Synergistic Lubricating Performance of Graphene Oxide and Modified Biodiesel Soot as Water Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tribological Tests
2.3. Analysis Methods
3. Results and Discussion
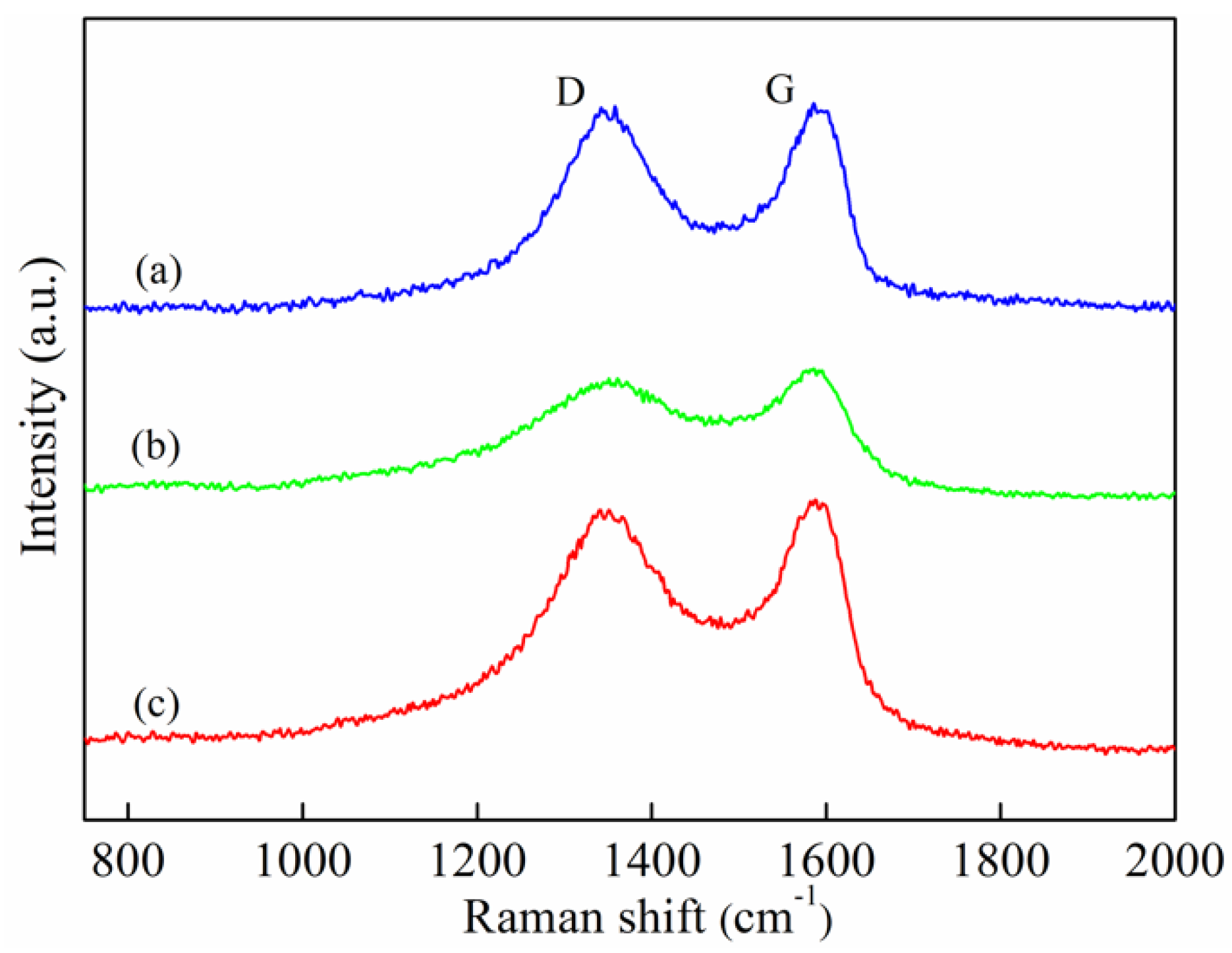
3.1. Characterizations
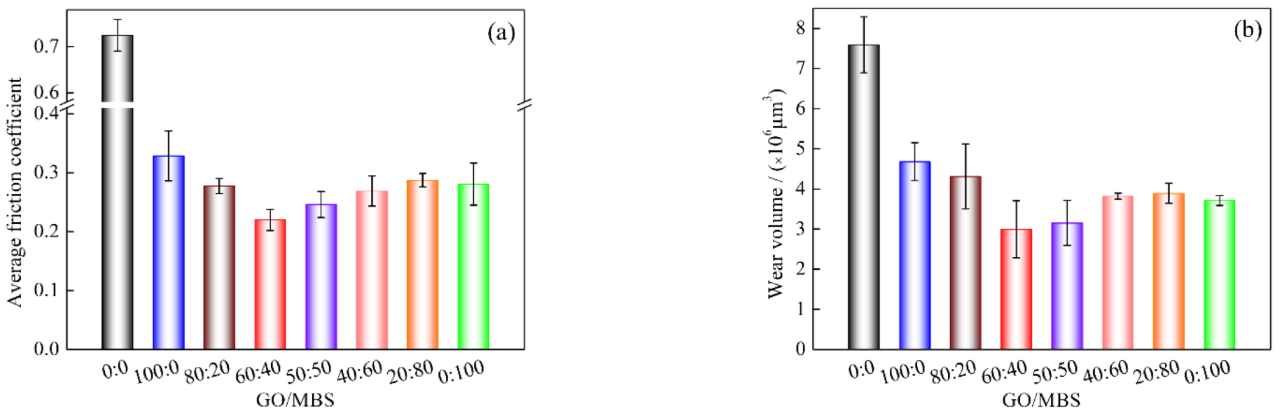
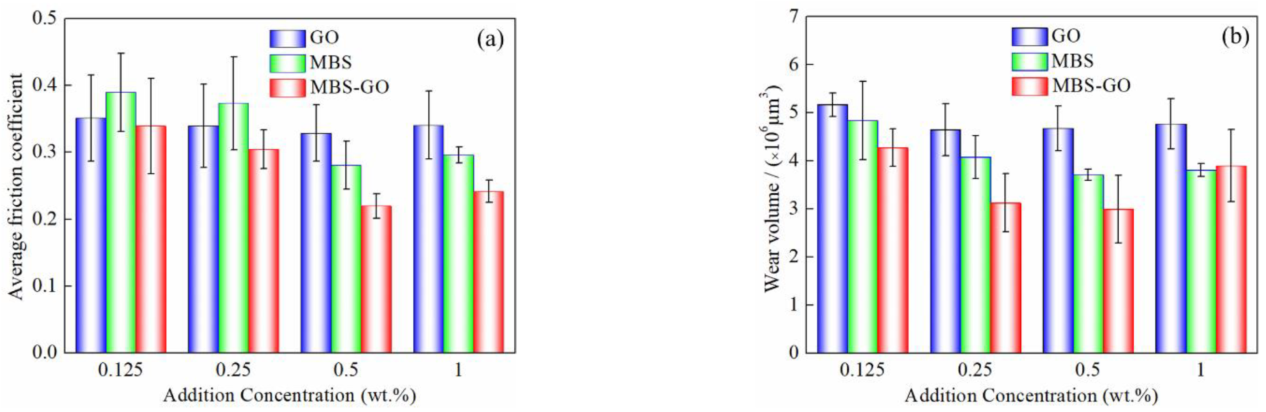
3.2. Antiwear and Friction Reduction
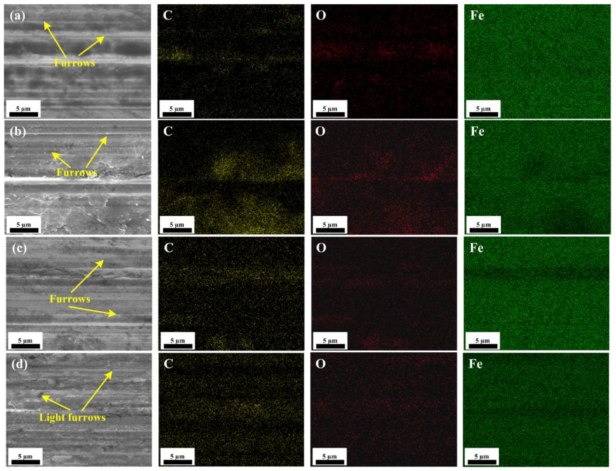
3.3. Worn Surface Analysis
3.4. Mechanism Analysis
4. Conclusions
- (1)
- GO nanosheets have a typical layered structure. When GO nanosheets are used as lubricating additives, a protective graphene tribofilm forms on the rubbing surfaces, improving the tribological properties of water.
- (2)
- MBS nanoparticles have a typical spherical structure. The ball bearing-lubricating effect of the MBS nanoparticles enhances the friction-reduction and antiwear capabilities of water.
- (3)
- In comparison to the GO nanosheets and MBS nanoparticles, water-containing MBS–GO nanoparticles show better lubrication properties. When the additive content is 0.5 wt% and GO−to−MBS mass ratio is 60:40, the friction coefficient and wear volume are reduced by 69.7% and 60.5%, respectively, compared to water. The synergistic effect of the GO nanosheets and MBS nanoparticles during the friction process is primarily responsible for the significant improvement in the lubricating performance of water.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Y.M.; Mandelli, D.; Hod, O.; Urbakh, M.; Ma, M.; Zheng, Q.S. Robust microscale superlubricity in graphite/hexagonal boron nitride layered heterojunctions. Nat. Mater. 2018, 17, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, A.A.; Akl, S.; Elsoudy, S. Investigation of the tribological behavior of mineral lubricant using copper oxide nano additives. Lubricants 2021, 9, 16. [Google Scholar] [CrossRef]
- Sharma, R.V.; Somidi, A.K.; Dalai, A.K. Preparation and properties evaluation of biolubricants derived from canola oil and canola biodiesel. J. Agric. Food. Chem. 2015, 63, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Li, S.W.; Bai, P.P.; Jia, W.P.; Xu, Q.; Meng, Y.G.; Ma, L.R.; Tian, Y. Surface wettability effect on aqueous lubrication: Van der Waals and hydration force competition induced adhesive friction. J. Colloid Interface Sci. 2021, 599, 667–675. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-based lubricants: Development, properties, and performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Morshed, A.; Wu, H.; Jiang, Z. A comprehensive review of water-based nanolubricants. Lubricants 2021, 9, 89. [Google Scholar] [CrossRef]
- Chen, S.Q.; Ding, Q.; Gu, Y.; Quan, X.; Ma, Y.; Jia, Y.L.; Xie, H.M.; Tang, J.Z. Study of tribological properties of fullerenol and nanodiamonds as additives in water-based lubricants for amorphous carbon (a-C) coatings. Nanomaterials 2022, 12, 139. [Google Scholar] [CrossRef]
- Mirzaamiri, R.; Akbarzadeh, S.; Ziaei-Rad, S.; Shin, D.G.; Kim, D.E. Molecular dynamics simulation and experimental investigation of tribological behavior of nanodiamonds in aqueous suspensions. Tribol. Int. 2021, 156, 106838. [Google Scholar] [CrossRef]
- Mou, Z.; Zhao, B.; Wang, B.; Xiao, D. Integration of functionalized polyelectrolytes onto carbon dots for synergistically improving the tribological properties of polyethylene glycol. ACS Appl. Mat. Interfaces 2021, 13, 8794–8807. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.Y.; He, Y.Y.; Shi, Y.J. Nanolubricant additives: A review. Friction 2021, 9, 891–917. [Google Scholar] [CrossRef]
- Fan, K.; Liu, X.K.; Liu, Y.; Li, Y.; Chen, Y.; Meng, Y.Q.; Liu, X.Y.; Feng, W.; Luo, L.B. Covalent functionalization of fluorinated graphene through activation of dormant radicals for water-based lubricants. Carbon 2020, 167, 826–834. [Google Scholar] [CrossRef]
- He, Y.X.; Chen, Q.Y.; Liu, H.; Zhang, L.; Wu, D.Y.; Lu, C.; Yang, W.O.; Jiang, D.F.; Wu, M.F.; Zhang, J.X.; et al. Friction and wear of MoO3/graphene oxide modified glass fiber reinforced epoxy nanocomposites. Macromol. Mater. Eng. 2019, 304, 1900166. [Google Scholar] [CrossRef]
- Song, H.; Wang, Z.; Yang, J. Tribological properties of graphene oxide and carbon spheres as lubricating additives. Appl. Phys. A 2016, 122, 933. [Google Scholar] [CrossRef]
- Su, F.H.; Chen, G.F.; Huang, P. Lubricating performances of graphene oxide and onion-like carbon as water-based lubricant additives for smooth and sand-blasted steel discs. Friction 2020, 8, 47–57. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zeng, X.Q.; Ren, T.H.; van der Heide, E. Tribological properties of graphene oxide sheets as water-based lubricant additive. Ind. Lubr. Tribol. 2018, 70, 1025–1036. [Google Scholar] [CrossRef]
- Gan, C.L.; Liang, T.; Li, X.P.; Li, W.; Li, H.; Fan, X.Q.; Zhu, M.H. Ultra-dispersive monolayer graphene oxide as water-based lubricant additive: Preparation, characterization and lubricating mechanisms. Tribol. Int. 2021, 155, 106768. [Google Scholar] [CrossRef]
- Gan, C.L.; Liang, T.; Li, W.; Fan, X.Q.; Li, X.; Li, D.S.; Zhu, M.H. Hydroxyl-terminated ionic liquids functionalized graphene oxide with good dispersion and lubrication function. Tribol. Int. 2020, 148, 106350. [Google Scholar] [CrossRef]
- Min, C.Y.; He, Z.B.; Song, H.J.; Liang, H.Y.; Liu, D.D.; Dong, C.K.; Jia, W. Fluorinated graphene oxide nanosheet: A highly efficient water-based lubricated additive. Tribol. Int. 2019, 140, 105867. [Google Scholar] [CrossRef]
- Wu, D.Y.; Xu, Y.F.; Yao, L.L.; You, T.; Hu, X.G. Tribological behaviour of graphene oxide sheets as lubricating additives in bio-oil. Ind. Lubr. Tribol. 2018, 70, 1396–1401. [Google Scholar] [CrossRef]
- Wu, P.; Chen, X.C.; Zhang, C.H.; Luo, J.B. Synergistic tribological behaviors of graphene oxide and nanodiamond as lubricating additives in water. Tribol. Int. 2019, 132, 177–184. [Google Scholar] [CrossRef]
- Huang, S.Q.; He, A.S.; Yun, J.; Xu, X.F.; Jiang, Z.Y.; Jiao, S.H.; Huang, H. Synergistic tribological performance of a water based lubricant using graphene oxide and alumina hybrid nanoparticles as additives. Tribol. Int. 2019, 135, 170–180. [Google Scholar] [CrossRef]
- Du, S.N.; Sun, J.L.; Wu, P. Preparation, characterization and lubrication performances of graphene oxide-TiO2 nanofluid in rolling strips. Carbon 2018, 140, 338–351. [Google Scholar] [CrossRef]
- Min, C.Y.; Zhang, Q.Q.; Shen, C.; Liu, D.D.; Shen, X.J.; Song, H.J.; Li, S.J.; Xu, D.; Lin, X.Y.; Zhang, K. Graphene oxide/carboxyl-functionalized multi-walled carbon nanotube hybrids: Powerful additives for water-based lubrication. RSC Adv. 2017, 7, 32574–32580. [Google Scholar] [CrossRef]
- Guo, P.F.; Chen, L.; Wang, J.J.; Geng, Z.R.; Lu, Z.B.; Zhang, G.G. Enhanced tribological performance of aminated nano-silica modified graphene oxide as water-based lubricant additive. ACS Appl. Nano Mater. 2018, 1, 6444–6453. [Google Scholar] [CrossRef]
- Green, D.A.; Lewis, R.; Dwyer-Joyce, R.S. Wear effects and mechanisms of soot-contaminated automotive lubricants. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 159–169. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Hu, X.G.; Duan, F.Q.; Meng, Y.G. An efficient lubrication approach to mitigate soot-induced wear: Synergistic repair effect of magnetic MoS2 composites and magnetic field. Wear 2022, 488, 204182. [Google Scholar] [CrossRef]
- Hu, E.Z.; Hu, X.G.; Liu, T.X.; Liu, Y.M.; Song, R.H.; Chen, Y.Z. Investigation of morphology, structure and composition of biomass-oil soot particles. Appl. Surf. Sci. 2013, 270, 596–603. [Google Scholar] [CrossRef]
- Li, C.; Wei, D.Z.; Zhuang, Y.; Song, R.H.; Hu, X.G. Effect of biodiesel soot on the tribological behavior of liquid paraffin. China Pet. Process. Petrochem. Technol. 2018, 20, 106–113. [Google Scholar]
- Mulay, M.R.; Chauhan, A.; Patel, S.; Balakrishnan, V.; Halder, A.; Vaish, R. Candle soot: Journey from a pollutant to a functional material. Carbon 2019, 144, 684–712. [Google Scholar] [CrossRef]
- Liu, T.X.; Wang, J.; Kang, K.; Qin, J.; Tang, Z.Q. Preparation, characterization and tribological properties of coal indirect liquefied diesel soot modified by oleylamine. Appl. Surf. Sci. 2021, 550, 149351. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Chaushev, T.A.; Mohammadi, R.; Vladkova, T.G. Silver-doped superhydrophobic carbon soot coatings with enhanced wear resistance and anti-microbial performance. Colloids Surf. A 2019, 582, 123880. [Google Scholar] [CrossRef]
- Li, C.; Li, M.L.; Wang, X.Y.; Feng, W.M.; Zhang, Q.Q.; Wu, B.; Hu, X.G. Novel carbon nanoparticles derived from biodiesel soot as lubricant additives. Nanomaterials 2019, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.Y.; Wu, F.Y.; Liu, T.X.; Hu, X.G. Preparation, characterization and water-based lubricity of modified biodiesel soot. Chem. J. Chin. Univ. 2020, 41, 987–994. [Google Scholar]
- Bondarev, A.V.; Fraile, A.; Polcar, T.; Shtansky, D.V. Mechanisms of friction and wear reduction by h-BN nanosheet and spherical W nanoparticle additives to base oil: Experimental study and molecular dynamics simulation. Tribol. Int. 2020, 151, 106493. [Google Scholar] [CrossRef]
- Fei, J.; Qi, Y.; Luo, L.; Gu, Y.F.; Huang, J.F. Synergistic effect of talc/carbon spheres composite as oil-based additive enhancing the lubricating properties for steel-steel contact. Lubr. Sci. 2020, 32, 80–89. [Google Scholar] [CrossRef]
- Tian, X.; Song, N.N.; Yang, G.B.; Zhou, C.H.; Zhang, S.M.; Zhang, P.Y. Organic-sulfonate functionalized graphene as a high temperature lubricant for efficient antifriction and antiwear in water based drilling fluid. Tribol. Lett. 2022, 70, 32. [Google Scholar] [CrossRef]
- Hao, L.; Hao, W.D.; Li, P.P.; Liu, G.M.; Li, H.Y.; Aljabri, A.; Xie, Z.L. Friction and wear properties of a nanoscale ionic liquid-like GO@ SiO2 hybrid as a water-based lubricant additive. Lubricants 2022, 10, 125. [Google Scholar] [CrossRef]
- Liu, C.C.; Guo, Y.B.; Wang, D.G. PEI-RGO nanosheets as a nanoadditive for enhancing the tribological properties of water-based lubricants. Tribol. Int. 2019, 140, 105851. [Google Scholar] [CrossRef]
- Li, C.; Hu, X.G. Biodiesel Soot: Tribology, Properties, and Formation; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Wu, B.; Song, H.; Li, C.; Song, R.H.; Zhang, T.M.; Hu, X.G. Enhanced tribological properties of diesel engine oil with nano-lanthanum hydroxide/reduced graphene oxide composites. Tribol. Int. 2020, 141, 105951. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.; Zhang, Q.Q.; Hu, X.G. Controllable synthesis and friction reduction of ZnFe2O4@C microspheres with diverse core-shell architectures. Tribol. Int. 2021, 153, 106614. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Song, H.; Wu, B.; Feng, W.M.; Li, X.Y.; Jiao, Y.; Hu, X.G. Effect of magnetic field on the tribological behaviors of Fe3O4@ MoS2 as polyalphaolefin additive in the steel/steel friction interface. Wear 2021, 466, 203586. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.X. Study on the Tribological properties of F-T DS/ZnFe-LDH composite lubricating material. Appl. Sci. 2022, 12, 599. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, R.L.; Rao, L.Z.; Kim, D.C.; Kook, S. The influence of a large methyl ester on in-flame soot particle structures in a small-bore diesel engine. Fuel 2017, 194, 423–435. [Google Scholar] [CrossRef]
- Xu, Y.F.; Geng, J.; Peng, Y.B.; Liu, Z.C.; Yu, J.Y.; Hu, X.G. Lubricating mechanism of Fe3O4@MoS2 core-shell nanocomposites as oil additives for steel/steel contact. Tribol. Int. 2018, 121, 241–251. [Google Scholar] [CrossRef]
- Liu, H.L.; Huang, Y.J.; Wang, Y.Z.; Zhao, X.M.; Chen, D.Q.; Chen, G.H. Study of tribological properties and lubrication mechanism of surfactant-coated anthracite sheets used as lubricant additives. Friction 2021, 9, 524–537. [Google Scholar] [CrossRef]
- Li, X.M.; Deng, J.X.; Liu, L.L.; Duan, R.; Ge, D.L. Fabrication of WS2/C composite coatings via electrohydrodynamic atomization and their tribology behaviours. Appl. Surf. Sci. 2021, 538, 148128. [Google Scholar] [CrossRef]
- Wei, J.X.; Cai, M.R.; Zhou, F.; Liu, W.M. Candle soot as particular lubricant additives. Tribol. Lett. 2014, 53, 521–531. [Google Scholar] [CrossRef]
- Fan, K.; Liu, J.; Wang, X.; Liu, Y.; Lai, W.C.; Gao, S.S.; Qin, J.Q.; Liu, X.Y. Towards enhanced tribological performance as water-based lubricant additive: Selective fluorination of graphene oxide at mild temperature. J. Colloid Interface Sci. 2018, 531, 138–147. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Q.Q.; Song, H.; Yang, B.X.; Tian, M.; Hu, X.G. Preparation, dispersion and tribological properties of oleophilic lanthanum hydroxide/graphene oxide nanocomposites. China Pet. Process. Petrochem. Technol. 2020, 22, 111. [Google Scholar]
- Zhang, L.L.; Pu, J.B.; Wang, L.P.; Xue, Q.J. Synergistic effect of hybrid carbon nanotube–graphene oxide as nanoadditive enhancing the frictional properties of ionic liquids in high vacuum. ACS Appl. Mat. Interfaces 2015, 7, 8592–8600. [Google Scholar] [CrossRef]
- Tang, J.Z.; Chen, S.Q.; Jia, Y.L.; Ma, Y.; Xie, H.M.; Quan, X.; Ding, Q. Carbon dots as an additive for improving performance in water-based lubricants for amorphous carbon (a-C) coatings. Carbon 2020, 156, 272–281. [Google Scholar] [CrossRef]
- Wang, W.; Dong, S.W.; Gao, Y.; Zhang, G.L.; Wang, K.S. Tribological behaviours of black phosphorus/MoS2 composites as water-based lubrication additives. Lubr. Sci. 2021, 33, 404–416. [Google Scholar] [CrossRef]
- Hu, Y.W.; Wang, Y.X.; Zeng, Z.X.; Zhao, H.C.; Li, J.L.; Ge, X.W.; Wang, L.P.; Xue, Q.J.; Mao, C.L.; Chen, S.J. BLG-RGO: A novel nanoadditive for water-based lubricant. Tribol. Int. 2019, 135, 277–286. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Etacheri, V.; Dysart, A.D.; Stacke, L.E.; Pol, V.G.; Sadeghi, F. Ultrasmooth submicrometer carbon spheres as lubricant additives for friction and wear reduction. ACS Appl. Mat. Interfaces 2015, 7, 5514–5521. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, Y.X.; Liu, D.; Tong, D.Y.; Liu, S.G. Nanostructured molybdenum/heteroatom-doped carbon dots nanohybrids for lubrication by direct carbonization route. Mater. Lett. 2019, 250, 20–24. [Google Scholar] [CrossRef]
- Zhang, J.S.; Chen, Z.X.; Wu, H.; Zhao, J.W.; Jiang, Z.Y. Effect of graphene on the tribolayer of aluminum matrix composite during dry sliding wear. Surf. Coat. Technol. 2019, 358, 907–912. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Hu, H.; Khadem, M.; Penkov, O.V. Tribology of 2D nanomaterials: A review. Coatings 2020, 10, 897. [Google Scholar] [CrossRef]
- Ye, M.T.; Cai, T.; Shang, W.J.; Zhao, L.N.; Zhang, Y.X.; Liu, D.; Liu, S.G. Friction-induced transfer of carbon quantum dots on the interface: Microscopic and spectroscopic studies on the role of inorganic–organic hybrid nanoparticles as multifunctional additive for enhanced lubrication. Tribol. Int. 2018, 127, 557–567. [Google Scholar] [CrossRef]
- Zhang, W.L.; Cao, Y.L.; Tian, P.Y.; Guo, F.; Tian, Y.; Zheng, W.; Ji, X.Q.; Liu, J.Q. Soluble, exfoliated two-dimensional nanosheets as excellent aqueous lubricants. ACS Appl. Mat. Interfaces 2016, 8, 32440–32449. [Google Scholar] [CrossRef]
- Guo, P.F.; Qi, S.S.; Chen, L.; Gou, C.X.; Lin, B.; Lu, Z.B.; Wu, Z.G.; Zhang, G.G. Black phosphorus–graphene oxide hybrid nanomaterials toward advanced lubricating properties under water. Adv. Mater. Interfaces 2019, 6, 1901174. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wu, B.; Chen, X.; Li, L.; Wang, X.; Gao, X.; Wang, X.; Hu, K.; Hu, X. Synergistic Lubricating Performance of Graphene Oxide and Modified Biodiesel Soot as Water Additives. Lubricants 2022, 10, 175. https://doi.org/10.3390/lubricants10080175
Li C, Wu B, Chen X, Li L, Wang X, Gao X, Wang X, Hu K, Hu X. Synergistic Lubricating Performance of Graphene Oxide and Modified Biodiesel Soot as Water Additives. Lubricants. 2022; 10(8):175. https://doi.org/10.3390/lubricants10080175
Chicago/Turabian StyleLi, Chuan, Bo Wu, Xiaoju Chen, Lei Li, Xinyun Wang, Xiaobao Gao, Xiaodong Wang, Kunhong Hu, and Xianguo Hu. 2022. "Synergistic Lubricating Performance of Graphene Oxide and Modified Biodiesel Soot as Water Additives" Lubricants 10, no. 8: 175. https://doi.org/10.3390/lubricants10080175





