; Luo, D.Q.; Li, F.H.; Li, J.H.; Ran, L. Neutrophil-to-Lymphocyte Ratio (NLR) and Monocyte-to-Lymphocyte Ratio (MLR) Predict Clinical Outcome in Patients with Stage IIB Cervical Cancer. J. Oncol. 2021, 2021, 2939162. [Google Scholar] [CrossRef]
- Mizunuma, M.; Yokoyama, Y.; Futagami, M.; Aoki, M.; Takai, Y.; Mizunuma, H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int. J. Clin. Oncol. 2015, 20, 989–996. [Google Scholar] [CrossRef]
- Onal, C.; Guler, O.C.; Yildirim, B.A. Prognostic Use of Pretreatment Hematologic Parameters in Patients Receiving Definitive Chemoradiotherapy for Cervical Cancer. Int. J. Gynecol. Cancer 2016, 26, 1169–1175. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Bai, Z.L.; He, J.L.; Yang, Y.; Zhao, R.; Hai, P.; Zhe, H. Prognostic Value of Neutrophil-Related Factors in Locally Advanced Cervical Squamous Cell Carcinoma Patients Treated with Cisplatin-Based Concurrent Chemoradiotherapy. Dis. Markers 2016, 2016, 3740794. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, M.; He, F.; Han, B.; Ma, K.; Zeng, X.; Liu, Z.; Liu, X.; Li, J.; Cao, H.; et al. Pretreatment neutrophil-lymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical Cancer. Clin. Chim. Acta 2018, 483, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Holub, K.; Biete, A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin. Transl. Oncol. 2019, 21, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, Y.S.; Shin, J.W.; Osong, B.; Lee, S.H. Prediction scoring system based on clinicohematologic parameters for cervical cancer patients undergoing chemoradiation. Int. J. Gynecol. Cancer 2020, 30, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Jonska-Gmyrek, J.; Gmyrek, L.; Zolciak-Siwinska, A.; Kowalska, M.; Fuksiewicz, M.; Kotowicz, B. Pretreatment neutrophil to lymphocyte and platelet to lymphocyte ratios as predictive factors for the survival of cervical adenocarcinoma patients. Cancer Manag. Res. 2018, 10, 6029–6038. [Google Scholar] [CrossRef]
- Chauhan, R.; Trivedi, V.; Rani, R.; Singh, U.; Singh, K. Pre-treatment hematological parameters as a cost effective predictive marker for response to concurrent chemo radiation in locally advanced cervical cancer. Cancer Treat. Res. Commun. 2022, 31, 100539. [Google Scholar] [CrossRef]
- Li, S.W.; Yuan, W.; Zhao, B.; He, Z.K.; Guo, X.; Xia, W.X.; Xu, L.H. Positive effect of HPV status on prognostic value of blood lymphocyte-to-monocyte ratio in advanced cervical carcinoma. Cancer Cell Int. 2016, 16, 54. [Google Scholar] [CrossRef]
- Gangopadhyay, A. Prognostic Nutritional Index and Clinical Response in Locally Advanced Cervical Cancer. Nutr. Cancer 2020, 72, 1438–1442. [Google Scholar] [CrossRef]
- Ferioli, M.; Benini, A.; Malizia, C.; Forlani, L.; Medici, F.; Laghi, V.; Ma, J.; Galuppi, A.; Cilla, S.; Buwenge, M.; et al. Classical Prognostic Factors Predict Prognosis Better than Inflammatory Indices in Locally Advanced Cervical Cancer: Results of a Comprehensive Observational Study including Tumor-, Patient-, and Treatment-Related Data (ESTHER Study). J. Pers. Med. 2023, 13, 1229. [Google Scholar] [CrossRef]
- Medici, F.; Ferioli, M.; Forlani, L.; Laghi, V.; Ma, J.; Cilla, S.; Buwenge, M.; Macchia, G.; Deodato, F.; Vadalà, M.; et al. Decoding the Complexity of Systemic Inflammation Predictors in Locally Advanced Cervical Cancer, with Hemoglobin as the Hidden Key (the ESTHER Study). Cancers 2023, 15, 5056. [Google Scholar] [CrossRef]
- Medici, F.; Ferioli, M.; Cammelli, S.; Forlani, L.; Laghi, V.; Ma, J.; Cilla, S.; Buwenge, M.; Macchia, G.; Deodato, F.; et al. Sarcopenic Obesity in Cervical Carcinoma: A Strong and Independent Prognostic Factor beyond the Conventional Predictors (ESTHER Study—AFRAID Project). Cancers 2024, 16, 929. [Google Scholar] [CrossRef]
- Deodato, F.; Cilla, S.; Massaccesi, M.; Macchia, G.; Ippolito, E.; Caravatta, L.; Picardi, V.; Romanella, M.; Di Falco, C.; Bartollino, A.; et al. Daily on-line set-up correction in 3D-conformal radiotherapy: Is it feasible? Tumori J. 2012, 98, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points. In StatPearls; StatPearls Publishing: Tampa/St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 9 October 2023).
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.H.; Gebski, V.; Narayan, K.; King, M.T.; Bradshaw, N.; Lee, Y.C.; Diamante, K.; Fyles, A.W.; et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 468–482. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Sukhin, V.; Cloven, N.; Pereira de Santana Gomes, A.J.; et al. ENGOT-cx11/GOG-3047/KEYNOTE-A18 investigators. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind, phase 3 clinical trial. Lancet 2024, 403, 1341–1350. [Google Scholar]
- Monk, B.J.; Toita, T.; Wu, X.; Vázquez Limón, J.C.; Tarnawski, R.; Mandai, M.; Shapira-Frommer, R.; Mahantshetty, U.; Del Pilar Estevez-Diz, M.; Zhou, Q.; et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1334–1348. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, X. An Energy-Efficient Test and Predictive Model for Recurrence After Radiotherapy in Localized Intermediate and Advanced Cervical Cancer Were Created Using Thymidine Kinase 1 in Conjunction with Inflammatory Markers and Tumor Markers. Int. J. Gen. Med. 2023, 16, 5789–5797. [Google Scholar] [CrossRef]
- Hua, L.; Wei, M.; Feng, C.; Li, S.; Wen, X.; Chen, S. Online ahead of print. Nomogram for Predicting Survival in Locally Advanced Cervical Cancer with Concurrent Chemoradiotherapy plus or Not Adjuvant Chemotherapy: A Retrospective Analysis Based on 2018 FIGO Staging. Cancer Biother. Radiopharm. 2024, 39, 690–705. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; Zhao, Q.; Zhang, L.; Yin, M.; Zhou, J.; Zhu, J.; Qin, S. CT-based radiomics nomogram for overall survival prediction in patients with cervical cancer treated with concurrent chemoradiotherapy. Front. Oncol. 2023, 13, 1287121. [Google Scholar] [CrossRef]
- Abdalvand, N.; Sadeghi, M.; Mahdavi, S.R.; Abdollahi, H.; Qasempour, Y.; Mohammadian, F.; Birgani, M.J.T.; Hosseini, K. Brachytherapy outcome modeling in cervical cancer patients: A predictive machine learning study on patient-specific clinical, physical and dosimetric parameters. Brachytherapy 2022, 21, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Leetanaporn, K.; Hanprasertpong, J. Predictive Value of the Hemoglobin-Albumin-Lymphocyte-Platelet (HALP) Index on the Oncological Outcomes of Locally Advanced Cervical Cancer Patients. Cancer Manag. Res. 2022, 14, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Chen, Q.; Zhang, X.; Zhong, X.; Chen, J.; Fang, Y.; Lin, A.; Wang, M. Nomogram Predicting Overall Survival in Patients with FIGO II to III Squamous Cell Cervical Carcinoma Under Radical Radiotherapy: A Retrospective Analysis Based on 2018 FIGO Staging. Cancer Manag. Res. 2021, 13, 9391–9400. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, X.; Sun, C.R. Predictive value of serum albumin levels on cancer survival: A prospective cohort study. Front. Oncol. 2024, 14, 1323192. [Google Scholar] [CrossRef]
- Takenaka, Y.; Takemoto, N.; Yasui, T.; Yamamoto, Y.; Uno, A.; Miyabe, H.; Ashida, N.; Shimizu, K.; Nakahara, S.; Hanamoto, A.; et al. Transaminase activity predicts survival in patients with head and neck cancer. PLoS ONE 2016, 11, e0164057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.L.; Xue, N.; Wu, M.T.; Chen, H.; He, X.; Li, J.P.; Liu, W.L.; Dai, S.Q. Influence of preoperative serum aspartate aminotransferase (AST) level on the prognosis of patients with non-small cell lung cancer. Int. J. Mol. Sci. 2016, 17, 1474. [Google Scholar] [CrossRef]
- Alves, A.; Dias, M.; Campainha, S.; Barroso, A. Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy. J. Thorac. Dis. 2021, 13, 2716–2727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moreira, A.; Leisgang, W.; Schuler, G.; Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 2017, 9, 115–121. [Google Scholar] [CrossRef]
- Herrmann, T.; Ginzac, A.; Molnar, I.; Bailly, S.; Durando, X.; Mahammedi, H. Eosinophil counts as a relevant prognostic marker for response to nivolumab in the management of renal cell carcinoma: A retrospective study. Cancer Med. 2021, 10, 6705–6713. [Google Scholar] [CrossRef]
- Forni, F.; Ferrandina, G.; Deodato, F.; Macchia, G.; Morganti, A.G.; Smaniotto, D.; Luzi, S.; D’Agostino, G.; Valentini, V.; Cellini, N.; et al. Squamous cell carcinoma antigen in follow-up of cervical cancer treated with radiotherapy: Evaluation of cost-effectiveness. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1145–1149. [Google Scholar] [CrossRef]
- Ferrandina, G.; Macchia, G.; Legge, F.; Deodato, F.; Forni, F.; Digesù, C.; Carone, V.; Morganti, A.G.; Scambia, G. Squamous cell carcinoma antigen in patients with locally advanced cervical carcinoma undergoing preoperative radiochemotherapy: Association with pathological response to treatment and clinical outcome. Oncology 2008, 74, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Chung, J.Y.; Byeon, S.J.; Kim, C.J.; Lee, Y.Y.; Kim, T.J.; Lee, J.W.; Kim, B.G.; Chae, Y.L.; Oh, S.Y.; et al. Validation of Potential Protein Markers Predicting Chemoradioresistance in Early Cervical Cancer by Immunohistochemistry. Front. Oncol. 2021, 11, 665595. [Google Scholar] [CrossRef] [PubMed]
- Lizano, M.; Carrillo-García, A.; De La Cruz-Hernández, E.; Castro-Muñoz, L.J.; Contreras-Paredes, A. Promising predictive molecular biomarkers for cervical cancer (Review). Int. J. Mol. Med. 2024, 53, 50. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Chatzikonstantinou, G.; Fokas, E.; Wichmann, J.; Christiansen, H.; Strebhardt, K.; Rödel, C.; Tselis, N.; Rödel, F. Molecular Markers to Predict Prognosis and Treatment Response in Uterine Cervical Cancer. Cancers 2021, 13, 5748. [Google Scholar] [CrossRef]
- Cheung, E.S.; Wu, P.Y. Current Paradigm and Future Directions in the Management of Nodal Disease in Locally Advanced Cervical Cancer. Cancers 2025, 17, 202. [Google Scholar] [CrossRef]

| Characteristic | Number of Patients (%) |
|---|---|
| Total patients | 173 (100) |
| Median age (range), years | 56 (27–85) |
| Histological type | |
| Squamous cell carcinoma | 147 (85.0) |
| Adenocarcinoma | 26 (15.0) |
| FIGO stage | |
| IB | 1 (0.6) |
| IIA | 3 (1.7) |
| IIB | 73 (42.2) |
| IIIA | 9 (5.2) |
| IIIB | 3 (1.7) |
| IIIC1 | 39 (22.5) |
| IIIC2 | 22 (12.7) |
| IVA | 23 (13.3) |
| Radiotherapy technique | |
| 3D conformal radiotherapy | 87 (50.3) |
| Intensity-modulated radiotherapy | 66 (38.1) |
| Volumetric modulated arc therapy | 20 (11.6) |
| Median radiotherapy dose (range), Gy | |
| Pelvic nodes (prophylactic) | 46.0 (26.0–50.4) |
| Metastatic nodes | 57.5 (52.5–61.0) |
| Brachytherapy boost | 28.0 (4.0–42.0) |
| Authors, Year | Aims | Methods | Results | Conclusions |
|---|---|---|---|---|
| Zang L, 2021 [41] | Develop a nomogram to predict OS in FIGO II-III CC treated with RT. | Retrospective study (469 patients). Cox regression and nomogram model creation. | C-index for nomogram = 0.71, better than FIGO staging. | Nomogram outperformed FIGO staging in predicting OS, offering a valuable clinical tool. |
| Leetanaporn K, 2022 [43] | Evaluate the predictive value of the HALP index on oncological outcomes in LACC patients. | Retrospective study (1588 patients). HALP cutoff identified using X-tile for survival model building. | HALP > 22.2 associated with better PFS and OS; improved model accuracy. | HALP is an independent predictor of survival, enhancing oncological outcome predictions. |
| Abdalvand N, 2022 [42] | Predict BRT response in LACC using clinical, physical, and dosimetric parameters via ML models. | Retrospective study (111 patients). ML models (LASSO, Ridge, SVM, Random Forest). | Random Forest models (AUC 0.82) outperformed reference models for response prediction. | ML models, especially Random Forest, improve BRT outcome prediction. |
| Ferioli M, 2023 [28] | Compare classical prognostic factors versus II in LACC. | Comprehensive retrospective analysis of IIs and survival (multivariate Cox). | Classical factors (age, tumor stage, Hb) were better predictors of OS than IIs. | Classical factors outperformed IIs for survival prediction in LACC patients. |
| Medici F, 2023 [29] | Assess the impact of systemic IIs on survival outcomes in LACC. | Retrospective study (173 patients). Multivariate Cox regression analysis of pretreatment IIs. | Hb levels, CRT dose, and age were significant predictors of OS; no IIs correlated with DFS or OS. | Classical prognostic factors outperform systemic IIs in predicting survival. |
| Luo Y, 2023 [39] | Develop a nomogram using TK1, inflammatory markers, and tumor markers to predict recurrence post-RT in intermediate-advanced CC. | Retrospective study (114 patients). Logistic regression for nomogram creation and validation (C-index and calibration curves). | TK1 and SCC antigen were independent predictors of recurrence (C-index 0.79). | Nomogram based on TK1 and inflammatory markers is more reliable than TNM staging for recurrence prediction. |
| Xu C, 2023 [41] | Develop a hybrid radiomics model to predict OS in CC patients receiving CCRT. | Retrospective study (367 patients). Handcrafted and DL-based radiomics features from CT for hybrid nomogram. | AUCs for OS = 0.83, 0.77, and 0.87 (1, 3, 5-year). | Hybrid radiomics model predicts OS effectively, aiding risk stratification in CC patients. |
| Hua L, 2024 [40] | Construct a survival prediction model for LACC patients treated with CCRT ± adjuvant chemotherapy. | Retrospective analysis (482 patients). Cox and LASSO regression for model building. | Validated risk factors for PFS and OS (AUC for OS = 0.94 at 1 year). | Supports accurate survival prediction and potential benefits of adjuvant chemotherapy for high-risk LACC. |
| Medici F, 2024 [30] | Evaluate sarcopenic obesity as a prognostic factor in CC outcomes. | Retrospective study (173 patients). Kaplan-Meier and Cox regression analysis. | Sarcopenic obesity was an independent predictor of worse DFS and OS. | Sarcopenic obesity is a strong prognostic factor and should be considered in treatment planning. |
| Present study | Evaluate prognostic significance of pretreatment, nutritional, systemic inflammatory markers, and body composition in LACC. | Retrospective analysis of 173 patients using LASSO and CART models to predict LC, MFS, DFS, and OS | Hemoglobin levels, ECOG status, and tumor size were key predictors of outcomes. ROC AUCs ranged from moderate to strong (AUC up to 0.851 for 2-year OS). | Predictive models effectively identified patients at a higher risk of poor outcomes, supporting personalized treatment strategies in LACC. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medici, F.; Ferioli, M.; Zamfir, A.A.; Buwenge, M.; Macchia, G.; Deodato, F.; Castellucci, P.; Tagliaferri, L.; Perrone, A.M.; De Iaco, P.; et al. Integrating Novel and Classical Prognostic Factors in Locally Advanced Cervical Cancer: A Machine Learning-Based Predictive Model (ESTHER Study). J. Pers. Med. 2025, 15, 153. https://doi.org/10.3390/jpm15040153
Medici F, Ferioli M, Zamfir AA, Buwenge M, Macchia G, Deodato F, Castellucci P, Tagliaferri L, Perrone AM, De Iaco P, et al. Integrating Novel and Classical Prognostic Factors in Locally Advanced Cervical Cancer: A Machine Learning-Based Predictive Model (ESTHER Study). Journal of Personalized Medicine. 2025; 15(4):153. https://doi.org/10.3390/jpm15040153
Chicago/Turabian StyleMedici, Federica, Martina Ferioli, Arina Alexandra Zamfir, Milly Buwenge, Gabriella Macchia, Francesco Deodato, Paolo Castellucci, Luca Tagliaferri, Anna Myriam Perrone, Pierandrea De Iaco, and et al. 2025. "Integrating Novel and Classical Prognostic Factors in Locally Advanced Cervical Cancer: A Machine Learning-Based Predictive Model (ESTHER Study)" Journal of Personalized Medicine 15, no. 4: 153. https://doi.org/10.3390/jpm15040153
APA StyleMedici, F., Ferioli, M., Zamfir, A. A., Buwenge, M., Macchia, G., Deodato, F., Castellucci, P., Tagliaferri, L., Perrone, A. M., De Iaco, P., Strigari, L., Bazzocchi, A., Rizzo, S. M. R., Donati, C. M., Arcelli, A., Fanti, S., Morganti, A. G., & Cilla, S. (2025). Integrating Novel and Classical Prognostic Factors in Locally Advanced Cervical Cancer: A Machine Learning-Based Predictive Model (ESTHER Study). Journal of Personalized Medicine, 15(4), 153. https://doi.org/10.3390/jpm15040153













