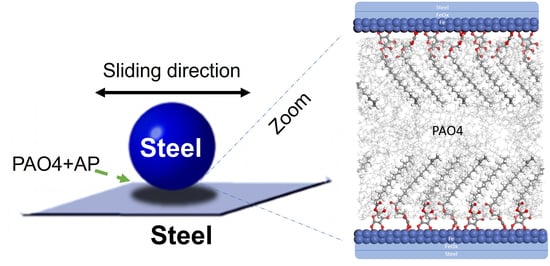
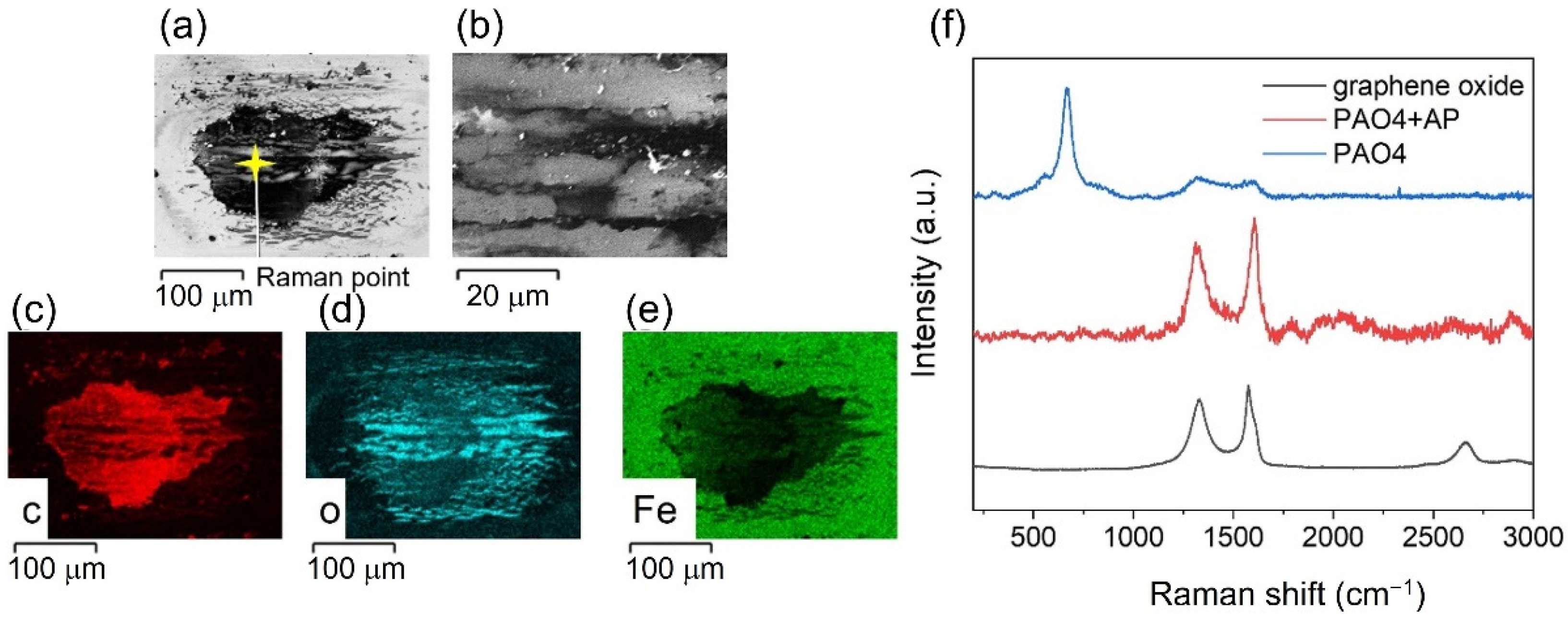
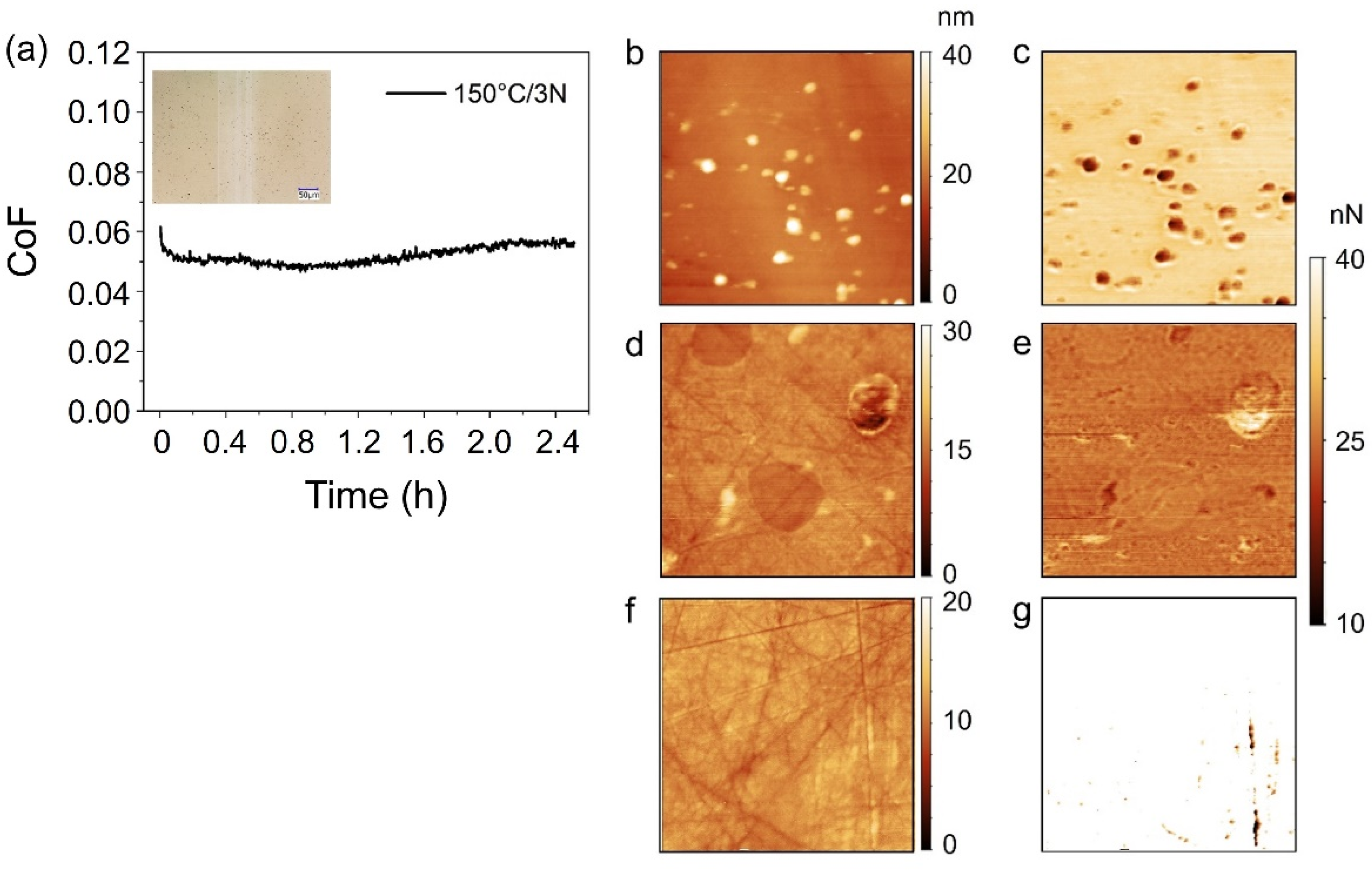
Ascorbyl Palmitate-Vitamin C Effective Friction Modifier and Wear Inhibitor for Steel in a PAO Base Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials & Tribometer
2.2. Surface Analysis
3. Results
3.1. Friction and Wear Results
3.2. Antioxidant Properties of AP under Pmax ≥ 2.12 GPa
3.3. Low Friction Properties of AP under Pmax ≤ 2.12 GPa
3.4. Comparison of AP with Conventional Oil Additives
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spikes, H. Friction modifier additives. Tribol. Lett. 2015, 60, 5. [Google Scholar] [CrossRef] [Green Version]
- Kania, D.; Yunus, R.; Omar, R.; Rashid, S.A.; Jan, B.M. A review of biolubricants in drilling fluids: Recent research, performance, and applications. J. Pet. Sci. Eng. 2015, 135, 177–184. [Google Scholar] [CrossRef]
- Syahrullail, S.; Hariz, M.A.M.; Hamid, M.A.; Bakar, A.A. Friction characteristic of mineral oil containing palm fatty acid distillate using four ball tribo-tester. Procedia Eng. 2013, 68, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Bouchet, M.I.D.B.; Martin, J.M.; Forest, C.; Le Mogne, T.; Mazarin, M.; Avila, J.; Asensio, M.C.; Fisher, G.L. Tribochemistry of unsaturated fatty acids as friction modifiers in (bio) diesel fuel. RSC Adv. 2017, 7, 33120–33131. [Google Scholar] [CrossRef] [Green Version]
- Wood, M.H.; Casford, M.T.; Steitz, R.; Zarbakhsh, A.; Welbourn, R.J.L.; Clarke, S.M. Comparative Adsorption of Saturated and Unsaturated Fatty Acids at the Iron Oxide/Oil Interface. Langmuir 2016, 32, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Cottington, R.L.; Shafrin, E.G.; Zisman, W.A. Physical properties of monolayers at the solid/air interface. III. Friction and durability of films on stainless steel. J. Phys. Chem. C 1958, 62, 513–518. [Google Scholar] [CrossRef]
- Bowden, F.P.; Tabor, D. The Friction and Lubrication of Solids; Clarendon Press: Oxford, UK, 1971. [Google Scholar]
- Abouhadid, F.; Crespo, A.; Morgado, N.; Mazuyer, D.; Cayer-Barrioz, J. Friction Laws for Saturated/Unsaturated Fatty Acid Layers. Tribol. Lett. 2021, 69, 46. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Doig, M.; Warrens, C.P.; Camp, P.J. Structure and friction of stearic acid and oleic acid films adsorbed on iron oxide surfaces in squalane. Langmuir 2014, 30, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Loehlé, S.; Matta, C.; Minfray, C.; Le Mogne, T.; Iovine, R.; Obara, Y.; Miyamoto, A.; Martin, J.M. Mixed lubrication of steel by C18 fatty acids revisited. Part I: Toward the formation of carboxylate. Tribol. Int. 2015, 82, 218–227. [Google Scholar] [CrossRef]
- Jahanmir, S.; Beltzer, M. Effect of additive molecular structure on friction coefficient and adsorption. J. Tribol.-T. Asme J. 1986, 108, 109–116. [Google Scholar] [CrossRef]
- Fry, B.M.; Chui, M.Y.; Moody, G.; Wong, J.S. Interactions between organic friction modifier additives. Tribol. Int. 2020, 151, 106438. [Google Scholar] [CrossRef]
- Pominov, A.; Müller-Hillebrand, J.; Träg, J.; Zahn, D. Interaction Models and Molecular Simulation Systems of Steel–Organic Friction Modifier Interfaces. Tribol. Lett. 2021, 69, 14. [Google Scholar] [CrossRef]
- Zachariah, Z.; Nalam, P.C.; Ravindra, A.; Raju, A.; Mohanlal, A.; Wang, K.; Castillo, R.V.; Espinosa-Marzal, R.M. Correlation between the adsorption and the nanotribological performance of fatty acid-based organic friction modifiers on stainless steel. Tribol. Lett. 2020, 68, 11. [Google Scholar] [CrossRef]
- Guibert, M.; Nauleau, B.; Kapsa, P.; Rigaud, E. Design and Manufacturing of a Reciprocating Linear Tribometer. In Journée Francophones de Tribologie: Tribologie et Couplages Multi-Physique, Lille; Presse Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 2006. [Google Scholar]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Gunawardana, B.; Swedlund, P.J.; Singhal, N.; Nieuwoudt, M.K. Pentachlorophenol dechlorination with zero valent iron: A Raman and GCMS study of the complex role of surficial iron oxides. Environ. Sci. Pollut. Res. 2018, 25, 17797–17806. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Chagas, P.; Da Silva, A.C.; Passamani, E.C.; Ardisson, J.D.; de Oliveira, L.C.A.; Fabris, J.D.; Paniago, R.M.; Monteiro, D.S.; Pereira, M.C. δ-FeOOH: A superparamagnetic material for controlled heat release under AC magnetic field. J. Nanopart. Res. 2013, 15, 1544. [Google Scholar] [CrossRef]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.; da Costa, J.C.D. Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 2014, 4, 4594. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Song, H.; Ma, L.; Chen, X. Magnetite/graphene nanosheet composites: Interfacial interaction and its impact on the durable high-rate performance in lithium-ion batteries. RSC Adv. 2011, 1, 782–791. [Google Scholar] [CrossRef]
- Kim, T.S.; Decker, E.A.; Lee, J. Antioxidant capacities of α-tocopherol, trolox, ascorbic acid, and ascorbyl palmitate in riboflavin photosensitized oil-in-water emulsions. Food Chem. 2012, 133, 68–75. [Google Scholar] [CrossRef]
- Cort, W.M. Antioxidant activity of tocopherols, ascorbyl palmitate, and ascorbic acid and their mode of action. JAOCS 1974, 51, 321. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.E.; Schade, S.G. Ascorbic acid chelates in iron absorption: A role for hydrochloric acid and bile. Gastroenterology 1968, 55, 35–45. [Google Scholar] [CrossRef]
- Simič, R.; Kalin, M. Adsorption mechanisms for fatty acids on DLC and steel studied by AFM and tribological experiments. Appl. Surf. Sci. 2013, 283, 460–470. [Google Scholar] [CrossRef]
- Campen, S.; Green, J.H.; Lamb, G.D.; Atkinson, D.; Spikes, H.A. On the increase in boundary friction with sliding speed. Tribol. Lett. 2012, 48, 237–248. [Google Scholar] [CrossRef]
- Nijenbanning, G.; Venner, C.H.; Moes, H. Film Thickness in Elastohydrodynamically Lubricated Elliptic Contacts. Wear 1994, 176, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Ewen, J.P.; Restrepo, S.E.; Morgan, N.; Dini, D. Nonequilibrium molecular dynamics simulations of stearic acid adsorbed on iron surfaces with nanoscale roughness. Tribol. Int. 2017, 107, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Benedini, L.; Schulz, E.P.; Messina, P.V.; Palma, S.D.; Allemandi, D.A.; Schulz, P.C. The ascorbyl palmitate-water system: Phase diagram and state of water. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 178–185. [Google Scholar] [CrossRef]
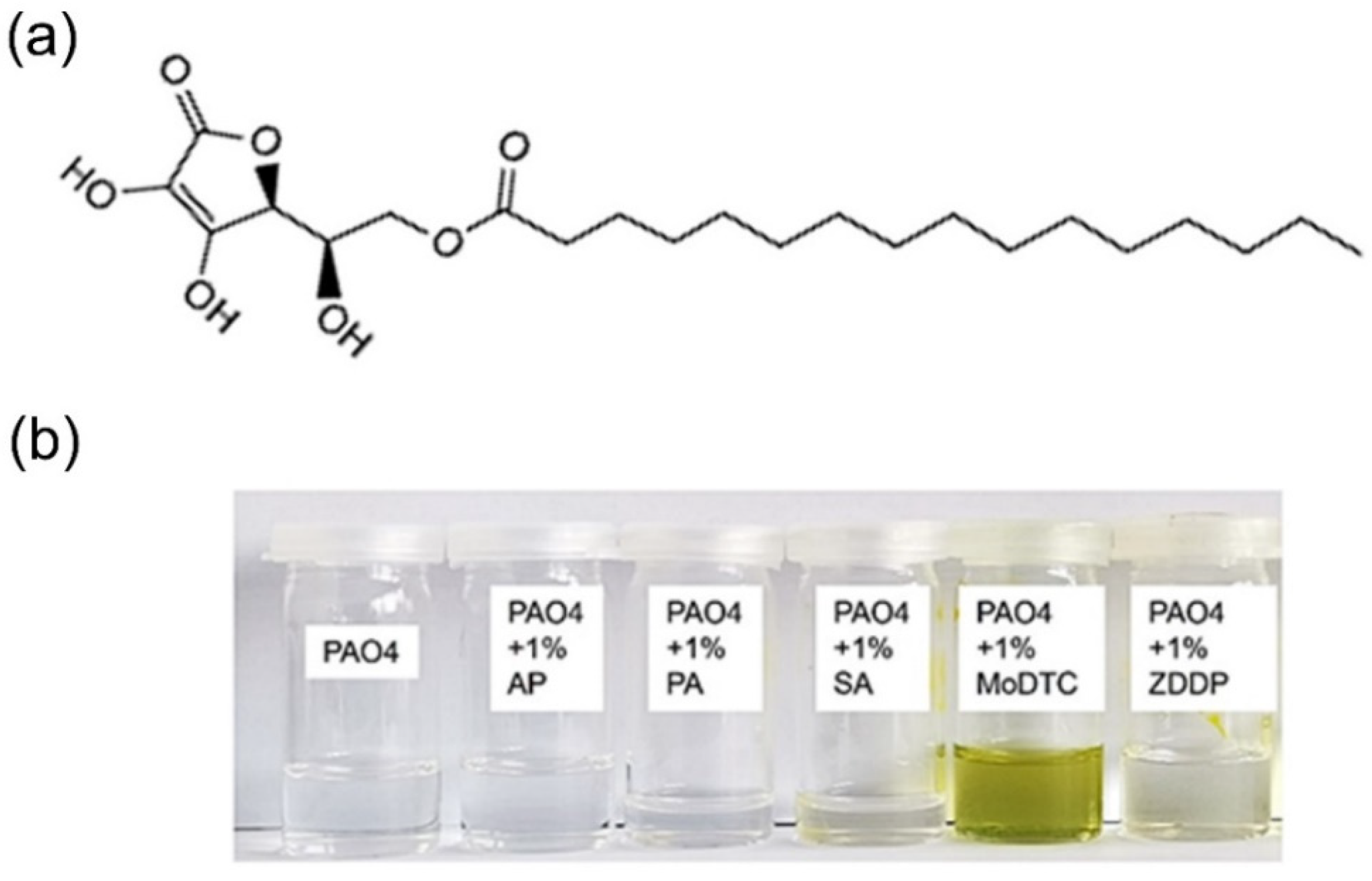


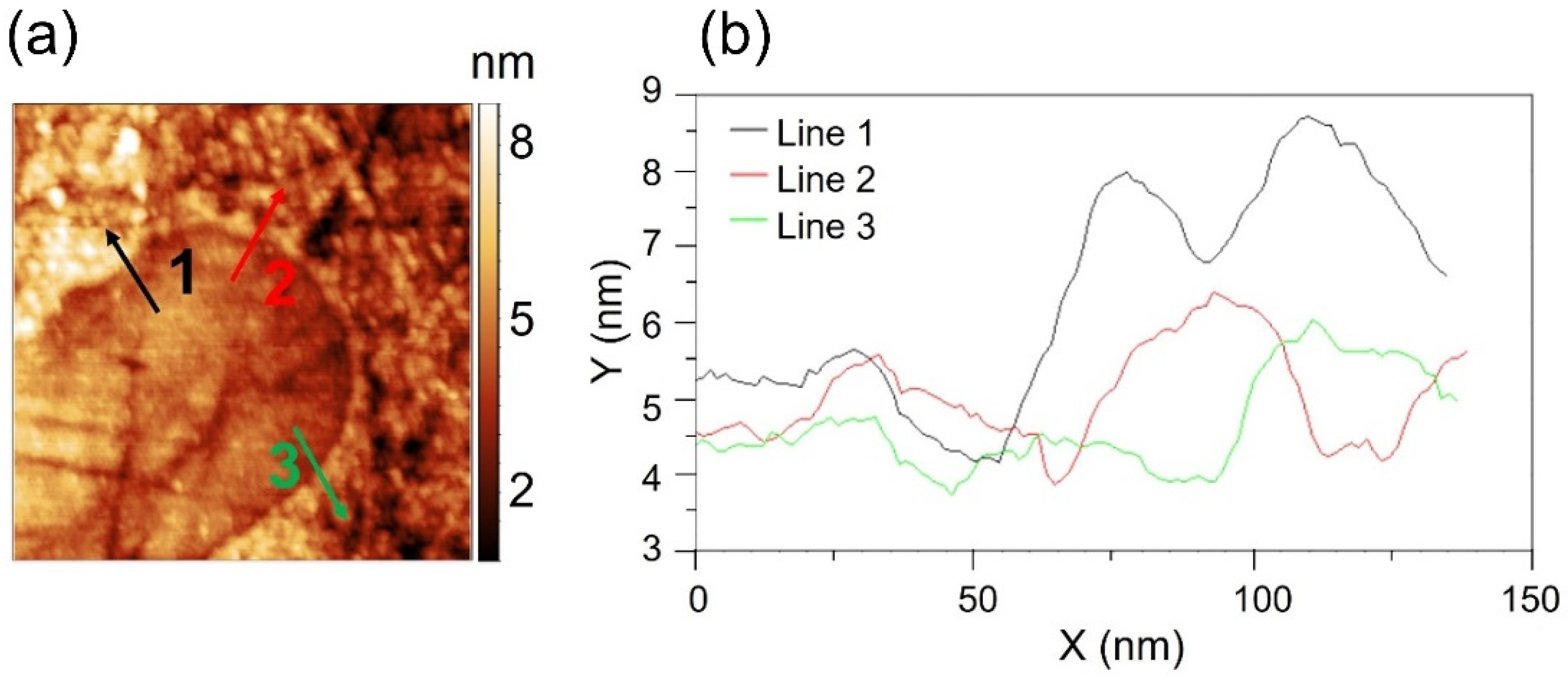
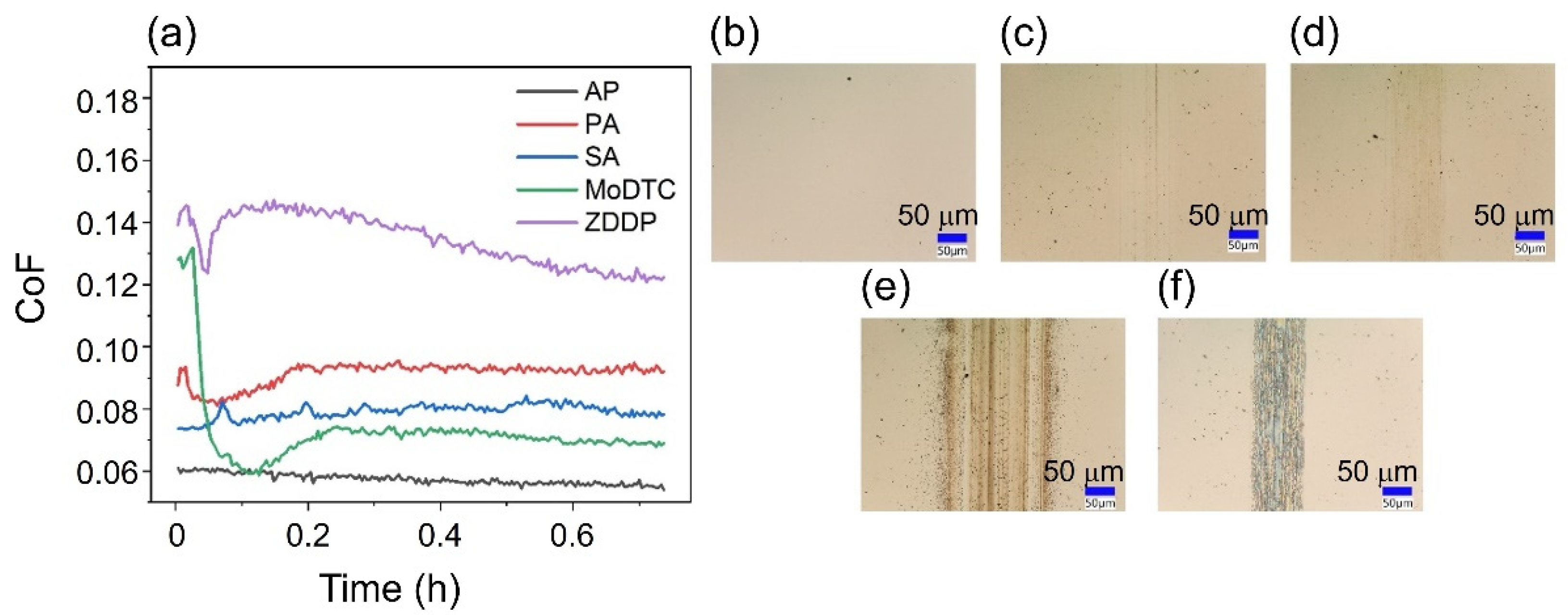
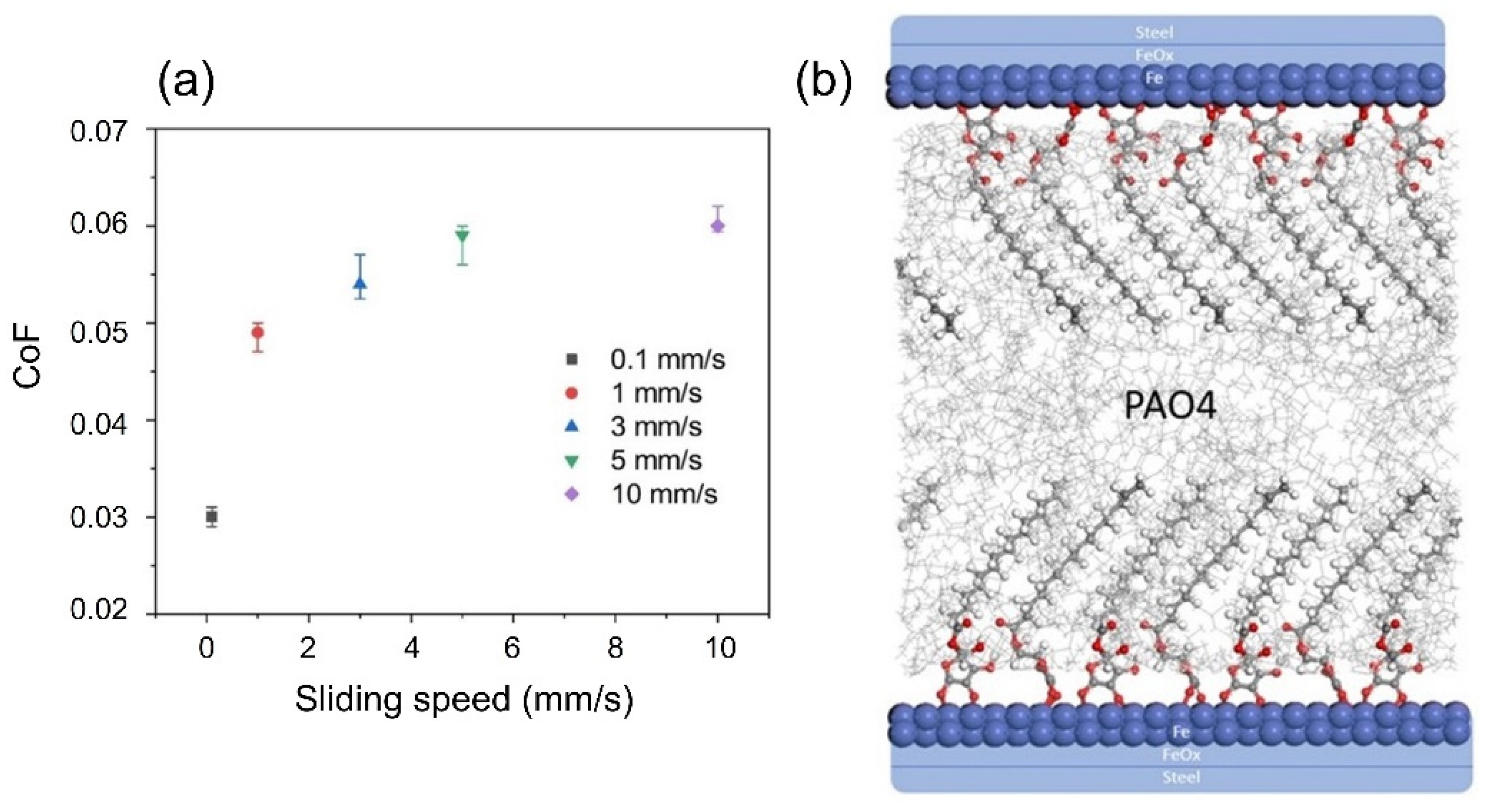
| Load (N) | 3 | 10 | 50 | 100 | 150 | 200 |
| Pmax (GPa) | 0.58 | 0.86 | 1.47 | 1.86 | 2.12 | 2.34 |
| DHertzian (μm) | 100 | 174 | 260 | 320 | 360 | 400 |
| PAO4 + 1 wt% AP | ||||||
| Dball (μm) | - | - | - | - | 195 | 363 |
| Dball/DHertzian | - | - | - | - | 0.54 | 0.91 |
| PAO4 | ||||||
| Dball (μm) | 126 | 183 | 400 | 412 | 494 | 516 |
| Dball/DHertzian | 1.26 | 1.05 | 1.54 | 1.28 | 1.37 | 1.29 |
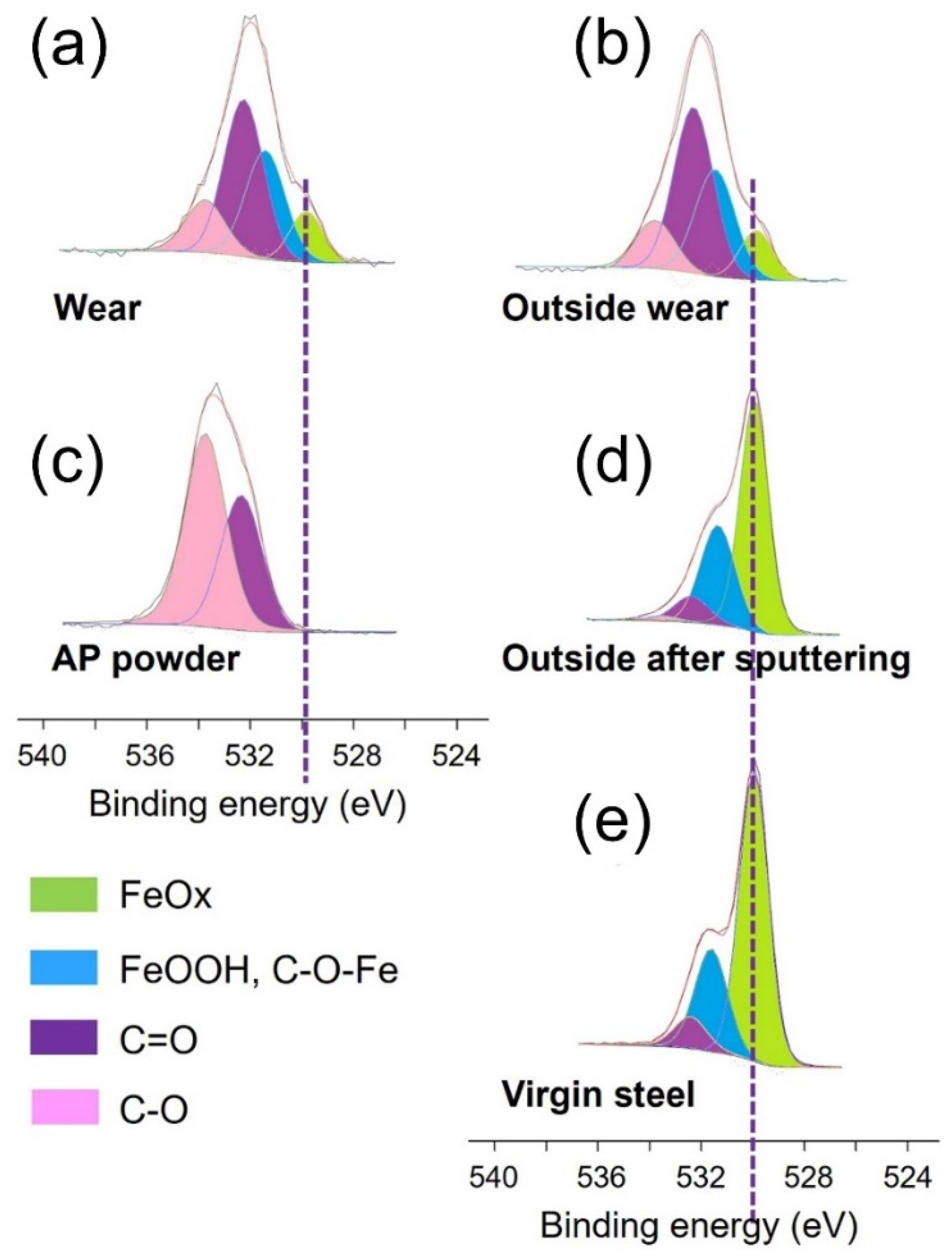
| Inside Steel Wear | O1s | C1s | Fe2p3/2 | |||
|---|---|---|---|---|---|---|
| Percentage (%) | 36.6 | 59.2 | 4.2 | |||
| FeOx | FeOH, C-O-Fe | C=O | C-O | - | - | |
| Position (eV) | 529.9 | 531.4 | 532.4 | 533.7 | - | - |
| FHWM (eV) | 1.3 | 1.6 | 1.6 | 1.6 | ||
| Percentage (%) | 3.8 | 10.9 | 15.5 | 6.4 | ||
| AP powder | O1s | C1s | Fe2p3/2 | |||
| Percentage (%) | 23.0 | 77.0 | - | |||
| FeOx | FeOH, C-O-Fe | C=O | C-O | - | - | |
| Position (eV) | - | - | 532.3 | 533.7 | - | - |
| FHWM (eV) | - | - | 1.9 | 1.9 | ||
| Percentage (%) | - | - | 9.7 | 13.3 | ||
| Outside steel wear | O1s | C1s | Fe2p3/2 | |||
| Percentage (%) | 35.4 | 61.4 | 3.2 | |||
| FeOx | FeOH, C-O-Fe | C=O | C-O | - | - | |
| Position (eV) | 529.9 | 531.4 | 532.4 | 533.7 | - | - |
| FHWM (eV) | 1.3 | 1.6 | 1.6 | 1.6 | ||
| Percentage (%) | 3.4 | 8.9 | 16.6 | 6.5 | ||
| Outside steel wear after sputtering | O1s | C1s | Fe2p3/2 | |||
| Percentage (%) | 62.1 | 5.9 | 32.0 | |||
| FeOx | FeOH, C-O-Fe | C=O | C-O | - | - | |
| Position (eV) | 530.0 | 531.4 | 532.4 | 533.6 | - | - |
| FHWM (eV) | 1.3 | 1.6 | 1.6 | 1.6 | ||
| Percentage (%) | 37.0 | 18.7 | 5.3 | 1.1 | ||
| Virgin steel | O1s | C1s | Fe2p3/2 | |||
| Percentage (%) | 57.2 | 22.8 | 20.0 | |||
| FeOx | FeOH, C-O-Fe | C=O | C-O | - | - | |
| Position (eV) | 530.0 | 531.5 | 532.5 | - | ||
| FHWM (eV) | 1.3 | 1.6 | 1.6 | - | ||
| Percentage (%) | 36.5 | 14.9 | 5.8 | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Martin, J.M.; Dubreuil, F.; Thiebaut, B.; Loehle, S.; Lacassagne, C.; De Barros Bouchet, M.-I. Ascorbyl Palmitate-Vitamin C Effective Friction Modifier and Wear Inhibitor for Steel in a PAO Base Oil. Lubricants 2022, 10, 253. https://doi.org/10.3390/lubricants10100253
Long Y, Martin JM, Dubreuil F, Thiebaut B, Loehle S, Lacassagne C, De Barros Bouchet M-I. Ascorbyl Palmitate-Vitamin C Effective Friction Modifier and Wear Inhibitor for Steel in a PAO Base Oil. Lubricants. 2022; 10(10):253. https://doi.org/10.3390/lubricants10100253
Chicago/Turabian StyleLong, Yun, Jean Michel Martin, Frederic Dubreuil, Benoit Thiebaut, Sophie Loehle, Corinne Lacassagne, and Maria-Isabel De Barros Bouchet. 2022. "Ascorbyl Palmitate-Vitamin C Effective Friction Modifier and Wear Inhibitor for Steel in a PAO Base Oil" Lubricants 10, no. 10: 253. https://doi.org/10.3390/lubricants10100253
APA StyleLong, Y., Martin, J. M., Dubreuil, F., Thiebaut, B., Loehle, S., Lacassagne, C., & De Barros Bouchet, M.-I. (2022). Ascorbyl Palmitate-Vitamin C Effective Friction Modifier and Wear Inhibitor for Steel in a PAO Base Oil. Lubricants, 10(10), 253. https://doi.org/10.3390/lubricants10100253