The Prognostic Impact of the Tumor Immune Microenvironment in Synovial Sarcoma: An Immunohistochemical Analysis Using Digital Pathology and Conventional Interpretation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Histopathology
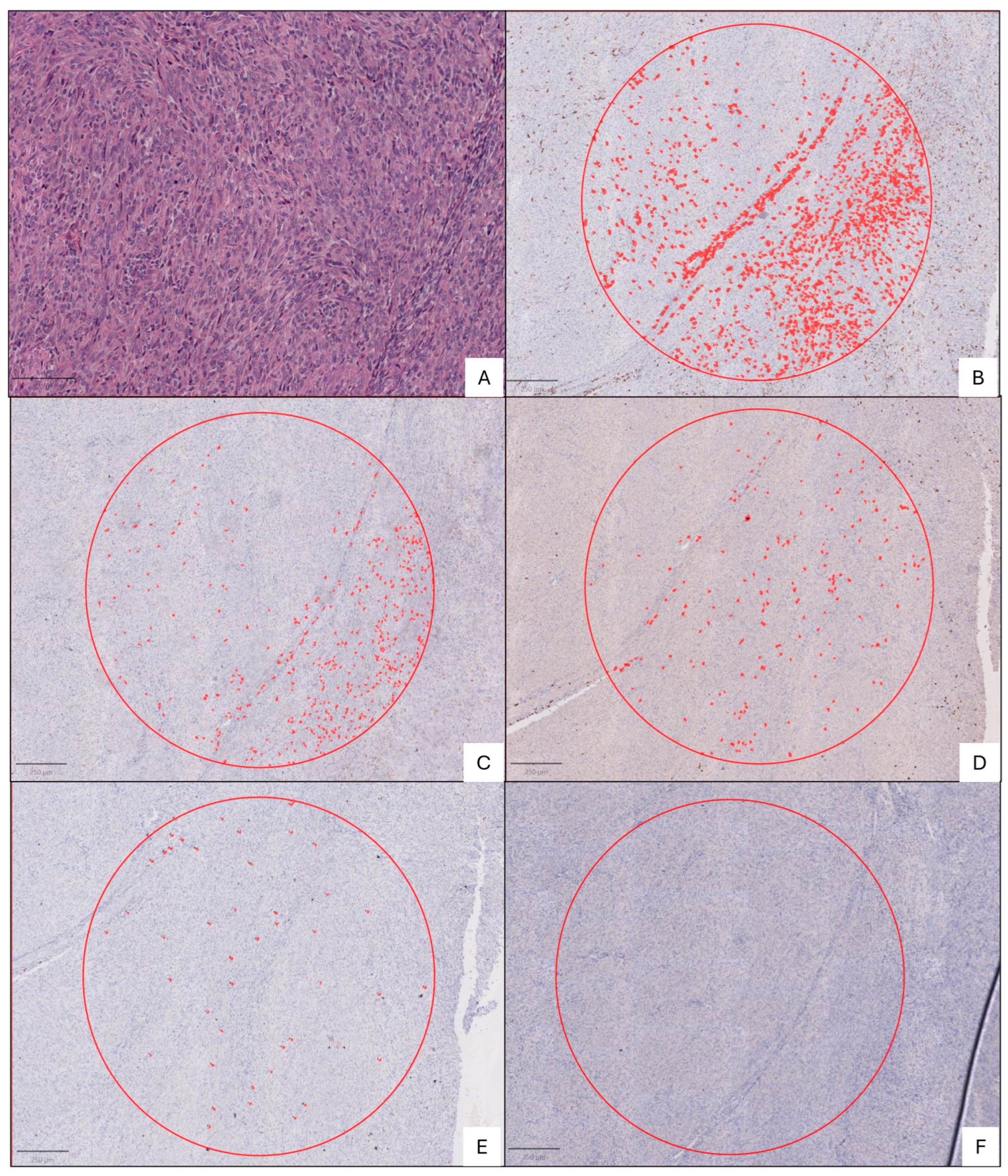
2.2. Immunohistochemical Analysis
2.3. Statistical Analysis
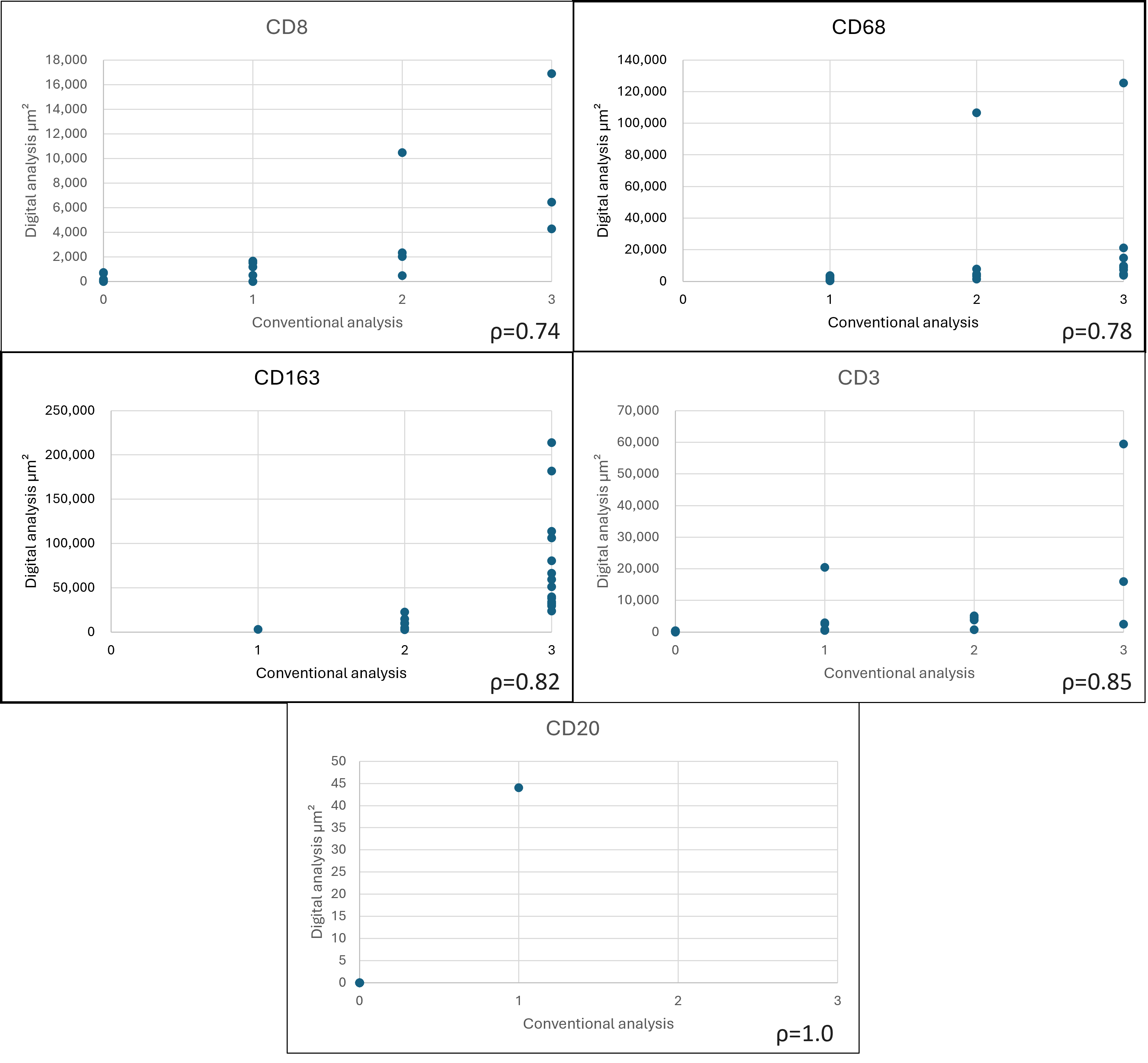
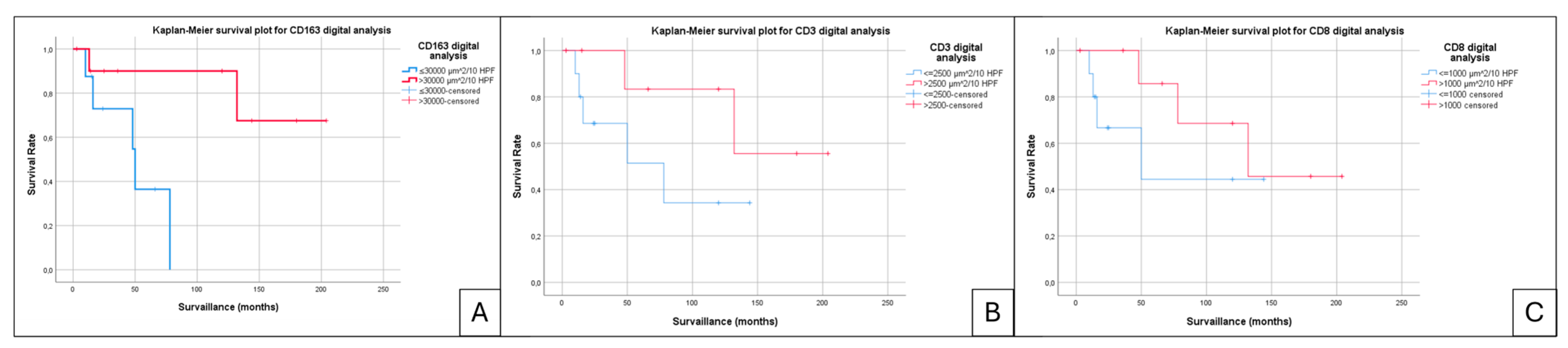
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suurmeijer, A.J.H.; Nielsen, T.O.; Ladanyi, M. Synovial Sarcoma. In World Health Organization Classification of Soft Tissue and Bone Tumours; The WHO Classification of Tumorours Editorial Bard, Ed.; IARC Press: Lyon, France, 2020; pp. 290–293. [Google Scholar]
- Gazendam, A.M.; Popovic, S.; Munir, S.; Parasu, N.; Wilson, D.; Ghert, M. Synovial Sarcoma: A Clinical Review. Curr. Oncol. 2021, 28, 1909–1920. [Google Scholar] [CrossRef]
- Sultan, I.; Rodriguez-Galindo, C.; Saab, R.; Yasir, S.; Casanova, M.; Ferrari, A. Comparing Children and Adults with Synovial Sarcoma in the Surveillance, Epidemiology, and End Results Program, 1983 to 2005: An Analysis of 1268 Patients. Cancer 2009, 115, 3537–3547. [Google Scholar] [CrossRef]
- De Logu, F.; Ugolini, F.; Caporalini, C.; Palomba, A.; Simi, S.; Portelli, F.; Campanacci, D.A.; Beltrami, G.; Massi, D.; Nassini, R. TRPA1 Expression in Synovial Sarcoma May Support Neural Origin. Biomolecules 2020, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, F.; Alsina, A.C.; Muratori, F.; Scoccianti, G.; Neri, E.; Kaya, H.; Sabah, D.; Capanna, R.; Campanacci, D.A. Tumor Size and Surgical Margins Are Important Prognostic Factors of Synovial Sarcoma—A Retrospective Study. J. Orthop. 2023, 42, 74. [Google Scholar] [CrossRef]
- Soft Tissue Sarcoma—NCCN Guidelines, version 4.2024; National Comprehensive Cancer Nework®: Plymouth Meeting, PA, USA, 2024.
- PDQ Pediatric Treatment Editorial Board. Childhood Soft Tissue Sarcoma Treatment (PDQ®). In PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD, USA, 2023. [Google Scholar]
- Espinoza, A.F.; Shetty, P.B.; Jacobson, J.C.; Todd, H.; Harrell, K.; Trappey, A.F.; Doski, J.; Castro, E.C.; Montgomery, N.I.; Okcu, M.F.; et al. The Impact of Margins and Re-Resection in Pediatric Synovial Sarcoma. Cancer Med. 2024, 13, e70207. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B Cells Are Associated with Survival and Immunotherapy Response in Sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef]
- Giner, F.; Medina-Ceballos, E.; López-Reig, R.; Machado, I.; López-Guerrero, J.A.; Navarro, S.; Rubio-Martínez, L.A.; Espino, M.; Mayordomo-Aranda, E.; Llombart-Bosch, A. The Combined Immunohistochemical Expression of GLI1 and BCOR in Synovial Sarcomas for the Identification of Three Risk Groups and Their Prognostic Outcomes: A Study of 52 Patients. Int. J. Mol. Sci. 2024, 25, 7615. [Google Scholar] [CrossRef]
- Balduit, A.; Agostinis, C.; Bulla, R. Beyond the Norm: The Emerging Interplay of Complement System and Extracellular Matrix in the Tumor Microenvironment. Semin. Immunol. 2025, 77, 101929. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, Z.; Wang, Q.; Deng, H. Prognostic Value of Tumor-Infiltrating Lymphocyte Subtypes and Microorganisms in Triple-Negative Breast Cancer. J. Cancer Res. Ther. 2024, 20, 1983–1990. [Google Scholar] [CrossRef]
- Ikeda, H. Cancer Immunotherapy in Progress—An Overview of the Past 130 Years. Int. Immunol. 2025, 37, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Cancer Immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef]
- Wood, G.E.; Meyer, C.; Petitprez, F.; D’Angelo, S.P. Immunotherapy in Sarcoma: Current Data and Promising Strategies. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432234. [Google Scholar] [CrossRef]
- Somaiah, N.; Conley, A.P.; Parra, E.R.; Lin, H.; Amini, B.; Solis Soto, L.; Salazar, R.; Barreto, C.; Chen, H.; Gite, S.; et al. Durvalumab plus Tremelimumab in Advanced or Metastatic Soft Tissue and Bone Sarcomas: A Single-Centre Phase 2 Trial. Lancet Oncol. 2022, 23, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Luk, S.J.; IJsselsteijn, M.E.; Somarakis, A.; Acem, I.; de Bruijn, I.B.; Szuhai, K.; Bovee, J.V.M.G.; de Miranda, N.F.C.C.; Falkenburg, J.H.F.; Heemskerk, M.H.M. Immunological Differences between Monophasic and Biphasic Synovial Sarcoma with Implications for Immunotherapy. Cancer Immunol. Immunother. 2024, 74, 31. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.; Büclein, V.; Kampmann, E.; Knösel, T.; Nössner, E.; Subklewe, M.; Lindner, L. Sarcoma 1412P- Dissecting the Role of Tumor-Infiltrating Lymphocytes (TIL) in Patients with High-Risk Soft-Tissue Sarcoma (STS) Receiving Neo-Adjuvant Chemotherapy (NAC) with Regional Hyperthermia (RHT). Annals of Oncology 2016, 27, vi483–vi492. [Google Scholar] [CrossRef]
- Issels, R.D.; Noessner, E.; Lindner, L.H.; Schmidt, M.; Albertsmeier, M.; Blay, J.Y.; Stutz, E.; Xu, Y.; Buecklein, V.; Altendorf-Hofmann, A.; et al. Immune Infiltrates in Patients with Localised High-Risk Soft Tissue Sarcoma Treated with Neoadjuvant Chemotherapy without or with Regional Hyperthermia: A Translational Research Program of the EORTC 62961-ESHO 95 Randomised Clinical Trial. Eur. J. Cancer 2021, 158, 123–132. [Google Scholar] [CrossRef]
- Sorbye, S.W.; Kilvaer, T.; Valkov, A.; Donnem, T.; Smeland, E.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.T. Prognostic Impact of Lymphocytes in Soft Tissue Sarcomas. PLoS ONE 2011, 6, e14611. [Google Scholar] [CrossRef]
- Raj, S.; Miller, L.D.; Triozzi, P.L. Addressing the Adult Soft Tissue Sarcoma Microenvironment with Intratumoral Immunotherapy. Sarcoma 2018, 2018, 9305294. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Shoushtari, A.N.; Agaram, N.P.; Kuk, D.; Qin, L.X.; Carvajal, R.D.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of Tumor-Infiltrating Lymphocytes and PD-L1 Expression in the Soft Tissue Sarcoma Microenvironment. Hum. Pathol. 2015, 46, 357–365. [Google Scholar] [CrossRef]
- Oike, N.; Kawashima, H.; Ogose, A.; Hotta, T.; Hatano, H.; Ariizumi, T.; Sasaki, T.; Yamagishi, T.; Umezu, H.; Endo, N. Prognostic Impact of the Tumor Immune Microenvironment in Synovial Sarcoma. Cancer Sci. 2018, 109, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Nyström, H.; Jönsson, M.; Nilbert, M.; Carneiro, A. Immune-Cell Infiltration in High-Grade Soft Tissue Sarcomas; Prognostic Implications of Tumor-Associated Macrophages and B-Cells. Acta Oncol. 2023, 62, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dancsok, A.R.; Gao, D.; Lee, A.F.; Steigen, S.E.; Blay, J.Y.; Thomas, D.M.; Maki, R.G.; Nielsen, T.O.; Demicco, E.G. Tumor-Associated Macrophages and Macrophage-Related Immune Checkpoint Expression in Sarcomas. Oncoimmunology 2020, 9, 1747340. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, D.; Fujiwara, Y.; Horlad, H.; Saito, Y.; Iriki, T.; Tsuboki, J.; Cheng, P.; Nakagata, N.; Mizuta, H.; Bekki, H.; et al. CD163 Is Required for Protumoral Activation of Macrophages in Human and Murine Sarcoma. Cancer Res. 2018, 78, 3255–3266. [Google Scholar] [CrossRef]
- Ganjoo, K.N.; Witten, D.; Patel, M.; Espinosa, I.; La, T.; Tibshirani, R.; Van De Rijn, M.; Jacobs, C.; West, R.B. The Prognostic Value of Tumor-Associated Macrophages in Leiomyosarcoma: A Single Institution Study. Am. J. Clin. Oncol. 2011, 34, 82–86. [Google Scholar] [CrossRef]
- Kather, J.N.; Hörner, C.; Weis, C.A.; Aung, T.; Vokuhl, C.; Weiss, C.; Scheer, M.; Marx, A.; Simon-Keller, K. CD163+ Immune Cell Infiltrates and Presence of CD54+ Microvessels Are Prognostic Markers for Patients with Embryonal Rhabdomyosarcoma. Sci. Rep. 2019, 9, 9211. [Google Scholar] [CrossRef]
- Lee, C.H.; Espinosa, I.; Vrijaldenhoven, S.; Subramanian, S.; Montgomery, K.D.; Zhu, S.; Marinelli, R.J.; Peterse, J.L.; Poulin, N.; Nielsen, T.O.; et al. Prognostic Significance of Macrophage Infiltration in Leiomyosarcomas. Clin. Cancer Res. 2008, 14, 1423–1430. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, C.; Zhang, X.; Hua, Q. ALOX5AP Is an Indicator for High CD8 Lymphocyte Infiltration and “Hot” Tumor Microenvironment in Osteosarcoma: A Bioinformatic Study. Biochem. Genet. 2023, 61, 2363–2381. [Google Scholar] [CrossRef]
- Ren, E.; Deng, Y.; Yuan, W.; Wu, Z.; Zhang, G.; Xie, Q. An Immune-Related Gene Signature for Determining Ewing Sarcoma Prognosis Based on Machine Learning. J. Cancer Res. Clin. Oncol. 2021, 147, 153–165. [Google Scholar] [CrossRef]
- Wisdom, A.J.; Mowery, Y.M.; Hong, C.S.; Himes, J.E.; Nabet, B.Y.; Qin, X.; Zhang, D.; Chen, L.; Fradin, H.; Patel, R.; et al. Single Cell Analysis Reveals Distinct Immune Landscapes in Transplant and Primary Sarcomas That Determine Response or Resistance to Immunotherapy. Nat. Commun. 2020, 11, 6410. [Google Scholar] [CrossRef]
- Möller, K.; Knöll, M.; Bady, E.; Schmerder, M.J.; Rico, S.D.; Kluth, M.; Hube-Magg, C.; Blessin, N.C.; Mandelkow, T.; Lennartz, M.; et al. PD-L1 Expression and CD8 Positive Lymphocytes in Human Neoplasms: A Tissue Microarray Study on 11,838 Tumor Samples. Cancer Biomark. 2023, 36, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Liu, Y.; Yuan, T.; Wang, H.; Li, B.; Jiang, Y.; Mo, X.; Lei, Y.; Xiao, Y.; Dong, S.; et al. Predicted CD4+ T Cell Infiltration Levels Could Indicate Better Overall Survival in Sarcoma Patients. J. Int. Med. Res. 2021, 49, 1. [Google Scholar] [CrossRef] [PubMed]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guérin, M.; Biton, J.; Ouakrim, H.; et al. Macrophages Impede CD8 T Cells from Reaching Tumor Cells and Limit the Efficacy of Anti–PD-1 Treatment. Proc. Natl. Acad. Sci. USA 2018, 115, E4041–E4050. [Google Scholar] [CrossRef] [PubMed]
- Pell, R.; Oien, K.; Robinson, M.; Pitman, H.; Rajpoot, N.; Rittscher, J.; Snead, D.; Verrill, C.; Driskell, O.J.; Hall, A.; et al. The Use of Digital Pathology and Image Analysis in Clinical Trials. J. Pathol. Clin. Res. 2019, 5, 81. [Google Scholar] [CrossRef]
- Moscalu, M.; Moscalu, R.; Dascălu, C.G.; Țarcă, V.; Cojocaru, E.; Costin, I.M.; Țarcă, E.; Șerban, I.L. Histopathological Images Analysis and Predictive Modeling Implemented in Digital Pathology—Current Affairs and Perspectives. Diagnostics 2023, 13, 2379. [Google Scholar] [CrossRef]
- Fassler, D.J.; Abousamra, S.; Gupta, R.; Chen, C.; Zhao, M.; Paredes, D.; Batool, S.A.; Knudsen, B.S.; Escobar-Hoyos, L.; Shroyer, K.R.; et al. Deep Learning-Based Image Analysis Methods for Brightfield-Acquired Multiplex Immunohistochemistry Images. Diagn. Pathol. 2020, 15, 100. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Sirinukunwattana, K.; Rittscher, J.; Mertz, K.D. Precision Immunoprofiling by Image Analysis and Artificial Intelligence. Virchows Arch. 2018, 474, 511. [Google Scholar] [CrossRef]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital Pathology and Artificial Intelligence in Translational Medicine and Clinical Practice. Mod. Pathol. 2021, 35, 23–32. [Google Scholar] [CrossRef]
- Capobianco, E. High-Dimensional Role of AI and Machine Learning in Cancer Research. Br. J. Cancer 2022, 126, 523–532. [Google Scholar] [CrossRef]


| Score | CD163 | CD68 | CD8 | CD20 | CD3 |
|---|---|---|---|---|---|
| 0 | 0 | 6 | 25 | 50 | 20 |
| 1 | 3 | 18 | 11 | 1 | 18 |
| 2 | 17 | 13 | 9 | 0 | 8 |
| 3 | 30 | 15 | 7 | 1 | 6 |
| Total cases | 50 | 52 | 52 | 52 | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Ceballos, E.; Giner, F.; Machado, I.; Heras-Morán, B.; Espino, M.; Navarro, S.; Llombart-Bosch, A. The Prognostic Impact of the Tumor Immune Microenvironment in Synovial Sarcoma: An Immunohistochemical Analysis Using Digital Pathology and Conventional Interpretation. J. Pers. Med. 2025, 15, 169. https://doi.org/10.3390/jpm15050169
Medina-Ceballos E, Giner F, Machado I, Heras-Morán B, Espino M, Navarro S, Llombart-Bosch A. The Prognostic Impact of the Tumor Immune Microenvironment in Synovial Sarcoma: An Immunohistochemical Analysis Using Digital Pathology and Conventional Interpretation. Journal of Personalized Medicine. 2025; 15(5):169. https://doi.org/10.3390/jpm15050169
Chicago/Turabian StyleMedina-Ceballos, Emilio, Francisco Giner, Isidro Machado, Begoña Heras-Morán, Mónica Espino, Samuel Navarro, and Antonio Llombart-Bosch. 2025. "The Prognostic Impact of the Tumor Immune Microenvironment in Synovial Sarcoma: An Immunohistochemical Analysis Using Digital Pathology and Conventional Interpretation" Journal of Personalized Medicine 15, no. 5: 169. https://doi.org/10.3390/jpm15050169
APA StyleMedina-Ceballos, E., Giner, F., Machado, I., Heras-Morán, B., Espino, M., Navarro, S., & Llombart-Bosch, A. (2025). The Prognostic Impact of the Tumor Immune Microenvironment in Synovial Sarcoma: An Immunohistochemical Analysis Using Digital Pathology and Conventional Interpretation. Journal of Personalized Medicine, 15(5), 169. https://doi.org/10.3390/jpm15050169









