Implications of the Use of Biodiesel on the Longevity and Operation of Particle Filters
Abstract
:1. Introduction
2. Experimental Methods and Materials
2.1. DPF Ash Samples
2.2. X-ray Diffraction
2.3. SEM and EDX
2.4. Focused Ion Beam (FIB) Milling
2.5. X-ray Computed Tomography (CT)
2.6. Differential Scanning Calorimetry (DSC)
3. Results
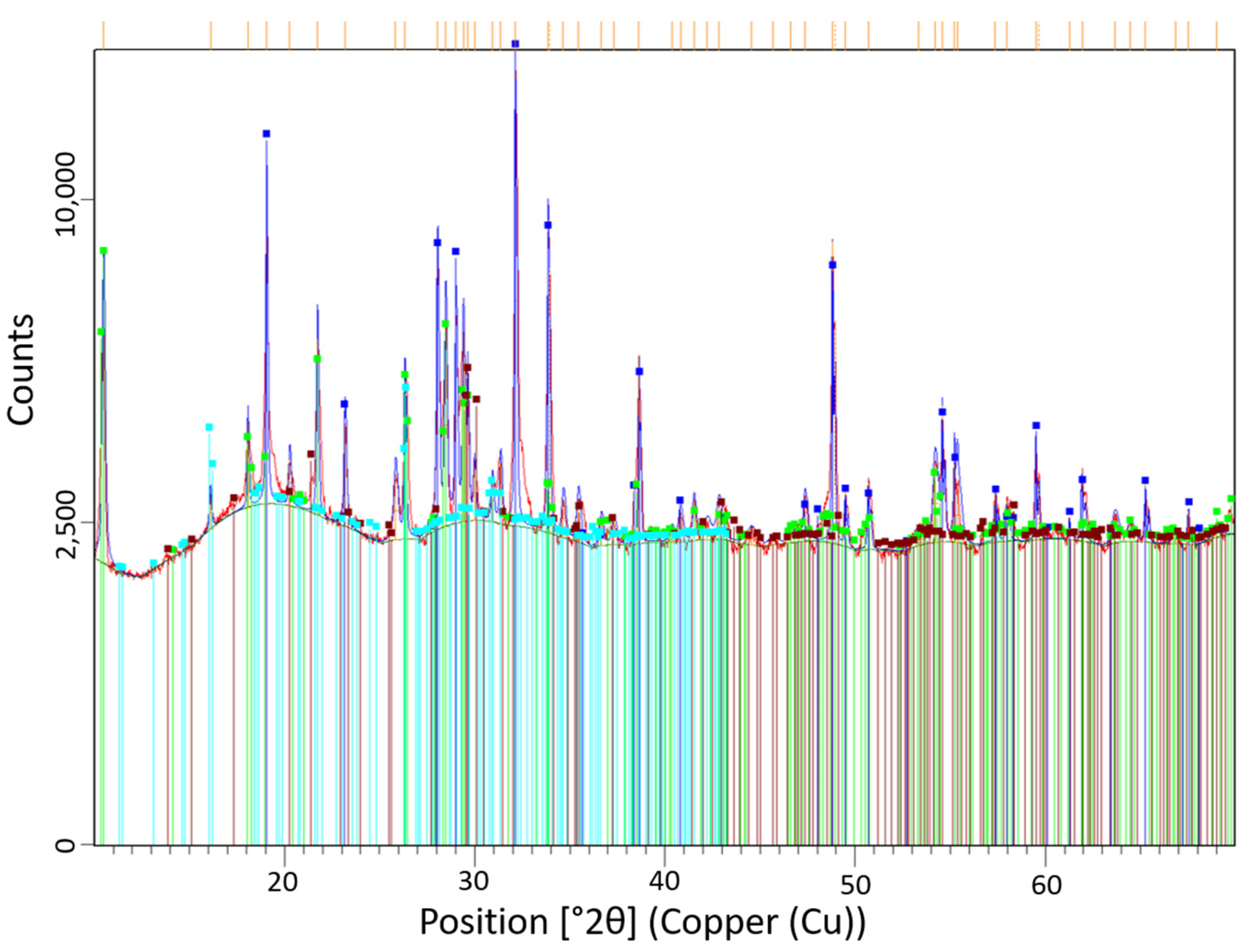
3.1. DPF Ash Composition
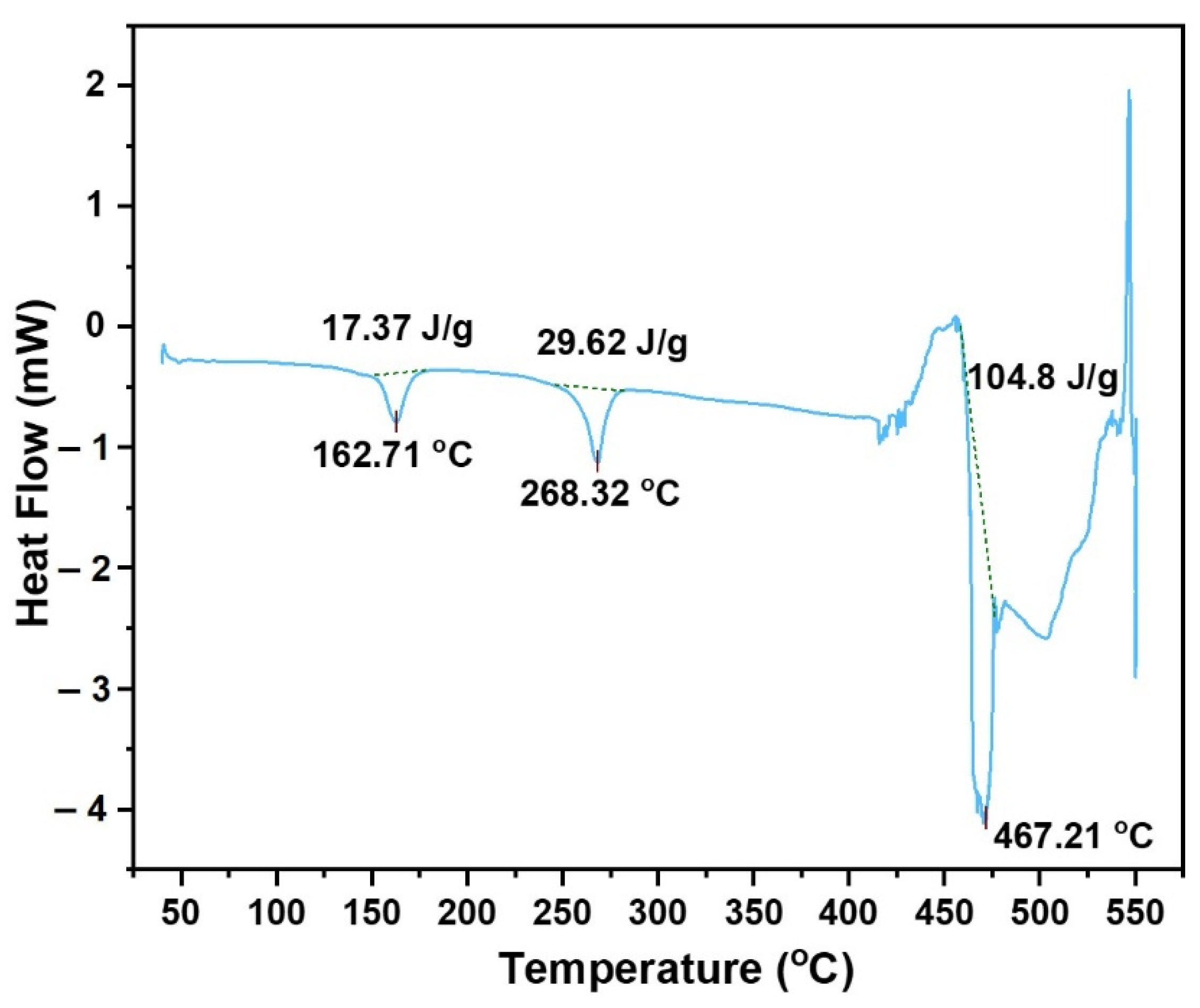
3.2. DSC (Differential Scanning Calorimeter) Analysis
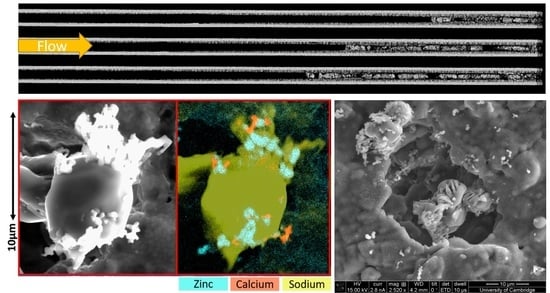
3.3. Na-ash Imaging and Elemental Analysis
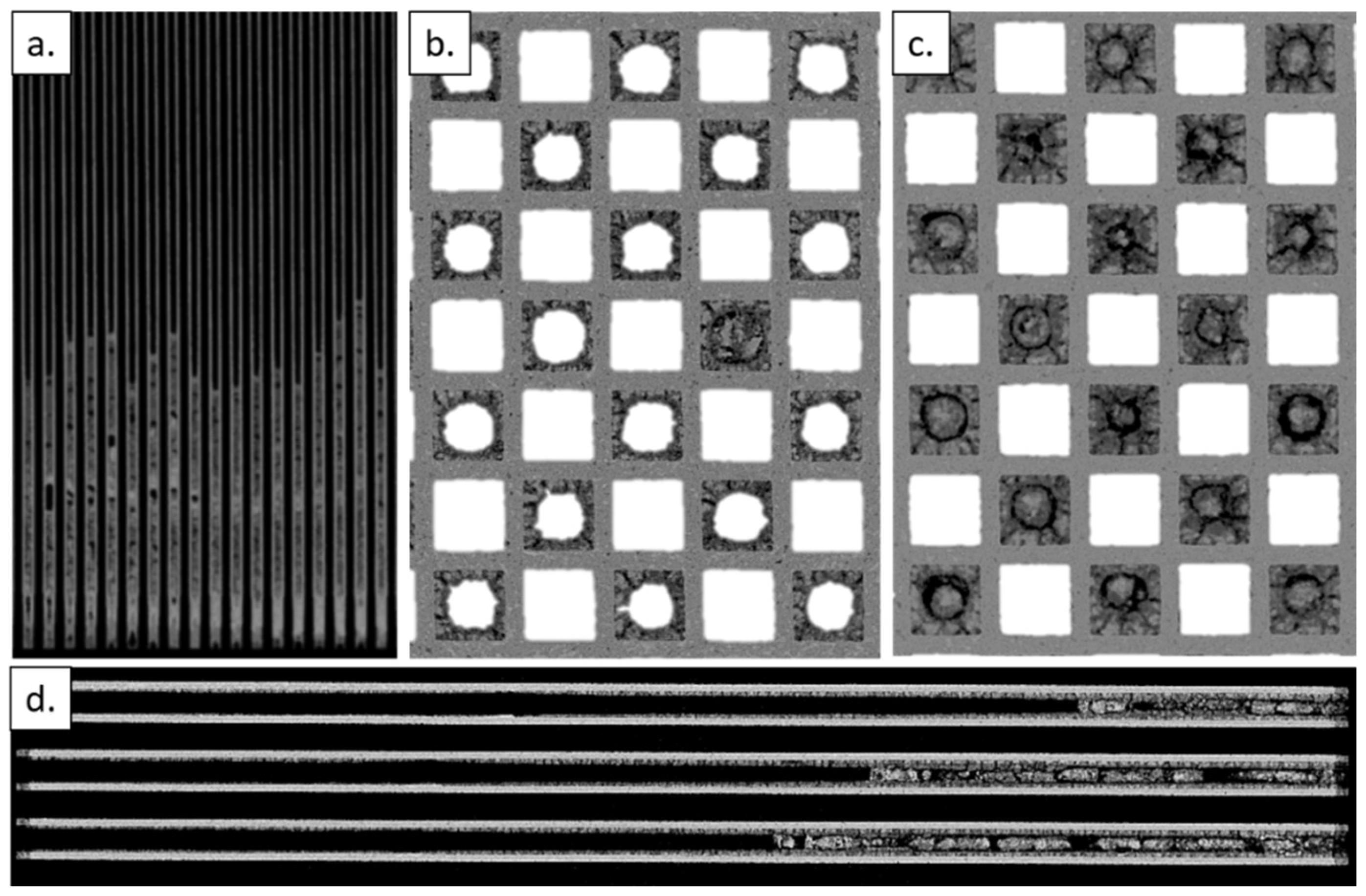
3.4. Ash Agglomeration Profiles
3.5. Na-Ash Penetration
4. Discussion
5. Conclusions
- The ash in this study was found to have a bimodal particle size distribution, where the Ca/Zn/Mg-ash primary particle size was in the 0.5–2 µm range, while Na-ash was around 10 µm and larger. This finding was observed with SEM imaging and validated by EDX mapping. These ash particles were sufficiently large enough to fill filter surface pores with a small number of particles.
- The large NaSO4 ash particles were observed to melt and sinter to the substrate surface, which is explained by the lower melting temperature of NaSO4 (approximately 600 °C lower melting temperature compared to CaSO4). XRD data showed the existence of Na2Si3O7, indicating the chemical binding of sodium ash to the cordierite substrate surface. DSC measurements show a peak at 467.21 °C, which suggests the onset of ash melting at a temperature significantly lower than ash primarily from inorganic lubricant additives.
- The doped biofuel was found to produce additional ash (compared to ULSD), resulting in significantly thick wall ash layers (up to 500 µm in some areas), as shown by X-ray CT axial and radial internal cross sections.
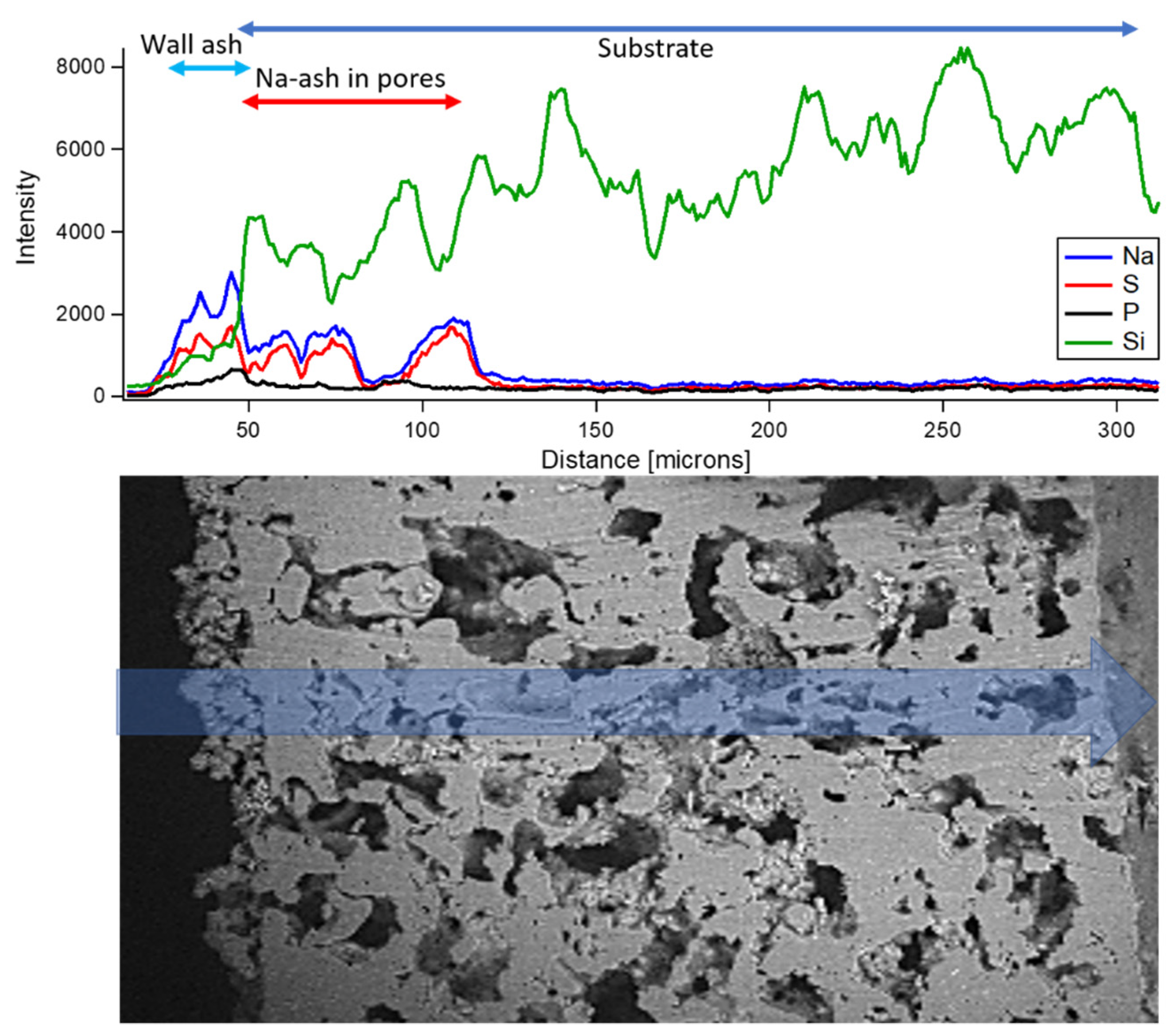
- Ion milling enabled filter cross-sectioning, showing that Na-ash penetrates over 75 µm into the filter wall thickness, which is approximately three times deeper than typical ULSD ash.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| CSIM | cross-section ion milling |
| CT | X-ray computed tomography |
| DOCF | diesel oxidation catalyst on filter |
| DPF | diesel particulate filter |
| DSC | differential scanning calorimeter |
| EBSD | electron backscatter detector |
| EDX | energy dispersive X-ray spectroscopy |
| FIB | focused ion beam milling |
| FUL | full useful life |
| GPF | gasoline particulate filter |
| ICDD | International Centre for Diffraction Data |
| ISN | Institute for Soldier Nanotechnologies |
| MPG | miles per gallon |
| NOx | nitrous oxides |
| OEC | open Eularian cradle |
| powder diffraction file | |
| SAE | Society of Automotive Engineers |
| SCR | selective catalytic reduction |
| SCRF | selective catalytic reduction on filter |
| SEM | scanning electron microscopy |
| STEM | scanning transmission electron microscopy |
| ULSD | ultra-low sulfur diesel |
| XRD | X-ray diffraction |
| XRPD | X-ray powder diffraction |
Appendix A
Appendix A.1. Phase Identification with HighScore Plus Using XRD Pattern
References
- Wang, Y.; Kamp, C.J.; Wang, Y.; Toops, T.J.; Su, C.; Wang, R. The origin, transport, and evolution of ash in engine particulate filters. Appl. Energy 2020, 263, 114631. [Google Scholar] [CrossRef]
- Zhang, B.; Zuo, H.; Huang, Z.; Tan, J.; Zuo, Q. Endpoint forecast of different diesel-biodiesel soot filtration process in diesel particulate filters considering ash deposition. Fuel 2020, 272, 117678. [Google Scholar] [CrossRef]
- André, M.; Hickman, A.J.; Hassel, D.; Joumard, R. Driving Cycles for Emission Measurements Under European Conditions; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1995. [Google Scholar]
- Kim, H.; Chung, J.; Kang, J.; Lee, J.; Park, J. Study on the high efficiency cleaning performance of the diesel vehicle DPF. J. Korea Acad. Ind. Coop. Soc. 2016, 17, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- ASTM-D6751; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2012.
- Alleman, T.L.; Fouts, L.; Chupka, G. Quality Parameters and Chemical Analysis for Biodiesel Produced in the United States in 2011; TP-5400-576622013; NREL: Golden, CO, USA, 2007. [Google Scholar]
- Williams, A.; McCormick, R.; Luecke, J.; Brezny, R.; Geisselmann, A.; Voss, K.; Hallstrom, K.; Leustek, M.; Parsons, J.; Abi-Akar, H. Impact of Biodiesel Impurities on the Performance and Durability of DOC, DPF and SCR Technologies. SAE Int. J. Fuels Lubr. 2011, 4, 110–124. [Google Scholar] [CrossRef]
- Ahari, H.; Andra, R.; Pauly, T. Case Study of Diesel Catalyst Performance Sensitivity and Degradation due to Alkali Metal Poisoning from Suspicious Use of Unregulated Fuel; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2021. [Google Scholar]
- Alleman, T.L.; McCormick, R.L. Results of the 2007 B100 Quality Survey; NREL Technical Report No. 540-42787; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Lance, M.; Wereszczak, A.; Toops, T.; Ancimer, R.; An, H.; Li, J.; Rogoski, L.; Sindler, P.; Williams, A.; Ragatz, A.; et al. Evaluation of Fuel-Borne Sodium Effects on a DOC-DPF-SCR Heavy-Duty Engine Emission Control System: Simulation of Full-Useful Life. SAE Int. J. Fuels Lubr. 2016, 9, 683–694. [Google Scholar] [CrossRef]
- Cavataio, G.; Jen, H.-W.; Dobson, D.A.; Warner, J.R. Laboratory Study to Determine Impact of Na and K Exposure on the Durability of DOC and SCR Catalyst Formulations; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Dou, D.; Balland, J. Impact of Alkali Metals on the Performance and Mechanical Properties of NOx Adsorber Catalysts; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Tatur, M.; NanjundaSwamy, H.; Tomazic, D.; Thornton, M. Effects of Biodiesel Operation on Light-Duty Tier 2 Engine and Emission Control Systems. SAE Int. J. Fuels Lubr. 2008, 1, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Tatur, M.; NanjundaSwamy, H.; Tomazic, D.; Thornton, M.; McCormick, R. Biodiesel Effects on U.S. Light-Duty Tier 2 Engine and Emission Control Systems—Part 2. SAE Int. J. Fuels Lubr. 2009, 2, 88–103. [Google Scholar] [CrossRef]
- Williams, A.; Burton, J.; McCormick, R.L.; Toops, T.J.; Wereszczak, A.A.; Fox, E.E.; Lance, M.J.; Cavataio, G.; Dobson, D.; Warner, J.; et al. Impact of Fuel Metal Impurities on the Durability of a Light-Duty Diesel Aftertreatment System; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Montanaro, L. Durability of ceramic filters in the presence of some diesel soot oxidation additives. Ceram. Int. 1999, 25, 437–445. [Google Scholar] [CrossRef]
- Choi, B.; Kang, H.; Son, G.; Hwang, C. Thermal Aging Behavior of SiC Substrate in the Presence of Ash Materials and Alkali Metals; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Kamp, C.J.; Sappok, A.; Wong, V. Soot and Ash Deposition Characteristics at the Catalyst-Substrate Interface and Intra-Layer Interactions in Aged Diesel Particulate Filters Illustrated using Focused Ion Beam (FIB) Milling. SAE Int. J. Fuels Lubr. 2012, 5, 696–710. [Google Scholar] [CrossRef]
- Bagi, S.; Bowker, R.; Andrew, R. Understanding Chemical Composition and Phase Transitions of Ash from Field Returned DPF Units and Their Correlation with Filter Operating Conditions. SAE Int. J. Fuels Lubr. 2016, 9, 239–259. [Google Scholar] [CrossRef]
- Bagi, S.; Kamp, C.J.; Sharma, V.; Aswath, P.B. Multiscale characterization of exhaust and crankcase soot extracted from heavy-duty diesel engine and implications for DPF ash. Fuel 2020, 282, 118878. [Google Scholar] [CrossRef]
- Bagi, S.; Sharma, V.; Aswath, P.B. Role of dispersant on soot-induced wear in Cummins ISB engine test. Carbon 2018, 136, 395–408. [Google Scholar] [CrossRef]
- Bagi, S.; Singh, N.; Andrew, R. Investigation into Ash from Field Returned DPF Units: Composition, Distribution, Cleaning Ability and DPF Performance Recovery; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2016. [Google Scholar]
- Sappok, A.; Wang, Y.; Wang, R.-Q.; Kamp, C.; Wong, V. Theoretical and Experimental Analysis of Ash Accumulation and Mobility in Ceramic Exhaust Particulate Filters and Potential for Improved Ash Management. SAE Int. J. Fuels Lubr. 2014, 7, 511–524. [Google Scholar] [CrossRef]
- Sappok, A.G. Ash Accumulation in Diesel Particulate Filters. DieselNet. 2013. Available online: www.dieselnet.com (accessed on 24 June 2022).
- Rudnick, L.R. (Ed.) Lubricant Additives: Chemistry and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Kamp, C.J.; Bagi, S. Perspectives on Current and Future Requirements of Advanced Analytical and Characterization Methods in the Automotive Emissions Control Industry. SAE Int. J. Sustain. Transp. Energy Environ. Policy 2021, 2, 141–160. [Google Scholar] [CrossRef]
- Kamp, C.J.; Bagi, S.; Wang, Y. Phenomenological Investigations of Mid-Channel Ash Deposit Formation and Characteristics in Diesel Particulate Filters; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2019. [Google Scholar]
- Morcos, M.; Ayyappan, P.; Harris, T. Characterization of DPF Ash for Development of DPF Regeneration Control and Ash Cleaning Requirements; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2011. [Google Scholar]
- Viswanathan, S.; Rakovec, N.; Foster, D.E. Microscale Study of Ash Accumulation Process in DPF Walls Using the Diesel Exhaust Filtration Analysis (DEFA) System. In Proceedings of the ASME 2012 Internal Combustion Engine Division Fall Technical Conference, Virtual, 25 July 2012; pp. 537–549. [Google Scholar]
- Aravelli, K.; Heibel, A. Improved Lifetime Pressure Drop Management for Robust Cordierite (RC) Filters with Asymmetric Cell Technology (ACT); SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Chen, K.; Martirosyan, K.; Luss, D. Temperature gradients within a soot layer during DPF regeneration. Chem. Eng. Sci. 2011, 66, 2968–2973. [Google Scholar] [CrossRef]
- Callister, W.D. Materials Science and Engineering: An Introduction, 10th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Devarakonda, S.; Groysman, A.; Myerson, A.S. THF–water hydrate crystallization: An experimental investigation. J. Cryst. Growth 1999, 204, 525–538. [Google Scholar] [CrossRef]
- Sappok, A.; Wong, V.W. Lubricant-Derived Ash Properties and Their Effects on Diesel Particulate Filter Pressure-Drop Performance. ASME. J. Eng. Gas Turbines Power. 2011, 133, 032805. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.-Y.; Wang, S.-X.; Chen, W. High temperature synthesis and effect of temperature on morphology of Na4Ca(SO4)3 double salt. Chin. J. Inorg. Chem. 2007, 23, 791–794. [Google Scholar]
- Sappok, A.; Kamp, C.; Wong, V. Sensitivity Analysis of Ash Packing and Distribution in Diesel Particulate Filters to Transient Changes in Exhaust Conditions; SAE International: Warrendale, PA, USA, 2012. [Google Scholar]
- Bagi, S.; Wright, A.M.; Oppenheim, J.; Dinca, M.; Román-Leshkov, Y. Accelerated Synthesis of a Ni2Cl2(BTDD) Metal–Organic Framework in a Continuous Flow Reactor for Atmospheric Water Capture. ACS Sustain. Chem. Eng. 2021, 9, 3996–4003. [Google Scholar] [CrossRef]
- Sonneveld, E.J.; Visser, J.W. Automatic collection of powder data from photographs. J. Appl. Crystallogr. 1975, 8, 1–7. [Google Scholar] [CrossRef]
- Bagi, S.D.; Myerson, A.S.; Román-Leshkov, Y. Solvothermal Crystallization Kinetics and Control of Crystal Size Distribution of MOF-808 in a Continuous Flow Reactor. Cryst. Growth Des. 2021, 21, 6529–6536. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamp, C.J.; Bagi, S.D. Implications of the Use of Biodiesel on the Longevity and Operation of Particle Filters. Lubricants 2022, 10, 259. https://doi.org/10.3390/lubricants10100259
Kamp CJ, Bagi SD. Implications of the Use of Biodiesel on the Longevity and Operation of Particle Filters. Lubricants. 2022; 10(10):259. https://doi.org/10.3390/lubricants10100259
Chicago/Turabian StyleKamp, Carl Justin, and Sujay Dilip Bagi. 2022. "Implications of the Use of Biodiesel on the Longevity and Operation of Particle Filters" Lubricants 10, no. 10: 259. https://doi.org/10.3390/lubricants10100259