Effects of an Electrical Double Layer and Tribo-Induced Electric Field on the Penetration and Lubrication of Water-Based Lubricants
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of Water-Based Lubricants
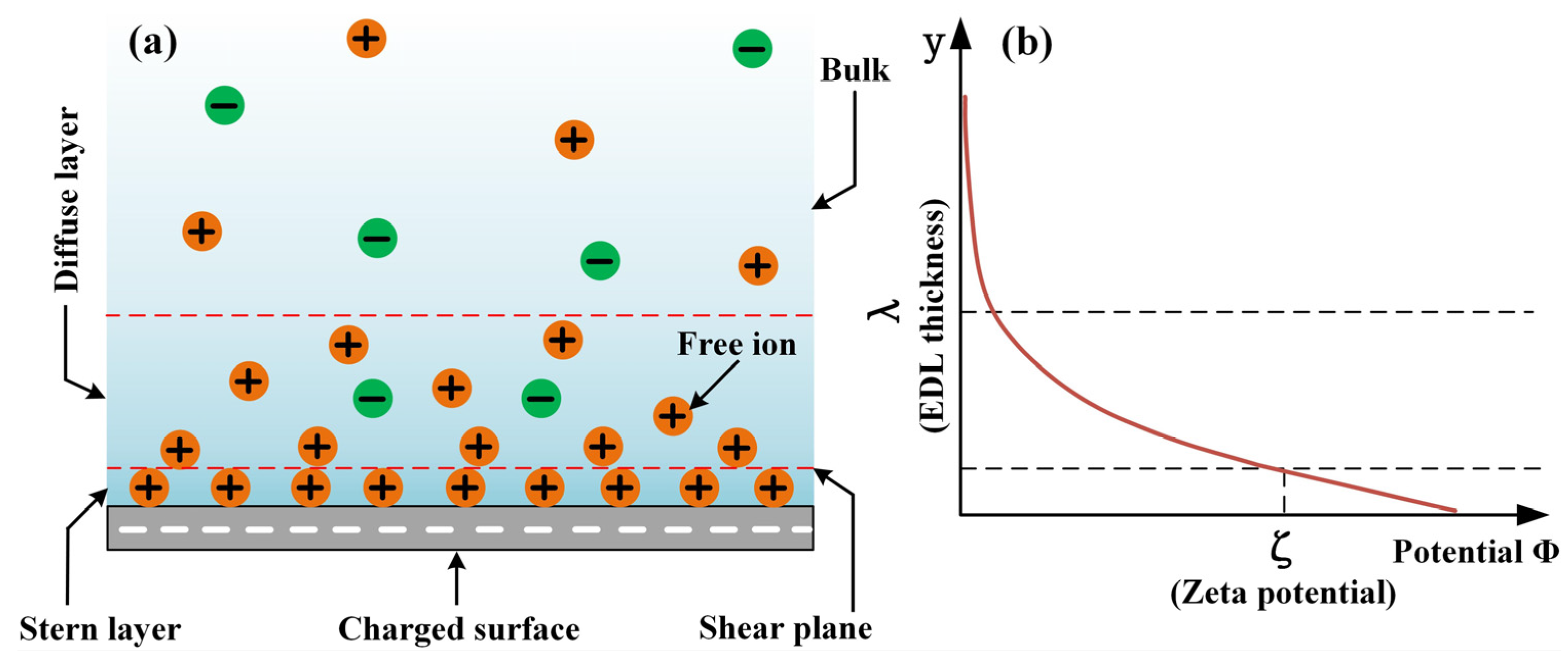
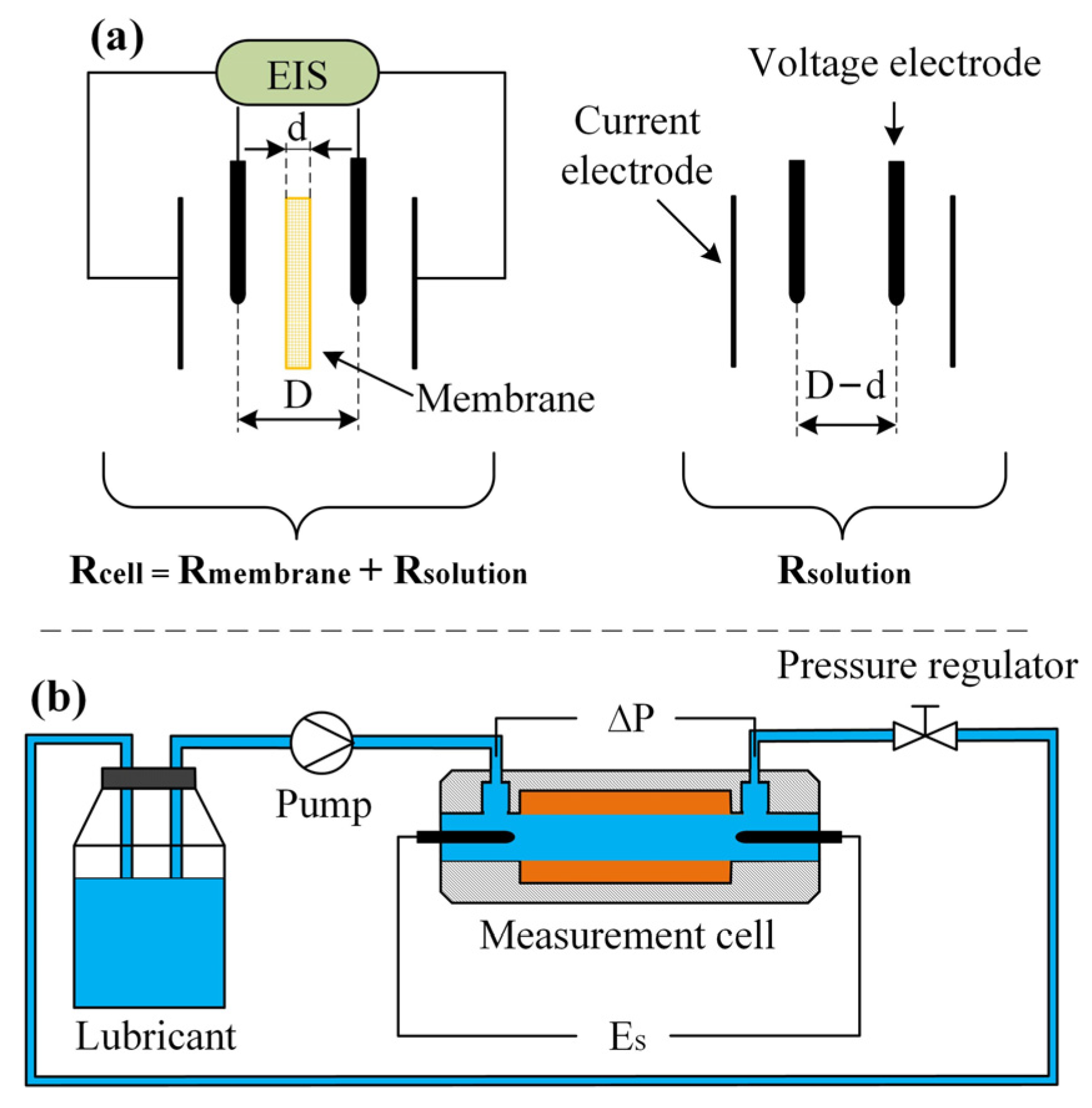
2.2. Measurement of EDL Conductivity and Zeta Potential
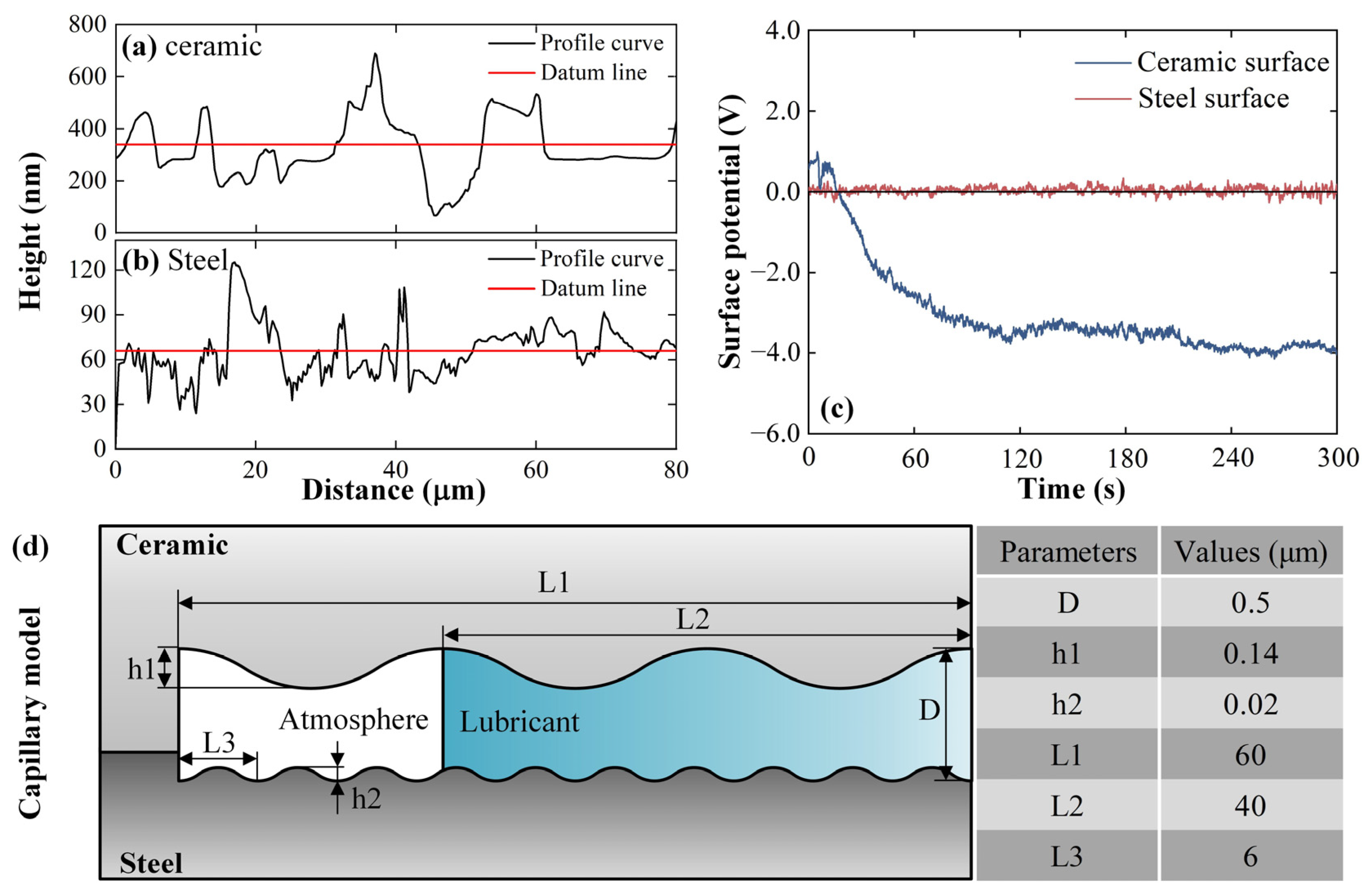
2.3. Analysis of Tribo-Induced Electric Field
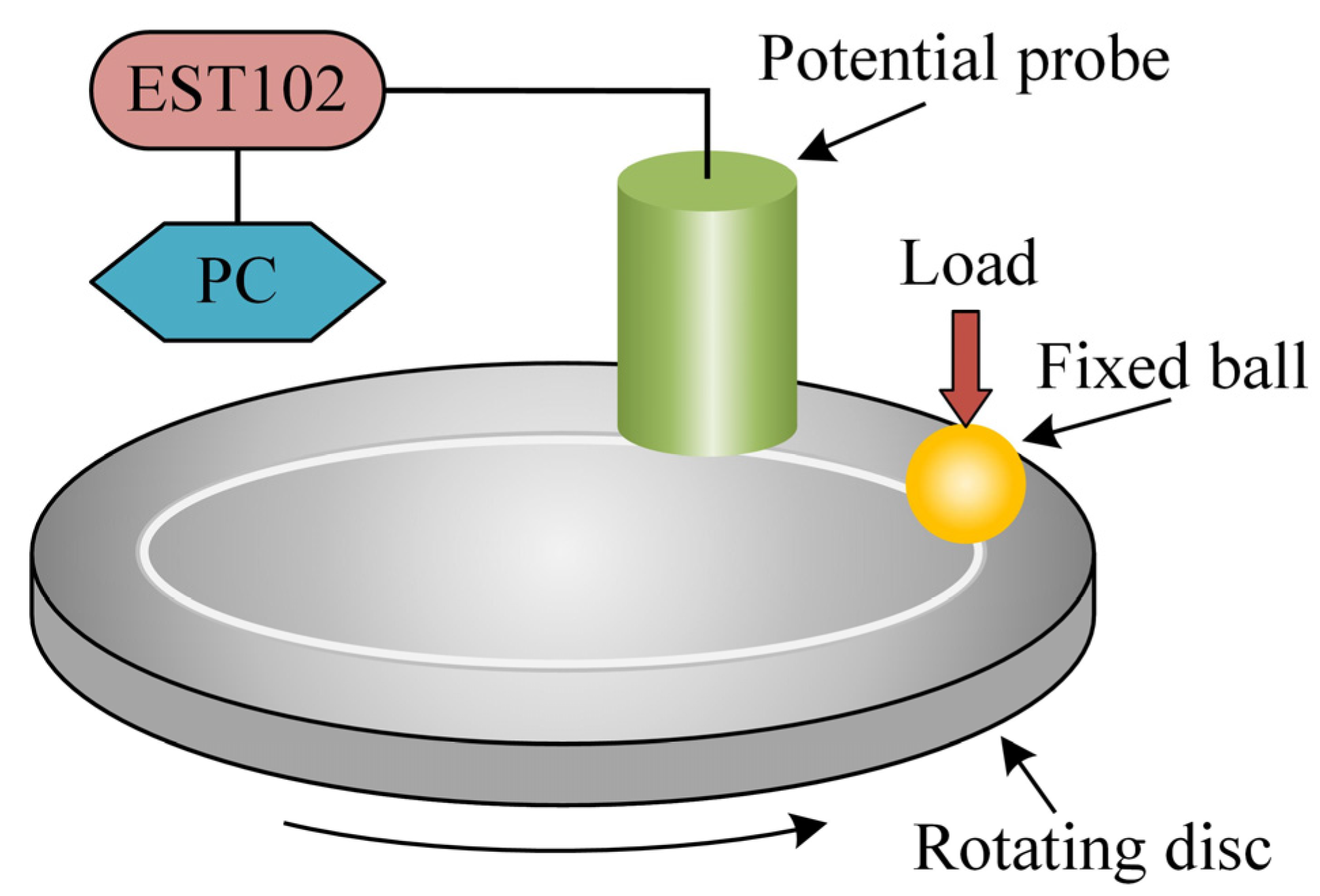
2.4. Tribological Tests
3. Results and Discussion
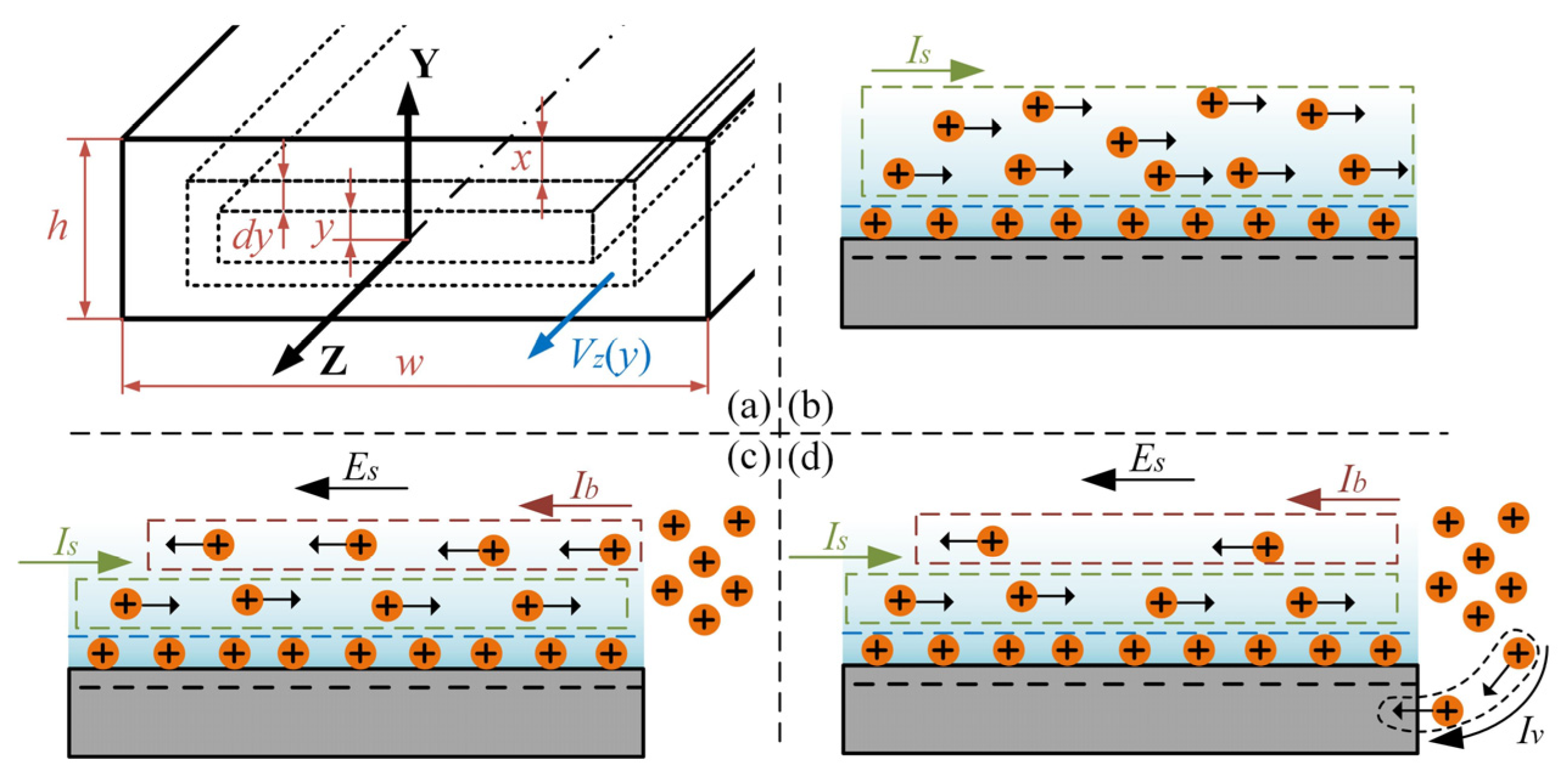
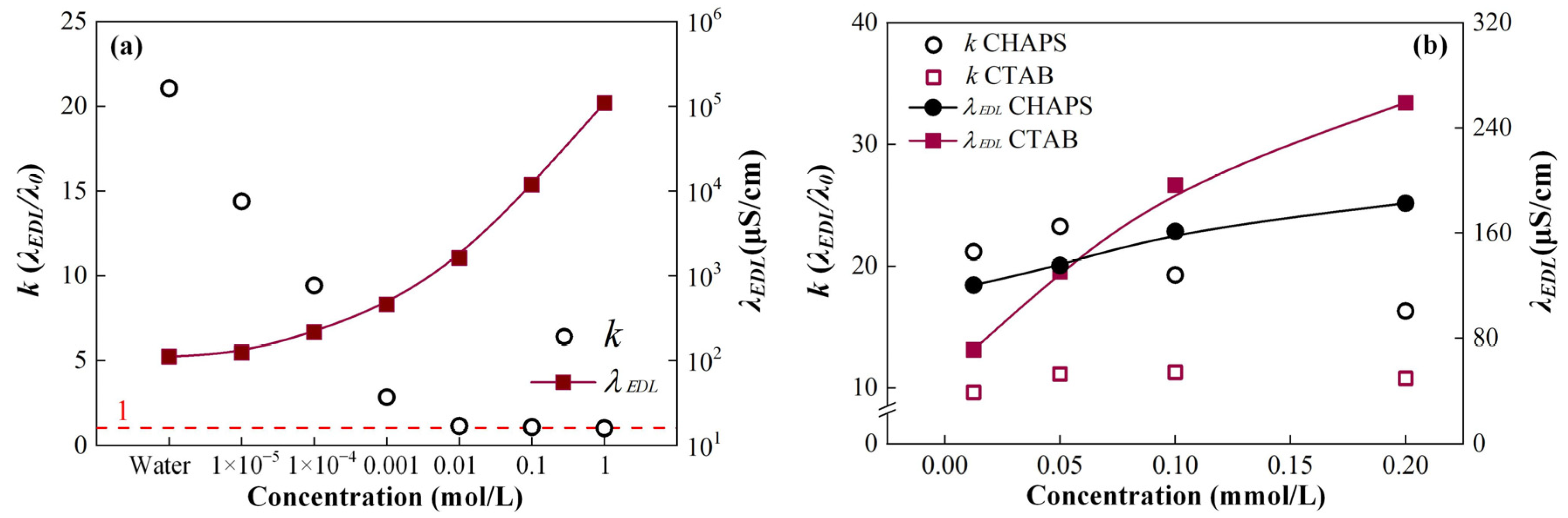
3.1. Electroosmotic Prerequisites
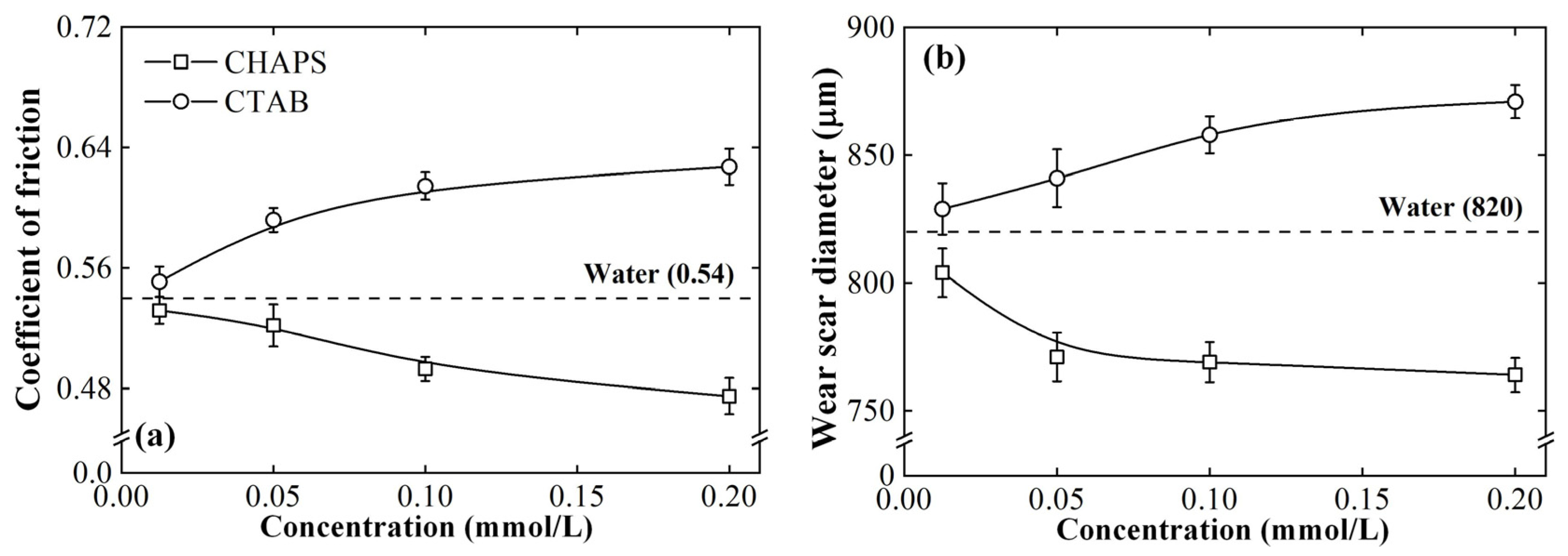
3.2. Tribological Behaviors
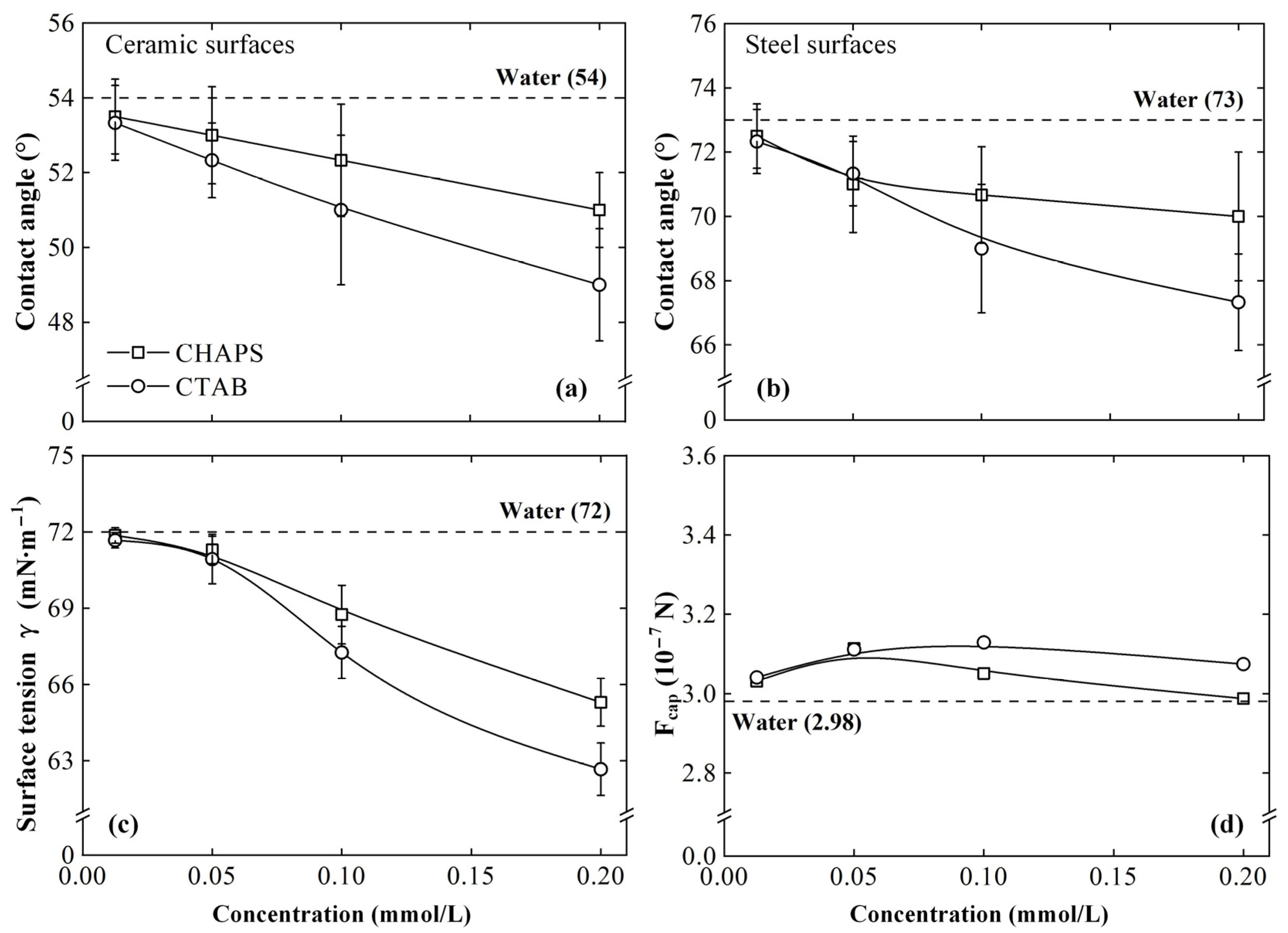
3.3. Mechanism Discussion
4. Conclusions
- The difference between the EDL conductivity and the bulk conductivity of a liquid (λEDL/λ0, k) is sensitive to the change in the ion concentration. The lower the ion concentration, the more obvious the difference. The k of 0.01 mol/L KCl solution is 1.13, while that of pure water is 21.05;
- Due to the charge transfer and ion adsorption at the solid/liquid interface, the alumina ceramic and AISI 52100 steel surfaces are negatively charged in the prepared lubricants. The charged surface attracts the counterions within the lubricant bulk to form an EDL, one of the electroosmotic prerequisites. Cause of the special molecular structure of surfactants CHAPS and CTAB, their adsorption at the solid/liquid interface changes the EDL structure, showing the different zeta potentials;
- The triboelectrification electrostatic potential during ceramic/steel friction was measured. The potential of the ceramic surface is −3.53 V, and that of the steel surface is 0.04 V. The distribution characteristics of the tribo-induced electric field within the capillary at the friction interface were analyzed using the numerical simulation method. The results show that the electric field direction in the lubricant is directed to the capillary inner end, and the intensity is maintained at about 300–600 V/cm, which satisfies the strength condition for driving the lubricant electroosmosis;
- The ceramic/steel friction interface possesses the conditions for inducing the capillary electroosmosis of the lubricant. CHAPS can promote the capillary penetration of the lubricant by improving its electroosmotic properties, presenting satisfactory anti-friction and anti-wear performances. CTAB can reverse the lubricant electroosmosis, thus suppressing its penetrability, showing poor tribological performance.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Calculation of EDL Conductivity from Membrane Resistance
Appendix B. Calculation of Zeta Potential from Streaming Potential
References
- Morshed, A.; Wu, H.; Jiang, Z. A Comprehensive Review of Water-Based Nanolubricants. Lubricants 2021, 9, 89. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-Based Lubricants: Development, Properties, and Performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.; Luo, L.; Huang, S.; Wang, L.; Zhang, S.; Jiao, S.; Huang, H.; Jiang, Z. Performance Evaluation and Lubrication Mechanism of Water-Based Nanolubricants Containing Nano-TiO2 in Hot Steel Rolling. Lubricants 2018, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Kamali, H.; Huo, M.; Lin, F.; Huang, S.; Huang, H.; Jiao, S.; Xing, Z.; Jiang, Z. Eco-Friendly Water-Based Nanolubricants for Industrial-Scale Hot Steel Rolling. Lubricants 2020, 8, 96. [Google Scholar] [CrossRef]
- Sagraloff, N.; Winkler, K.J.; Tobie, T.; Stahl, K.; Folland, C.; Asam, T. Investigations on the Scuffing and Wear Characteristic Performance of an Oil Free Water-Based Lubricant for Gear Applications. Lubricants 2021, 9, 24. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Control of surface forces through hydrated boundary layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Huang, S.; Wu, H.; Jiang, Z.; Huang, H. Water-based nanosuspensions: Formulation, tribological property, lubrication mechanism, and applications. J. Manuf. Processes 2021, 71, 625–644. [Google Scholar] [CrossRef]
- Godlevskiy, V.A. Technological lubricating means: Evolution of materials and ideas. Front. Mech. Eng. 2016, 11, 101–107. [Google Scholar] [CrossRef]
- Brinksmeier, E.; Meyer, D.; Huesmann-Cordes, A.G.; Herrmann, C. Metalworking fluids-Mechanisms and performance. CIRP Ann. 2015, 64, 605–628. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Naerheim, Y.; Lan, M.S. Theoretical analysis of cutting fluid interaction in machining. Tribol. Int. 1988, 21, 239–247. [Google Scholar] [CrossRef]
- Zeytounian, R.K. Interfacial Phenomena and the Marangoni Effect, 1st ed.; Springer: Vienna, Austria, 2002; pp. 123–190. [Google Scholar]
- Xu, X.; Luan, Z.; Zhang, T.; Liu, J.; Feng, B.; Lv, T.; Hu, X. Effects of electroosmotic additives on capillary penetration of lubricants at steel/steel and steel/ceramic friction interfaces. Tribol. Int. 2020, 151, 106441. [Google Scholar] [CrossRef]
- Feng, B.; Luan, Z.; Zhang, T.; Liu, J.; Hu, X.; Guan, J.; Xu, X. Capillary electroosmosis properties of water lubricants with different electroosmotic additives under a steel-on-steel sliding interface. Friction 2021, 10, 1019–1034. [Google Scholar] [CrossRef]
- Chen, L.X.; Ma, J.P.; Tan, F.; Guan, Y.F. Generating high-pressure sub-microliter flow rate in packed microchannel by electroosmotic force: Potential application in microfluidic systems. Sens. Actuators B Chem. 2003, 88, 260–265. [Google Scholar] [CrossRef]
- Li, D. EDL Potential. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: West Lafayette, IN, USA, 2008; pp. 444–453. [Google Scholar]
- Lucy, C.A.; Underhill, R.S. Characterization of the cationic surfactant induced reversal of electroosmotic flow in capillary electrophoresis. Anal. Chem. 1996, 68, 300–305. [Google Scholar] [CrossRef]
- MacDonald, A.M.; Sheppard, M.A.W.; Lucy, C.A. Enhancement of electroosmotic flow using zwitterionic additives. Electrophoresis 2005, 26, 4421–4428. [Google Scholar] [CrossRef]
- Buchberger, W.; Winna, K. Determination of free fatty acids by capillary zone electrophoresis. Mikrochim. Acta 1996, 122, 45–52. [Google Scholar] [CrossRef]
- Möckel, D.; Staude, E.; Dal-Cin, M.; Darcovich, K.; Guiver, M. Tangential flow streaming potential measurements: Hydrodynamic cell characterization and zeta potentials of carboxylated polysulfone membranes. J. Membr. Sci. 1998, 145, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Fievet, P.; Sbaï, M.; Szymczyk, A.; Vidonne, A. Determining the ζ-potential of plane membranes from tangential streaming potential measurements: Effect of the membrane body conductance. J. Membr. Sci. 2003, 226, 227–236. [Google Scholar] [CrossRef]
- Hunter, R.J. Chapter 3-The Calculation of Zeta Potential. In Zeta Potential in Colloid Science; Hunter, R.J., Ed.; Academic Press: Cambridge, MA, USA, 1981; pp. 59–124. [Google Scholar]
- Szymczyk, A.; Fievet, P.; Aoubiza, B.; Simon, C.; Pagetti, J. An application of the space charge model to the electrolyte conductivity inside a charged microporous membrane. J. Membr. Sci. 1999, 161, 275–285. [Google Scholar] [CrossRef]
- Exartier, C.; Maximovitch, S.; Baroux, B. Streaming potential measurements on stainless steels surfaces: Evidence of a gel-like layer at the steel/electrolyte interface. Corros. Sci. 2004, 46, 1777–1800. [Google Scholar] [CrossRef]
- Nakayama, K. Triboemission of charged particles from various solids under boundary lubrication conditions. Wear 1994, 178, 61–67. [Google Scholar] [CrossRef]
- Nakayama, K. The plasma generated and photons emitted in an oil-lubricated sliding contact. J. Phys. D Appl. Phys. 2007, 40, 1103–1107. [Google Scholar] [CrossRef]
- Chang, Y.P.; Chu, H.M.; Chou, H.M. Effects of mechanical properties on the tribo-electrification mechanisms of iron rubbing with carbon steels. Wear 2007, 262, 112–120. [Google Scholar] [CrossRef]
- Charlson, E.M.; Charlson, E.J.; Burkett, S.; Yasuda, H.K. Study of the contact electrification of polymers using contact and separation current. IEEE Trans. Electr. Insul. 1992, 27, 1144–1151. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Meng, Y.; Pei, Y. Superior lubrication performance of MoS2-Al2O3 composite nanofluid in strips hot rolling. J. Manuf. Processes 2020, 57, 312–323. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Meng, Y.; Tang, H.; Wu, P. Improved lubrication performance of MoS2-Al2O3 nanofluid through interfacial tribochemistry. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126428. [Google Scholar] [CrossRef]
- Xiong, S.; Zhang, B.; Luo, S.; Wu, H.; Zhang, Z. Preparation, characterization, and tribological properties of silica-nanoparticle-reinforced B-N-co-doped reduced graphene oxide as a multifunctional additive for enhanced lubrication. Friction 2021, 9, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.T.; Fernández-Mateo, R. Electric force between a dielectric sphere and a dielectric plane. J. Electrostat. 2021, 112, 103601. [Google Scholar] [CrossRef]
- Tatsumi, K.; Nishitani, K.; Fukuda, K.; Katsumoto, Y.; Nakabe, K. Measurement of electroosmotic flow velocity and electric field in microchannels by micro-particle image velocimetry. Meas. Sci. Technol. 2010, 21, 11. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H. Capillary Mechanics, 1st ed.; Science Press: Beijing, China, 2010; pp. 121–123. [Google Scholar]
- Huang, S.; Li, Z.; Yao, W.; Hu, J.; Xu, X. Tribological performance of charged vegetable lubricants. Tribology 2014, 34, 371–378. [Google Scholar]
- Wang, J.; Li, C.; Wang, J.; Zhao, G.; Wang, X. Synthesis and tribological properties of a water-soluble lubricant additive. Lubr. Eng. 2012, 37, 1–6. [Google Scholar]
- Zou, H.; Guo, L.; Xue, H.; Zhang, Y.; Wang, Z.L. Quantifying and understanding the triboelectric series of inorganic non-metallic materials. Nat. Commun. 2020, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Hashimoto, H. Effect of surrounding gas-pressure on triboemission of charged-particles and photons from wearing ceramic surfaces. Tribol. Trans. 1995, 38, 35–42. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, X.; Yang, F.; Wang, K.G.; Bai, J.T.; Qiao, R.; Wang, G.R. Study of Oscillating Electroosmotic Flows with High Temporal and Spatial Resolution. Anal. Chem. 2018, 90, 1652–1659. [Google Scholar] [CrossRef]
- Liu, J.Y.; Liu, H.P.; Han, R.D.; Wang, Y. The study on lubrication action with water vapor as coolant and lubricant in cutting ANSI 304 stainless steel. Int. J. Mach. Tool Manuf. 2010, 50, 260–269. [Google Scholar]
- Wang, S.; Li, X.; Liu, D. Surfactant Chemistry, 1st ed.; Chemical Industry Press: Beijing, China, 2005; pp. 8–13. [Google Scholar]
- Xu, X.F.; Lv, T.; Luan, Z.Q.; Zhao, Y.Y.; Wang, M.H.; Hu, X.D. Capillary penetration mechanism and oil mist concentration of Al2O3 nanoparticle fluids in electrostatic minimum quantity lubrication (EMQL) milling. Int. J. Adv. Manuf. Technol. 2019, 104, 1937–1951. [Google Scholar] [CrossRef]
- Chen, Y. Capillary Electrophoresis Technology and Its Application, 2nd ed.; Chemical Industry Press: Beijing, China, 2005; pp. 9–91. [Google Scholar]
- Hines, H.B.; Brueggemann, E.E. Factors affecting the capillary electrophoresis of ricin, a toxic glycoprotein. J. Chromatogr. A 1994, 670, 199–208. [Google Scholar] [CrossRef]
- Sbai, M.; Fievet, P.; Szymczyk, A.; Aoubiza, B.; Vidonne, A.; Foissy, A. Streaming potential, electroviscous effect, pore conductivity and membrane potential for the determination of the surface potential of a ceramic ultrafiltration membrane. J. Membr. Sci. 2003, 215, 1–9. [Google Scholar] [CrossRef]




| C (mmol/L) | 1 | 0.1 | 0.01 | 0.001 | 0.0001 | 0.00001 | Pure water |
| λ0 (μS/cm) | 109,600 | 11,080 | 1428 | 161.6 | 22.8 | 8.56 | 5.26 |
| Rm (Ω∙cm2) | 0.1 | 0.93 | 6.8 | 24 | 51 | 89 | 99 |
| Parameters | CHAPS Lubricants | CTAB Lubricants | ||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mmol/L) | 0.0125 | 0.05 | 0.1 | 0.2 | 0.0125 | 0.05 | 0.1 | 0.2 |
| λ0 (μS/cm) | 5.69 | 5.82 | 8.36 | 11.20 | 7.39 | 11.71 | 17.39 | 24.03 |
| Rm (Ω∙cm2) | 91 | 81 | 68 | 60 | 154 | 84 | 55.8 | 42.3 |
| Worn Surface | Ra (μm) | Rz (μm) | Rsm (μm) |
|---|---|---|---|
| Alumina ceramic | 0.07 | 0.51 | 20.05 |
| AISI 52100 steel | 0.01 | 0.08 | 5.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Z.; Liu, W.; Xia, Y.; Zhang, R.; Feng, B.; Hu, X.; Huang, S.; Xu, X. Effects of an Electrical Double Layer and Tribo-Induced Electric Field on the Penetration and Lubrication of Water-Based Lubricants. Lubricants 2022, 10, 111. https://doi.org/10.3390/lubricants10060111
Luan Z, Liu W, Xia Y, Zhang R, Feng B, Hu X, Huang S, Xu X. Effects of an Electrical Double Layer and Tribo-Induced Electric Field on the Penetration and Lubrication of Water-Based Lubricants. Lubricants. 2022; 10(6):111. https://doi.org/10.3390/lubricants10060111
Chicago/Turabian StyleLuan, Zhiqiang, Wenshuai Liu, Yu Xia, Ruochong Zhang, Bohua Feng, Xiaodong Hu, Shuiquan Huang, and Xuefeng Xu. 2022. "Effects of an Electrical Double Layer and Tribo-Induced Electric Field on the Penetration and Lubrication of Water-Based Lubricants" Lubricants 10, no. 6: 111. https://doi.org/10.3390/lubricants10060111





